Abstract
Gadfly petrels are among the widest-ranging birds and inhabit oceanic regions beyond the legislative protection of national jurisdictions (the High Seas). Detailed information on breeding phenology, at-sea distributions, and habitat requirements is crucial for understanding threats and designing conservation measures for this highly threatened group. We tracked 10 Stejneger’s petrels Pterodroma longirostris, endemic to Isla Alejandro Selkirk, Juan Fernández Islands in the southeast Pacific Ocean, with geolocator-immersion loggers over two years to examine year-round movements, phenology, habitat use, and activity patterns. Birds conducted round-trip trans-equatorial migrations of 54,725 km to the northwest Pacific Ocean between Hawaii and Japan. Across the boreal summer, birds followed the c. 1000 km northward movement of the North Pacific Transition Zone Chlorophyll Front, before their return migration which took a long detour south toward New Zealand before heading east at 40–50°S, presumably benefitting from Antarctic circumpolar winds. To our knowledge, a comparable triangular migration is unique among seabirds. During the pre-laying exodus, birds traveled southwest to the Sub-Antarctic Front, and unlike congeners, there was no evidence of sexual segregation. Foraging areas during incubation were similar to pre-laying, with trips lasting 13 d and taking birds up to 4810 km southwest of the colony. Petrels spent > 75% of their time flying during breeding and migration, yet flight activity was substantially lower during non-breeding, presumably due to flight feather molt. Birds spent 87% of their time at sea within the High Seas and their apparent preference for oceanic frontal regions demonstrates the importance of protecting these remote habitats.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seabirds play vital ecological roles within marine and terrestrial ecosystems (Brooke 2004a; Piatt and Sydeman 2007). As a result of their wide-ranging movements and use of marine and terrestrial habitats for feeding and breeding, respectively, they facilitate nutrient and pollutant transfer between distant habitats, and across ecosystem boundaries (Grant et al. 2022). Despite their ecological importance, seabirds are among the most threatened groups of birds (Croxall et al. 2012). Monitored seabird populations have declined by over 70% since the 1950s (Paleczny et al. 2015), mainly due to direct harvesting and introduced species at breeding sites, incidental mortality (bycatch) in fisheries and climate change (Brooke et al. 2018; Dias et al. 2019). A baseline understanding of their breeding and spatial ecology is important to characterize at-sea distributions and to assess the impacts of marine and terrestrial threats; yet, many aspects of seabird ecology remain poorly understood, especially for single-island endemic species or those breeding in remote and hard-to-access areas such as offshore islands (Rodríguez et al. 2019).
Among the most threatened and wide-ranging of all seabirds are the 37 species of gadfly petrel in the genera Pterodroma and Pseudobulweria (Adams and Flora 2009; Clay et al. 2017, 2023; Ramos et al. 2017; Ventura et al. 2020). These species are poorly understood for a variety of reasons (Spear et al. 1992): first, they can be very hard to distinguish at sea due to similarity in appearance, complicated taxonomy (particularly smaller taxa in the sub-genus Cookilaria), and fast, maneuverable flight style (Roberson and Bailey 1991; Spear et al. 1992). Second, they commonly breed on inaccessible mountains, cliffs, or remote islands far from human settlement. Third, they forage in open ocean habitats and are often not attracted to vessels so are not well documented by at-sea surveys (Roberson and Bailey 1991; Spear et al. 2007). Over the past two decades, technological advancements in the development of biologging devices, including their miniaturization, have facilitated the study of gadfly petrel movements and foraging behavior, and allowed increasingly smaller species to be tracked (Rodríguez et al. 2019; Bernard et al 2021). Yet, despite the wealth of information provided by seabird tracking efforts, studies have tended not to focus on species of conservation concern, and large data gaps remain in particular regions such as the Pacific Ocean (Bernard et al. 2021).
Research on gadfly petrels has shown that during breeding, they have some of the widest-ranging foraging trips of any taxa (Clay et al. 2019; Taylor et al. 2020; Ventura et al. 2020). Birds cover vast distances by spending a large proportion of their time in flight (during day and night; e.g., Ramirez et al. 2013; Clay et al. 2017; Bonnet-Lebrun et al. 2021; Rayner et al. 2023), often facilitated by their use of ocean basin-scale wind patterns (Adams and Flora 2009; Ventura et al. 2020; Clay et al. 2023). Species vary in migration strategies, including trans-equatorial or longitudinal migration, or residency (Warham 1990; Rayner et al. 2011; 2023; Pinet et al. 2011; Ramos et al. 2017; Bonnet-Lebrun et al. 2021). When not breeding, birds are generally less mobile and concentrate in discrete oceanic regions, with different species generally using non-overlapping areas (Rayner et al. 2016; 2023; Ramos et al. 2017). While gadfly petrels are more threatened on land than at sea, a complete knowledge of their year-round movements and at-sea distributions is important toward understanding the effects of climate change and emerging threats, such as plastic and other chemical pollution on populations (Dias et al. 2019; Clark et al. 2023). Indeed, their high mobility and propensity to forage in oceanic habitats present potential challenges for conservation as species are unlikely to be afforded protection by spatial management approaches such as marine protected areas (MPAs; Oppel et al. 2018; Beal et al. 2021).
Stejneger’s petrel Pterodroma longirostris is a small (c. 150 g; Spear et al. 1992) and poorly known gadfly petrel endemic to Isla Alejandro Selkirk in the Juan Fernández archipelago, 800 km west of central Chile. Only basic information is available on its breeding biology; birds lay their eggs in late December and early January, incubation shifts are thought to last around two to three weeks (Brooke 1987) and the peak of hatching occurs during the first half of February (Hodum and Wainstein 2003). In 1986, the breeding population was estimated to be around 131,000 pairs (Brooke 1987) but is thought to be lower or declining due to predation from introduced feral cats Felis catus and brown rats Rattus norvegicus, which appear to disproportionately prey upon Stejneger’s compared with the larger Juan Fernández petrel P. externa (Brooke 1987; Bourne et al. 1992). No reliable information is available on population trends, but the species is listed as Vulnerable by the IUCN due to its single-island endemic status, making it particularly susceptible to human impacts and stochastic events (Hodum and Wainstein 2003; BirdLife International 2019). Stejneger’s petrel is a migrant to the subtropical northwest Pacific Ocean as far west as Japan (Falla 1942; Tanaka et al. 1985) with birds apparently using a corridor passing the Hawaiian Islands for their outbound migrations during the boreal spring (Roberson and Bailey 1991), while they have also been recorded off California and in the eastern tropical Pacific during late summer and autumn and so may migrate back via the northeast Pacific (Spear et al. 1995). Regardless, the migration routes and foraging movements taken by birds, as well as the timing of major events and activity patterns across the annual cycle are unknown. Without basic at-sea distribution data, their habitat requirements and at-sea threats faced remain poorly understood. Sampling of Stejneger’s petrels in the early 1990s on their return southbound migrations via the eastern tropical Pacific found comparatively high plastic ingestion rates, suggesting that high burdens might be due to elevated exposure on the wintering grounds in the North Pacific (Spear et al. 1995).
We tracked Stejneger’s petrels with geolocator-immersion loggers (hereafter geolocators) over a two-year period from late 2019 to early 2022 to provide a detailed account of their year-round phenology, movements, activity patterns, and habitat use. Specifically, our aims were to characterize (1) the timing of major events across the annual cycle, (2) year-round at-sea distributions and major foraging grounds and (3) migration routes, (4) define use of major oceanographic features such as frontal zones, particularly during the non-breeding period in the North Pacific, (5) quantify their relative use of the High Seas, and (6) compare activity budgets across the year. Lastly, we investigated (7) whether there is evidence for sex differences in foraging strategies during the pre-laying exodus, as has been detected in some other gadfly petrel species (e.g., Pinet et al. 2012; Clay et al. 2017).
Methods
Study site and data collection
Stejneger’s petrels nest only on Isla Alejandro Selkirk in burrows at high altitude (approximately 800–1000 m) in a colony intermixed with the more numerous Juan Fernández petrel. In late December 2019 and early January 2020, burrows were opened on a grassy ridge (33°47.40′S, 80°47.16′W) using a standard procedure involving cutting a flowerpot-shaped sod (roughly 20 cm in maximum diameter) above the nest chamber. The incubating adult was then removed for around 10 min. A numbered metal ring was applied to one leg and a geolocator to the other. In total, we deployed 29 loggers (Intigeo C65-SUPER: 14 × 8 × 6 mm and 1 g; Migrate Technology Ltd, Cambridge, UK), each attached to a Darvic plastic ring on the tarsus using a cable tie. The total mass of rings plus logger (1.8 g) corresponded to approximately 1–1.5% of the mass of tracked birds (140–222 g at deployment, n = 28). In January 2022, we recaptured 13 (44.8%) of the tagged birds, of which three had lost the logger and Darvic ring. We therefore retrieved and successfully downloaded data from 10 birds (34% of total) and three pairs of birds (paired in both study seasons) provided six of the retrieved loggers. Each logger was calibrated in an open location without artificial light on Isla Alejandro Selkirk at night for 5.5 ± 0.5 d and 3.8 ± 0.4 d before and after retrieval, respectively. Loggers were programmed to record light intensity (lux) every min and store the maximum value every 5 min, and saltwater immersion every 6 s and store the sum of positive tests at 5-min intervals providing values between 0 (entirely dry) and 50 (entirely wet).
Data processing
All data processing, analysis, and visualization were conducted in R v. 4.1.2 (R Core Team 2021). Locations were estimated for each individual through transitions in the light curves (threshold method) following processing steps laid out in Lisovski et al. (2020). Twilights were defined from the raw light data using the preprocess function in the TwGeos package (Lisovski et al. 2016) using a light intensity threshold of 2 lux. We manually inspected light curves and, when there was suspected interference (e.g., due to shading of the logger), we adjusted the timings of sunrise and sunset by comparing to those on the previous and next days. We pooled pre-deployment and post-retrieval calibration periods and defined the sun zenith angle for each tag based on sunrise and sunset times (95.1–97.8°). As calibration periods were short relative to overall tagging durations (> 2 years), we compared angles with those derived from stationary periods exhibited by tagged birds during non-breeding (see Lisovski et al. 2020 for details), with negligible differences between the two.
We used the SGAT package (Sumner et al. 2009) to estimate and refine initial locations based on Markov chain Monte Carlo (MCMC) simulations. SGAT incorporates as priors: (1) a spatial probability mask to prevent locations occurring on land, (2) a flight speed distribution and (3) a twilight error distribution. SGAT also compares sea surface temperatures (SST) recorded by tags to remotely sensed SST maps, but due to our requirement for a long battery duration and small size, loggers did not record this information. Potential flight speeds were given a gamma distribution (shape: 2.0, scale: 0.10; based on Franklin et al. (2022) and roughly corresponding to the distribution of flight speeds of closely related black-winged petrels P. nigripennis (Halpin et al. 2022)). A gamma distribution was also selected for the twilight error model, with the parameters defined separately for each tag based on the calibration twilights (shape: 1.3 ± 0.3, scale: 0.11 ± 0.03). We selected 2000 runs for initial burn-in and then a further 300 samples were drawn three times to determine convergence, before four chains of 3000 samples were drawn to describe the posterior distribution (Lisovski et al. 2020). We ran the above process ten times per individual to determine the effect of the equinox on tracks and chose the “best” track as that which had the most consistently direct route between non-equinox locations (see Fig. S1 in Online Resource). Without SST information, latitudes during the equinox can be unreliable, and we noticed a tendency for tracks to drift southward for two to three weeks around the boreal spring equinox (March, when birds are in the Southern Hemisphere) and northward around the boreal autumn equinox (September) (Fig. S1). We took a conservative approach to remove potentially erroneous locations: for each individual, we plotted tracks and the change in latitude over time up to a month either side of the equinox, and flagged locations for removal if they exceeded the northerly and southerly latitudinal extent of locations in both the previous and subsequent months. This resulted in the removal of 21.4% of locations across the tracking period but only 11.0% of locations during non-breeding (including migration).
Stejneger’s petrels are burrow-nesting and extended periods spent at the colony are reflected in both the immersion (dry) and light (darkness) data. Nests were not monitored over the study period, so we used tag-specific data to characterize transitions among breeding stages; specifically, a combination of processed locations, raw light and immersion data and thresholds in travel speeds (see Online Resource). Data were split into six stages: outbound migration, non-breeding, return migration, pre-laying exodus, incubation and “late breeding” (from the end of incubation to the start of migration; Clay et al. 2017). Due to the effect of the equinox on locations and brief colony attendance after incubation making immersion data uninformative, it was not possible to assign a breeding stage or status during late breeding. Gadfly petrels have a characteristic incubation pattern whereby the female returns to lay the egg and the male then takes the first and longest incubation stint, followed by the female and then the male (Warham 1990). To assign sex, we identified the female as the bird present at the colony (defined as dry and dark periods within 500 km of the colony) for a short stint (0.6 ± 0.8 days) in mid-late December, before departing on a foraging trip. After the female left, the male incubated in the burrow for a long stint (15.2 ± 2.4 days) before departing. Sexes were assigned independently by both authors with complete agreement.
To characterize activity budgets, we first linked immersion and twilight data to split daylight (≥ 2 lux) and darkness periods. We summarized both the total and proportion of time spent wet per day during daylight and darkness periods separately and both combined (to control for seasonal variation in day length). We first removed dry periods at the colony during incubation and did not consider late-breeding as it was not possible to discern long flights or brief periods of colony attendance from the immersion data. We defined each day as a daylight period followed by a consecutive darkness period. Given that Stejneger’s petrels are thought to feed predominantly by seizing prey at or just below the sea surface (Spear et al. 2007), we also summarized the minimum number of wet bouts (i.e., wet immersion values [> 0] that followed a dry recording [0]), as a proxy for foraging activity of birds taking off and landing on the sea surface. One individual lacked reliable immersion data after May 2021 (see Online Resource for details on the assignment of phenology).
Data analysis
At-sea distributions
We summarized movement characteristics by calculating the maximum range from the colony during incubation and pre-laying exodus, and the cumulative distance traveled (as the consecutive straight-line distance between points) during incubation, pre-laying exodus and outbound and return migrations, using great circle distances in the R package fields (Nychka et al. 2021). We calculated travel speed (km/d) as the cumulative distance traveled during a foraging trip or migration bout divided the number of locations divided by two (as there were two locations/d). Utilization distribution (UD) kernels were created to map at-sea distributions across the annual cycle using the adehabitatHR package (Calenge 2006). A grid size of 50 km and a smoothing parameter of 186 km were selected, the latter to account for geolocator error (Phillips et al. 2004). To control for differences in the number of locations between individuals, UDs were generated for each bird and then merged to ensure equal representation.
To determine sex differences in foraging distributions during the pre-laying exodus, we calculated overlap between male and female core (50%) UDs using the kerneloverlaphr function in the adehabitatHR package. We selected the Bhattacharyya’s affinity (BA) index which measures the similarity of two UD estimates and ranges from 0–representing no similarity or overlap between UDs to 1–indicating identical UDs or complete overlap (Fieberg and Kochanny 2005). We used a randomization procedure to test whether males and females were segregated in space more than expected by chance (Clay et al. 2017). We randomly re-assigned bird identities among the two sexes without replacement 100 times, making sure that the randomized sequence of males and females did not match the observed, and calculated overlap scores. We considered male and female UDs to be significantly different if five or fewer (out of 100) randomized overlap values were smaller than the observed (i.e., P < 0.05). The duration of the pre-laying exodus and the maximum range of males and females were compared statistically using paired t-tests.
Habitat use
We determined how petrels distributed during the non-breeding period in relation to the Transition Zone Chlorophyll Front (TZCF), a basin-wide feature that serves as a foraging hotspot for diverse prey and predator species in the North Pacific (reviewed in Polovina et al. 2017). The front separates cool, nutrient-rich waters of the subarctic gyre from warmer, nutrient-poor waters of the subtropical gyre (Polovina et al. 2001). During the boreal summer, the southern boundary of the TZCF shifts as the area of warmer and stratified water expands, with the TZCF reaching its northernmost extent in September/October. The TZCF is characterized by surface chlorophyll values of 0.2 mgm3; however, the 18 °C isotherm also provides a proxy of its location, and given that blended SST products are available at a daily level (whereas satellite chlorophyll layers are limited by cloud cover), they are more suitable for investigation into intra-annual variability (Bograd et al. 2004). We downloaded daily SST layers, which were a remotely sensed product based on multiple sensors, from the Copernicus Climate Data Store (https://cds.climate.copernicus.eu/cdsapp#!/dataset/satellite-sea-surface-temperature) at a 0.05° resolution and used the rasterTocontour function in the raster package (Hijmans 2016) to extract the 18 °C isotherm. We plotted daily bird locations in relation to the position of the isotherm to determine whether birds tracked changes in its position across the boreal summer, and tested for associations between the median daily latitude of the isotherm and of bird locations using Spearman rank correlations. We also extracted SST values for each location and plotted the distribution of values to visualize broad use of frontal zones across the year.
Use of the High Seas and marine protected areas
To examine at-sea distributions in relation to jurisdictional boundaries and use of MPAs, we downloaded shapefiles of Exclusive Economic Zone (EEZ; 200 nm from the shore) contours from the Marine Regions portal (v. 11; https://www.marineregions.org/downloads.php; accessed 18/11/2019) and MPA shapefiles from Protected Planet (https://www.protectedplanet.net; accessed 20/04/2022). Bird locations were intersected with EEZ and MPA contours using the gIntersection function in the rgeos package (Bivand and Rundel 2020), and summarized across the year and for each stage as the proportion of locations (1) within EEZs versus the High Seas and (2) within versus outside of MPAs.
Activity patterns
We compared three daily activity metrics between breeding stages using generalized linear mixed models (GLMMs) in the lme4 package (Bates et al. 2015): time spent wet (both as a proportion and total number of hours) and the number of wet bouts. The factors breeding stage, year, and LoD (daylight or darkness) were included as covariates along with the two-way interaction between breeding stage and LoD to test for differing nocturnal activity according to breeding stage. Individual identity (ID) was also included as a random intercept term. Proportion of time wet, total time wet, and number of wet bouts were modeled using binomial, gamma, and Poisson distributions, respectively. An offset term of the log of the total daylight or darkness hours was included in the wet bouts model to control for variation in photoperiod. We built a set of nested candidate models and compared them using Akaike’s Information Criterion (AIC), whereby the best model was the most parsimonious, i.e., that with the AIC > 2 lower than the next best model (Burnham and Anderson 2002). We compared significant differences between stages using Tukey’s post hoc tests in the package multcomp (Hothorn et al. 2016), which was done separately for daylight and darkness periods as the interaction between LoD and stage was significant in all cases (Table S1). Unless otherwise specified, statistical comparisons were conducted using the stats package (R Core Team 2021), and all means are provided ± standard deviations with ranges in parentheses.
Results
Year-round movements and breeding cycle
Stejneger’s petrels spent the breeding season in the southeast Pacific and conducted trans-equatorial migrations to non-breeding grounds in the northwest Pacific (Fig. 1). Birds departed on northward migrations on 30 April (15 April–15 May) in 2020 and 3 May (18 April–14 May) in 2021 and headed north–northwest until they crossed the equator (at 90–120°W) after which they headed northwest, passing either side of the Hawaiian archipelago before arriving at their non-breeding grounds in the northwest Pacific between the north-western Hawaiian Islands, the Aleutian Islands, and Hokkaido Island (145°E–170°W, 28–46°N; Fig. 2a) on 24 May (11 May–4 June) in 2020 and 25 May (13 May–4 June) in 2021 (Table 1, Fig. 1a). Birds departed their non-breeding grounds on 23 September (13 September–11 October) in 2020 and 21 September (15–30 September) in 2021 and generally headed southeast, crossing the equator across a large longitudinal range in the central Pacific (180°–130°W) before continuing south or heading southwest toward the Chatham Islands east of New Zealand, in the southwest Pacific. After reaching 40°–50°S, birds turned due east, taking an almost straight-line route across the South Pacific, before completing the northward return to the colony at 80–90°W. Birds took over twice as long to achieve their return as their outbound migrations (paired t-test; t19 = 19.6, P < 0.001), both because they covered much greater distances (t19 = 10.3, P < 0.001) and had slower travel speeds (t19 = 5.5, P < 0.001; Table 1). The total distance covered during outward migration, return migration, and non-breeding periods combined (excluding potentially erroneous equinox locations) was 54,725 ± 6148 (47,742–66,149) km. Despite spending c. 20% of the year on migration, there was not any clear evidence of use of stopovers or residency periods en route (Fig. 1a).
a Migration routes and non-breeding areas of Stejneger’s petrels Pterodroma longirostris tracked with geolocators over two years from Isla Alejandro Selkirk, Juan Fernández Islands (yellow triangle). The outbound and return migrations and non-breeding movements are shown as orange, yellow, and green lines, respectively. Tracks during the autumn equinox when latitude estimation was unreliable are shown by dashed lines and the 500 km buffer around the colony used to define departure on migration is shown by a white dashed circle. b, c The non-breeding movements of two example individuals in both years of tracking (2020: pink, 2021: sky blue) are shown in two separate panels. Blue shading represents ocean floor depth with lighter shades indicating shallower waters. Maps were plotted using the ggmap R package in the Mercator projection, which stretches regions at higher latitudes
At-sea distributions of Stejneger’s petrels Pterodroma longirostris tracked with geolocators from Isla Alejandro Selkirk (yellow triangle), Juan Fernández Islands, during a non-breeding, b pre-laying exodus, c incubation and d late breeding, in relation to marine protected areas (MPAs; shaded in light gray and labeled in gray text) and Exclusive Economic Zones (EEZs; shown as dark gray lines and labeled in black text). Utilization distributions (UD) are displayed from darker to lighter shades of blue for 25%, 50%, 75% and 95% isopleths
The first night back at the colony signaling the end of migration was 11 (4–15) November in 2020 and 13 (9–18) November in 2021. Birds departed on pre-laying on 19 (11–28) and 21 (15–26) November in 2020 and 2021, respectively, and returned on 18 (14–21) and 18 (15–22) December in 2020 and 2021, respectively. The duration of pre-laying exodus was 27.3 ± 3.2 days (2020: 28.1 ± 4.0 [19.7–32.6] days; 2021: 27.3 ± 3.2 [20.8–30.8] days) and did not differ between males (26.5 ± 4.3 days) and females (29.0 ± 2.2 days) (t-test; t13.5 = 1.6, P = 0.135). Birds headed southwest of the colony to forage in a sector between 50° and 55°S and from 90° to 120°W and as far west as 140°W (Fig. 2b) around the northern edge of the Sub-Antarctic Front, with 51.8% of locations associated with SSTs considered to be within this frontal band (Fig. 3a). Although males appeared to range slightly further than females (Fig. S2a; males: 3824 ± 850 km; females: 3337 ± 318 km), the difference was not significant (t-test; t11.5 = 1.7, P = 0.117) and this pattern was not consistent among the three pairs for which both members were tracked (Fig. S2b–d). There was also no evidence of sexual segregation in core foraging areas during pre-laying (Fig. S2a; observed overlap: 0.73; randomized overlap [median ± interquartile range]: 0.68 ± 0.14; P = 0.790).
Sea surface temperature (SST, °C) values associated with locations of Stejneger’s petrels Pterodroma longirostris tracked with geolocators during a pre-laying exodus, b incubation and c non-breeding. Gray-shaded boxes represent the approximate range of SST values associated with a, b the Sub-Antarctic Front in the southeast Pacific Ocean (3–8 °C; Chaigneau and Pizarro 2005) and with c the North Pacific Transition Zone (NPTZ) in the northwest Pacific Ocean (18–21 °C; Watanabe et al. 2009). The mean SST value associated with waters around the breeding colony is shown by a dotted line
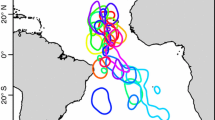
We recorded 45 incubation trips across three seasons, lasting 13.0 ± 2.6 d (range: 4.9–17.3 d) (Table 2). During the second and only fully monitored incubation season (2020/2021), all females took two foraging trips and 3 out of 5 males took two trips, with the other two males recording only one trip. Trips generally took the form of anticlockwise loops, with the majority (35/45) taking an initial southwest-westerly bearing before heading south(east) and then returning on a northerly bearing (Fig. 4), presumably to take advantage of prevailing southerly winds associated with the Humboldt Upwelling on the return route (Fig S3). Foraging distributions were broadly similar to pre-laying exodus with birds traveling 2953 ± 792 km (and up to 4810 km) away from the colony to a region just north of the Sub-Antarctic Front (Figs. 2c, 3b), but with some individuals traveling as far south as the Polar Front (at around 60°S) (Figs. 4, S2). There were no apparent differences between years. During late breeding, birds foraged closer to the colony in a band of habitat to the west of the Humboldt Upwelling (25°S–50°S) (Fig. 2d).
Incubation trips of Stejneger’s petrels Pterodroma longirostris tracked with geolocators from Isla Alejandro Selkirk, Juan Fernández Islands (yellow triangle), in three consecutive breeding seasons: 2019–2020 (n = 17), 2020–2021 (n = 18) and 2011–2022 (n = 10). The average position of major Southern Ocean fronts are shown by blue dotted lines
Non-breeding habitat and use of the Transition Zone Chlorophyll Front
The TZCF (indicated by the 18 °C isotherm) moved northwards across the summer from 34.5°N in May to 42.5°N in late August/September, a northward movement of 937 and 1,028 km in 2020 and 2021, respectively (Fig. 5c). The tracked birds also moved northwards between May and September by 1322 and 848 km on average during 2020 and 2021, respectively, and there was a high correlation between the daily mean latitude of tracked birds and the TZCF (Spearman rank correlation; 2020: r = 0.89, P < 0.001; 2021: r = 0.87, P < 0.001). Birds were generally distributed on the southerly, warmer trailing edge of the TZCF, with 34.6% of locations within the 18–21 °C SST band broadly indicative of the NPTZ (Fig. 3c). Birds did occasionally venture into colder northerly waters associated with the subarctic gyre (Fig. 5a, b). The tracked petrels appeared to be consistent in non-breeding site choice between years; for example, one individual consistently spent a portion of the non-breeding season in both years much further west than other birds, around the submarine canyon off Hokkaido, Japan (Fig. 1b, c).
Non-breeding locations of Stejneger’s petrels Pterodroma longirostris tracked with geolocators in relation to the position of the North Pacific Transition Zone (NPTZ) from May to September 2020 and 2021. a The locations of all birds are shown in each month (columns) and in both years (rows) in relation to sea surface temperature (SST) on the 15th day of each month. The position of the 18 °C isotherm (a proxy for the Transition Zone Chlorophyll Front) is shown by a black solid line. b The northwest Pacific Ocean is plotted with bathymetry in blue shading and the positions of major ocean currents shown by blue (cold) and red (warm) arrows. The rough latitudinal extent of the NPTZ is shown by a shaded box. c The mean daily latitude of the 18 °C isotherm is shown by a red line in each year in relation to the latitudes associated with bird locations in light gray. Dotted vertical lines represent the first day of each month from June to September
Use of the High Seas and marine protected areas
Birds spent 87.2 ± 4.8% of their time across the year in the High Seas. The proportion of time was the greatest during non-breeding and pre-laying exodus and lowest during late breeding (Table 3, Fig. 2). Birds spent 5.0 ± 1.4% of their time across the year within MPAs, with use greatest during outward migration (when birds migrated along the Papahānaumokuākea Marine National Monument) and late breeding (when distributions were more constrained within the Mar de Juan Fernández National Park) (Table 3, Fig. 2).
Activity patterns
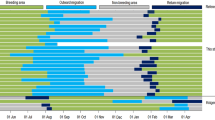
Stejneger’s petrels varied their activity patterns considerably across the year (Fig. 6), as indicated by the best model explaining variation in all metrics including the influence of daylight or darkness (LoD), breeding stage, and the two-way interaction between daylight or darkness and breeding stage (Table S1). The models for time on water (number of hours and %) also included the effect of year. The tracked petrels consistently spent 70–75% of their time in flight during breeding (Table 4, Fig. 6). Birds increased their flight time (c. 85%) during outward migration, and after arrival on non-breeding grounds substantially increased time spent on the water to 70–80% from June to early August, a period likely associated with flight feather molt (Fig. 6). From August onwards, birds gradually increased their flight time such that during return migrations, they had similar flight activity to breeding. The number of wet bouts per hour was the lowest during non-breeding and highest during incubation, and higher during darkness than during daylight during all stages except incubation (Tables 4, S1, S2). Birds spent a greater proportion of darkness than daylight hours in flight during pre-laying, incubation, and non-breeding, but not during migration. Results were similar when raw values (rather than proportions) were used, indicating that changes in day length did not substantially influence results (Tables 4, S1, S2).
Annual variation in the percentage of time spent on water during daylight (dashed line) and darkness (solid line) by Stejneger’s petrels Pterodroma longirostris tracked with geolocators. Means (± SE) of individual weekly averages are shown and the average start and end of each stage of the annual cycle are shown by vertical dotted lines. The period potentially associated with molt during non-breeding is labeled based on > 60% time spent on water during both daylight and darkness. Out mig = outward migration; Return mig = return migration
Discussion
Year-round movements and breeding cycle
We present the first tracking study of Stejneger’s petrels and provide detailed information on their breeding and migratory phenology, year-round movements, habitat use, and activity patterns. Our study accords with and expands upon the scant information previously available on the timing of breeding (Brooke 1987; Hodum and Wainstein 2003). Birds first returned to the colony after migration in early or mid-November and began to lay in mid-December after a pre-laying exodus of 27.3 days, comparable to that of other Cookilaria species (24–36 days; Grant et al. 1983; Imber et al. 2003; Brooke 2004b; Rayner et al. 2012; 2023) but shorter than larger Pterodroma species (40–68 days; Warham 1990; Pinet et al. 2012; Clay et al. 2017; Taylor et al. 2020). During pre-laying, birds universally used an oceanic region to the south-west of the colony around 3500 km away, on the northern edge of the Sub-Antarctic Front (~ 50°S, ~ 110°W). Birds traveled as far west as 140oW and likely overlapped to some degree with closely related Chatham petrels P. axillaris which travel as far east as 120–130°W from the Chatham Islands (Rayner et al. 2012). In most other gadfly petrels, pre-laying males generally travel further from the colony than females and feed in more biologically productive waters, which is presumed to be related to their need to build up energy stores before a long first incubation shift, while females may also have different nutritional requirements during egg formation (Pinet et al. 2012; Rayner et al. 2012; Clay et al. 2017). However, while males ranged further from the colony than females in two of the three tracked pairs, maximum ranges of males and females were not significantly different across all individuals, nor were there obvious differences in the foraging distributions of the two sexes. The reasons for a lack of sexual segregation are unclear, but could be explained by several factors, including (1) the lack of more productive habitats further from the colony for males to exploit (contra Pinet et al. 2012; Clay et al. 2017), or (2) the low sample size, given the only other gadfly petrel study not demonstrating sex differences also tracked a similarly low number of birds (white-headed petrels P. lessonii; Taylor et al. 2020).
During incubation, birds took trips lasting 13.0 days, a duration similar to other Cookilaria species, which generally have long incubation shifts for their small size (Rayner et al. 2012; 2023; Kim et al. 2017). The tracked petrels ranged as far as 3890–4810 km from the colony across the three breeding seasons tracked, indicating that the species has among the greatest foraging ranges of any seabird, comparable to larger gadfly petrels and shearwaters (Ronconi et al. 2018; Clay et al. 2019, 2023; Taylor et al. 2020); this is a particularly impressive feat given their smaller size and lower airspeeds, and hence, lesser ability to counter the effects of wind drift (Spear and Ainley 1997). The majority (78%) of trips followed an anticlockwise track, with birds generally initially heading southwest and then returning from a more southerly bearing with prevailing southerly winds associated with the Humboldt Upwelling. While this pattern is broadly similar to sympatric Juan Fernández petrels, which also take looping trips westwards (3404 ± 630 km from the colony) and then return via a more southerly route using stronger and more predictable westerlies (Clay et al. 2023), Stejneger’s petrel trips appeared to be more direct and birds foraged further south in waters associated with the Sub-Antarctic Front, and as far south as the Polar Front. Due to uncertainties in breeding fate and the effect of the March equinox, foraging distributions during late breeding should be examined with caution, yet birds appeared to forage closer to the colony and further east toward the Humboldt Current than during pre-laying and incubation.
Our study confirms that birds conduct round-trip trans-equatorial migrations to the northwest Pacific, covering 54,725 (47,742–66,149) km. This is one of the longest roundtrip migrations of any animal, exceeding landbird migrations (c. 30,000 km for bar tailed godwits Limosa lapponica, northern wheatears Oenanthe oenanthe and European nightjars Caprimulgus europaeus; Battley et al. 2012, Bairlein et al. 2012, Lathouwers et al. 2022) and other tracked gadfly petrels (c. 48,000 km for Cook’s petrel; Rayner et al. 2011), but is surpassed by sooty shearwaters Ardenna grisea in the Pacific (c. 64,000 km; Shaffer et al. 2006) and Arctic terns Sterna paradisaea (> 70,000 km; Egevang et al. 2010). As well as the notable distance traveled, which is underestimated due to the removal of potentially erroneous locations around the autumn equinox and the coarse spatiotemporal resolution of geolocator data, Stejneger’s petrels took a triangular route around the Pacific, which is distinct from other trans-equatorial migrations routes taken by seabirds. During their northbound route, birds appeared to follow a consistent bearing and rapidly (640 km/d) passed the Hawaiian Islands toward the Emperor seamount chain in the northwest Pacific. Doing so, they passed non-breeding habitats of other Cookilaria species including Cook’s and black-winged petrels (Rayner et al. 2011, 2023). In contrast, the return migration was twice as long (both in time and distance) and substantially slower (504 km/d), taking birds directly south past many tropical Pacific Island nations to waters east of New Zealand, before turning eastward across the southern portion of the Pacific at around 45°S. The detour is about twice the straight-line route, and likely allows birds to reduce energy costs by first using northeasterly trade winds to reach the equator, and then strong westerlies associated with the Antarctic Circumpolar Current (the “Roaring Forties”) to aid movements. While several species breeding in in the southwest Pacific also take a long figure-of-eight loop around the southeast Pacific on their way to wintering areas in the North Pacific (e.g., black-winged and Cook’s petrels, sooty shearwaters; Rayner et al. 2011, 2023; Shaffer et al. 2006), the southward return leg from the northwest Pacific down to New Zealand currently appears to be unique among petrels, though may well be matched by other species wintering in the northwest Pacific for which there are not published data, such as Providence petrels P. solandri.
Non-breeding habitat and use of the Transition Zone Chlorophyll Front
During non-breeding, birds were distributed in a region associated with the Emperor Seamounts and the NPTZ, which supports evidence from ship-based sightings in the northwest Pacific (c. 30–35°N) of birds feeding along warmer fronts (> 18 °C) associated with the Kuroshio Current (Nakamura and Tanaka 1977; Tanaka and Inaba 1981; Tanaka et al. 1985). Stejneger’s petrels apparently segregate latitudinally from other gadfly petrels, presumably to avoid competition; white-necked P. cervicalis, black-winged P. nigripennis and Bonin petrels P. hypoleuca tend to forage further south in subtropical waters between 15 and 25°N (Tanaka and Inaba 1981; Tanaka et al. 1985; Kuroda 1991), while mottled petrels P. inexpectata are distributed further north (> 40°N) in sub-Arctic waters toward the Aleutian Islands and in the Sea of Okhotsk (Ogi et al. 1999). The tracked birds appeared to target waters just south of the NPTZ, a biologically productive oceanic region where cool, vertically mixed, high chlorophyll surface waters sink below warm low chlorophyll water, forming the TZCF (Polovina et al. 2001). This region hosts a diversity of other marine predators, which predominantly feed on pelagic fish and squid, such as black-footed albatrosses Phoebastria nigripes, albacore tuna Thunnus alalunga, swordfish Xiphias gladius, and loggerhead turtles Caretta caretta (Watanabe et al. 2009; Hyrenbach et al. 2017; Polovina et al. 2017).
Over large spatial scales, studies have identified oceanic frontal regions as important for gadfly petrels in the Pacific, including the Subtropical Convergence Zone (e.g., Rayner et al. 2012, 2023; Clay et al. 2017) and the North and South Equatorial Countercurrents (Ballance et al. 2006). In contrast, finer-scale analyses show that petrels do not appear to target particular oceanographic or topographic features (e.g., Halpin et al. 2022) and that wind patterns play a major role in determining foraging destinations or routes taken to foraging areas (Ventura et al. 2020; Clay et al. 2023). While we did not conduct an analysis of habitat preference and our study is limited by the coarse resolution of geolocator data, we found that from May to August in both years, the tracked petrels consistently moved northwards by roughly the same distance (c. 1000 km) as the TZCF moved north (Bograd et al. 2004). This indicates that birds likely have specific preferences for prey associated with this dynamic frontal habitat and that the position of the TZCF is an important driver of their non-breeding distributions. Given that Stejneger’s petrels also appear to target the Sub-Antarctic Frontal Zone in the southern hemisphere for foraging during breeding, our study suggests that frontal regions are important habitats for this species year-round.
Activity patterns
The tracked petrels had fairly consistent activity patterns across breeding and migration, spending c. 70–85% of their time in flight. As birds did not appear to conduct stopovers during migration and had comparatively high landing rates during migration (similar to pre-laying, slightly lower than incubation), this suggests they search for and capture prey while en route. Our findings accord with other studies of gadfly petrels, which have high flight activity during breeding and migration, spending c. 73–95% and 56–83% of their time in flight, respectively (e.g.,Ramirez et al. 2013; Clay et al. 2017; Bonnet-Lebrun et al. 2021). Flight activity was substantially reduced (to around 20–30% of total time) for the first two months after arrival at non-breeding grounds in mid-June to mid-August, likely corresponding with the molt of flight feathers (Cherel et al. 2016). Stejneger’s petrels are thought to molt during non-breeding, following a similar pattern to most other Cookilaria petrels (Falla 1942; Roberson and Bailey 1991; Howell et al. 1996); indeed, feathers are most worn in March to June during outbound migration through the eastern tropical Pacific (Murphy 1936; Spear et al. 1992).
Although flight and foraging activity appeared to be similar between daylight and darkness hours, birds spent a greater proportion of darkness than daylight hours in flight during pre-laying, incubation, and non-breeding, similar to many other Pterodroma petrels (Bonnet-Lebrun et al. 2021; Rayner et al. 2023). As landing rates on the sea surface were also higher during darkness in all stages except incubation, it is likely that nocturnal flight activity relates to foraging behavior and that birds are more likely to be feeding at night or just before and after twilight periods, when diel vertically migrating fish and squid could still be present near the sea surface and visible to foraging birds. This is supported by a diet study of migrating birds in the eastern tropical Pacific, which showed myctophids (Myctophidae) and other fish formed an important component (frequency of occurrence [FO]: 73%; Spear et al. 2007). Most prey were also consumed at night (Spear et al 2007), though it is important to note that trans-equatorial migrants are generally more nocturnal in equatorial regions than in temperate non-breeding areas (Bonnet-Lebrun et al. 2021).
Implications for conservation
Our study shows that Stejneger’s petrels make extensive use of the High Seas, spending 87% of their time beyond national EEZs. It is unsurprising then that birds only spend 5% of their time within MPAs, which are mostly confined to national EEZs. It is unlikely then, that the designation of large (> 100,000 km2) MPAs across the Pacific over the last decade, including the Mar de Juan Fernández Marine Park, established in 2018 in the Chilean EEZ around the Juan Fernández Islands (area of 262,000 km2; Friedlander and Gaymer 2021; Wagner et al. 2021), affords protection to Stejneger’s petrels while at sea, similar to sympatric Juan Fernández petrels during incubation (Clay et al. 2023). Stejneger’s petrels are predominantly threatened by invasive mammals at Alejandro Selkirk (BirdLife International 2019) and while there are currently no established marine threats, the lack of colony-based monitoring and prior understanding of at-sea distributions and threats preclude a robust assessment. Like other Cookilaria petrels, Stejneger’s petrels do not appear to interact with fisheries. However, studies in the 1980s and 1990s documented high plastic occurrence (FO: 0.74) during the species’ return migration from the North Pacific (Ainley et al. 1990; Spear et al. 1995). Our study indicates that during non-breeding, birds forage at the northern edge of the western North Pacific Gyre, which is an area of globally high plastic concentrations and ingestion risk to seabirds (the “Western Garbage Patch”; Howell et al. 2012; Clark et al. 2023). Indeed, Laysan albatrosses Phoebastria immutabilis foraging in this region have higher plastic loads than elsewhere (Young et al. 2009), and the propensity for Cookilaria petrels to be attracted to flotsam (Roberson and Bailey 1991) may lead them to mistake small plastic particles for prey. We recommend studies that can quantify ingestion rates, in combination with more regular and expanded colony monitoring (Hodum and Wainstein 2003), to better understand individual- and population-level implications.
Overall, our study shows that Stejneger’s petrels are extremely mobile and use large swathes of the Pacific Ocean across their annual cycles. Birds spend almost 90% of their time at sea outside of the protection of national jurisdictions and their apparent preference for oceanic frontal regions in both hemispheres demonstrates the importance of protecting these remote habitats from extractive and polluting human activities.
Data availability
Raw light and twilight and processed geolocator data are available in the Movebank Data Repository, https://doi.org/https://doi.org/10.5441/001/1.301 (Clay and Brooke 2024). Processed geolocator data can also be viewed and requested on the BirdLife International Seabird Tracking Database (https://data.seabirdtracking.org/dataset/1872). R codes to process geolocator data are available via https://github.com/tommyclay/Stejnegers_movement.
References
Adams J, Flora S (2009) Correlating seabird movements with ocean winds: linking satellite telemetry with ocean scatterometry. Mar Biol 157:915–929
Ainley D, Spear LB, Ribic CA (1990) The incidence of plastic in the diets of pelagic seabirds in the Eastern Equatorial Pacific region. In: Proceedings of the Second International Conference on Marine Debris. US Dep. Comer., NOM Tech. Memo. NMFS
Bairlein F, Norris DR, Nagel R, Bulte M, Voigt CC et al (2012) Cross-hemisphere migration of a 25g songbird. Biol Let 8:505–507
Ballance LT, Pitman RL, Fiedler PC (2006) Oceanographic influences on seabirds and cetaceans of the eastern tropical Pacific: a review. Prog Oceanogr 69:360–390
Bates D, Maechler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48
Battley PF, Warnock N, Tibbitts TL, Gill RE Jr, Piersma T et al (2012) Contrasting extreme long-distance migration patterns in bar-tailed godwits Limosa lapponica. J Avian Biol 43:21–32
Beal M, Dias MP, Phillips RA, Oppel S, Hazin C et al (2021) Global political responsibility for the conservation of albatrosses and large petrels. Sci Adv 7:eabd7225
Bernard A, Rodrigues ASL, Cazalis V, Grémillet D (2021) Toward a global strategy for seabird tracking. Conserv Lett 14:e12804
BirdLife International (2019) Pterodroma longirostris. The IUCN red list of threatened species 2019:e.T22697997A152684668. https://doi.org/10.2305/IUCN.UK.2019-3.RLTS.T22697997A152684668.en
Bivand R, Rundel C (2020) Rgeos: interface to geometry engine - open source (‘GEOS’). R package version 0.5–9
Bograd SJ, Foley DG, Schwing FB, Wilson C, Laurs RM et al (2004) On the seasonal and interannual migrations of the transition zone chlorophyll front. Geophys Res Lett 31:L17204
Bonnet-Lebrun A-S, Dias MP, Phillips RA, Granadeiro JP, Brooke MdeL et al (2021) Seabird migration strategies: flight budgets, diel activity patterns, and lunar influence. Front Mar Sc 8:1543
Bourne WRP, Brooke MdeL, Clark GS, Stone T (1992) Wildlife conservation problems in the Juan Fernández Archipelago, Chile. Oryx 26:43–51
Brooke MdeL (1987) Population estimates and breeding biology of the Petrels Pterodroma externa and P. longirostris on Isla Alejandro Selkirk Juan Fernandez Archipelago. Condor 89:581–586
Brooke MdeL (2004a) The food consumption of the world’s seabirds. Proc R Soc Lond B Biol Sci 271:S246–S248
Brooke MdeL (2004b) Albatrosses and petrels across the world. Oxford University Press, Oxford, UK
Brooke MdeL, Bonnaud E, Dilley BJ, Flint EN, Holmes ND et al (2018) Seabird population changes following mammal eradications on islands. Anim Conserv 21:3–12
Burnham KP, Anderson DR (2002) Model selection and multimodel inference: a practical information: theoretic approach, 2nd edn. Springer-Verlag, New York, USA
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Model 197:516–519
Chaigneau A, Pizarro O (2005) Surface circulation and fronts of the South Pacific Ocean, east of 120°W. Geophys Res Lett 32:8
Cherel Y, Quillfeldt P, Delord K, Weimerskirch H (2016) Combination of at-sea activity, geolocation and feather stable isotopes documents where and when seabirds moult. Front Ecol Evol 4:3
Clark BL, Carneiro APB, Pearmain EJ, Rouyer M-M, Clay TA et al (2023) Global assessment of marine plastic exposure risk for oceanic birds. Nat Commun 14:3665
Clay TA, Brooke MdeL (2024) Data from: trans-equatorial migration links oceanic frontal habitats across the Pacific Ocean: year-round movements and foraging activity of a small gadfly petrel. Movebank Data Repos. https://doi.org/10.5441/001/1.301
Clay TA, Phillips RA, Manica A, Jackson HA, Brooke MdeL (2017) Escaping the oligotrophic gyre? The year-round movements, foraging behaviour and habitat preferences of Murphy’s petrels. Mar Ecol Prog Ser 579:139–155
Clay TA, Oppel S, Lavers JL, Phillips RA, Brooke MdeL (2019) Divergent foraging strategies during incubation of an unusually wide-ranging seabird, the Murphy’s petrel. Mar Biol 166:8
Clay TA, Hodum P, Hagen E, Brooke MdeL (2023) Adjustment of foraging trips and flight behaviour to own and partner mass and wind conditions by a far-ranging seabird. Anim Behav 198:165–179
Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ et al (2012) Seabird conservation status, threats and priority actions: a global assessment. Bird Conserv Int 22:1–34
Dias MP, Martin R, Pearmain EJ, Burfield IJ, Small C et al (2019) Threats to seabirds: a global assessment. Biol Cons 237:525–537
Egevang C, Stenhouse IJ, Phillips RA, Petersen A, Fox JW, Silk JR (2010) Tracking of Arctic terns Sterna paradisaea reveals longest animal migration. Proc Natl Acad Sci USA 107:2078–2081
Falla RA (1942) Review of the smaller Pacific forms of Pterodroma and Cookilaria. Emu 42:111–118
Fieberg J, Kochanny CO (2005) Quantifying home-range overlap: the importance of the utilization distribution. J Wildl Manag 69:1346–1359
Franklin KA, Norris K, Gill JA, Ratcliffe N, Bonnet-Lebrun A-S et al (2022) Individual consistency in migration strategies of a tropical seabird, the Round Island petrel. Mov Ecol 10:13
Friedlander AM, Gaymer CF (2021) Progress, opportunities and challenges for marine conservation in the Pacific Islands. Aquat Conserv Mar Freshwat Ecosyst 31:221–231
Grant GS, Warham J, Pettit TN, Whittow GC (1983) Reproductive behavior and vocalizations of the Bonin Petrel. Wilson Bull 95:522–539
Grant ML, Bond AL, Lavers JL (2022) The influence of seabirds on their breeding, roosting and nesting grounds: a systematic review and meta-analysis. J Anim Ecol 91:1266–1289
Halpin LR, Mott R, Clay TA, Humphries GRW, Chatwin TA et al (2022) Predicting the foraging habitats of sympatrically breeding gadfly petrels in the South Pacific Ocean. Front Mar Sci 9:853104
Hijmans RJ (2016) Raster: geographic data analysis and modeling. R package version 3.6–14
Hodum P, Wainstein M (2003) Biology and conservation of the Juan Fernández Archipelago Seabird community. Juan Fernández Islands Conservancy, Long Beach, USA.
Hothorn T, Bretz F, Westfall P (2016) Simultaneous inference in general parametric models. Biom J 50(3):346–363
Howell SN, Webb S, Spear LB (1996) Identification at sea of Cook’s, De Filippi’s, and Pycroft’s petrels. West Birds 27:57–64
Howell EA, Bograd SJ, Morishige C, Seki MP, Polovina JJ (2012) On North Pacific circulation and associated marine debris concentration. Mar Pollut Bull 65:16–22
Hyrenbach KD, Hester MM, Adams J, Titmus AJ, Michael PE et al (2017) Plastic ingestion by black-footed albatross Phoebastria nigripes from Kure atoll, Hawai’i: linking chick diet remains and parental at-sea foraging distributions. Mar Ornithol 45:225–236
Imber MJ, West JA, Cooper WJ (2003) Cook’s petrel (Pterodroma cookii): historic distribution, breeding biology and effects of predators. Notornis 50:221–230
Kim Y, Priddel D, Carlile N (2017) Incubation routine and associated changes in body mass of Gould’s Petrel (Pterodroma leucoptera). Emu 118:193–200
Kuroda N (1991) Distributional patterns and seasonal movements of procellariiformes in the North Pacific. J Yamashina Inst Ornithol 23:23–84
Lathouwers M, Nussbaumer R, Liechti F, Davaasuren B, Artois T et al (2022) Migration routes and timing of European Nightjars (Caprimulgus europaeus) breeding in eastern Mongolia. J Ornithol 163:881–890
Lisovski S, Bauer S, Briedis M, Davidson SC, Dhanjal-Adams KL et al (2020) Light-level geolocator analyses: a user’s guide. J Anim Ecol 89:221–236
Lisovski S, Wotherspoon S, Sumner M (2016) TwGeos: basic data processing for light-level geolocation archival tags. R package version 0.1.2
Murphy RC (1936) Oceanic birds of South America. American Museum of Natural History, New York, USA
Nakamura K, Tanaka Y (1977) Distribution and migration of two species of the genus Pterodroma in the North Pacific. J Yamashina Inst Ornithol 9:112–120
Nychka D, Furrer R, Paige J, Sain S, Gerber F et al (2021) Fields: tools for spatial data. R package version 13.3
Ogi H, Newcomer MW, Fujimura H, Shiratori S (1999) Seasonal distribution of the mottled Petrel in the Northwestern North Pacific. Hokkaido University, Japan
Oppel S, Bolton M, Carneiro APB, Dias MP, Green JA et al (2018) Spatial scales of marine conservation management for breeding seabirds. Mar Policy 98:37–46
Paleczny M, Hammill E, Karpouzi V, Pauly D (2015) Population trend of the World’s Monitored Seabirds, 1950–2010. PLoS ONE 10:e0129342
Phillips RA, Silk JR, Croxall JP, Afanasyev V, Briggs DR (2004) Accuracy of geolocation estimates for flying seabirds. Mar Ecol Prog Ser 266:265–272
Piatt IJF, Sydeman WJ (2007) Seabirds as indicators of marine ecosystems. Mar Ecol Prog Ser 352:199–204
Pinet P, Jaquemet S, Pinaud D, Weimerskirch H, Phillips RA, Corre ML (2011) Migration, wintering distribution and habitat use of an endangered tropical seabird, Barau’s petrel Pterodroma baraui. Mar Ecol Prog Ser 423:291–302
Pinet P, Jaquemet S, Phillips RA, Le Corre M (2012) Sex-specific foraging strategies throughout the breeding season in a tropical, sexually monomorphic small petrel. Anim Behav 83:979–989
Polovina JJ, Howell E, Kobayashi DR, Seki MP (2001) The transition zone chlorophyll front, a dynamic global feature defining migration and forage habitat for marine resources. Prog Oceanogr 49:469–483
Polovina JJ, Howell EA, Kobayashi DR, Seki MP (2017) The transition zone chlorophyll front updated: advances from a decade of research. Prog Oceanogr 150:79–85
R Core Team (2021) R: a language and environment for statistical computing.
Ramirez I, Paiva VH, Menezes D, Silva I, Phillips RA et al (2013) Year-round distribution and habitat preferences of the Bugio petrel. Mar Ecol Prog Ser 476:269–284
Ramos R, Carlile N, Madeiros J, Ramírez I, Paiva VH et al (2017) It is the time for oceanic seabirds: tracking year-round distribution of gadfly petrels across the Atlantic Ocean. Divers Distrib 23:794–805
Rayner MJ, Hauber ME, Steeves TE, Lawrence HA, Thompson DR et al (2011) Contemporary and historical separation of transequatorial migration between genetically distinct seabird populations. Nat Commun 2:332
Rayner MJ, Taylor GA, Gummer HD, Phillips RA, Sagar PM et al (2012) The breeding cycle, year-round distribution and activity patterns of the endangered Chatham Petrel (Pterodroma axillaris). Emu 112:107–116
Rayner MJ, Carlile N, Priddel D, Bretagnolle V, Miller MGR et al (2016) Niche partitioning by three Pterodroma petrel species during non-breeding in the equatorial Pacific Ocean. Mar Ecol Prog Ser 549:217–229
Rayner MJ, Gaskin CP, Taylor GA (2023) Seasonal movements, foraging activity, and breeding biology of black- winged petrel (Pterodroma nigripennis) from three New Zealand colonies. Notornis 70:111–123
Roberson D, Bailey TG (1991) Cookilaria Petrels in the eastern Pacific Ocean. Am Birds 45:1067–1081
Rodríguez A, Arcos JM, Bretagnolle V, Dias MP, Holmes ND et al (2019) Future directions in conservation research on petrels and shearwaters. Front Mar Sci 6:94
Ronconi RA, Schoombie S, Westgate AJ, Wong SNP, Koopman HN, Ryan PG (2018) Effects of age, sex, colony and breeding phase on marine space use by Great Shearwaters Ardenna gravis in the South Atlantic. Mar Biol 165:58
Shaffer SA, Tremblay Y, Weimerskirch H, Scott D, Thompson DR et al (2006) Migratory shearwaters integrate oceanic resources across the Pacific Ocean in an endless summer. Proc Natl Acad Sci USA 103:12799–12802
Spear LB, Ainley DG (1997) Flight speed of seabirds in relation to wind speed and direction. Ibis 139:234–251
Spear LB, Howell SNG, Ainley DG (1992) Notes on the at-sea identification of some Pacific Gadfly Petrels (Genus: Pterodroma). Colon Waterbirds 15:202–218
Spear LB, Ainley DG, Ribic CA (1995) Incidence of plastic in seabirds from the tropical pacific, 1984–1991: relation with distribution of species, sex, age, season, year and body weight. Mar Environ Res 40:123–146
Spear LB, Ainley DG, Walker WA (2007) Foraging dynamics of seabirds in the Eastern Tropical Pacific Ocean. Stud Avian Biol 35:1–99
Sumner MD, Wotherspoon SJ, Hindell MA (2009) Bayesian estimation of animal movement from archival and satellite tags. PLoS ONE 4:10
Tanaka Y, Inaba F (1981) The distribution and migration of White-necked petrel, Pterodroma externa cervicalis, in the west area of North Pacific Ocean and the Japanese waters. J Yamashina Inst Ornithol 13:207–214
Tanaka Y, Kaneko Y, Sato S (1985) Distribution and migration of smaller petrels of the genus Pterodroma in the Northwest Pacific. J Yamashina Inst Ornithol 17:23–31
Taylor G, Elliott G, Walker K, Bose S (2020) Year-round distribution, breeding cycle, and activity of white-headed petrels (Pterodroma lessonii) nesting on Adams Island, Auckland Islands. Notornis 67:369–386
Ventura F, Granadeiro JP, Padget O, Catry P (2020) Gadfly petrels use knowledge of the windscape, not memorized foraging patches, to optimize foraging trips on ocean-wide scales. Proc R Soc B: Biol Sci 287:20191775
Wagner D, van der Meer L, Gorny M, Sellanes J, Gaymer CF et al (2021) The Salas y Gómez and Nazca ridges: a review of the importance, opportunities and challenges for protecting a global diversity hotspot on the High Seas. Mar Policy 126:104377
Warham J (1990) The petrels: their ecology and breeding systems. Academic Press, San Diego
Watanabe H, Kubodera T, Yokawa K (2009) Feeding ecology of the swordfish Xiphias gladius in the subtropical region and transition zone of the western North Pacific. Mar Ecol Prog Ser 396:111–122
Young LC, Vanderlip C, Duffy DC, Afanasyev V, Shaffer SA (2009) Bringing home the trash: do colony-based differences in foraging distribution lead to increased plastic ingestion in Laysan Albatrosses? PLoS ONE 4:1–9
Acknowledgements
We would like to thank James Fox at Migrate Technology for advice and help with device management, Danilo and Guillermo Araya for crucial on-island support, the owner and crew of Tio Lalo for inter-island transport and Iva Vasquez and Valentina Colodro of Oikonos plus Jose Luis Cabello and María José Vilches of Island Conservation for logistical support. We thank Associate Editor Vitor Paiva and two anonymous reviewers who provided useful comments on an earlier version of the manuscript, John Horsfall for facilitating COVID-19-impeded transit through continental Chile, and Samantha Andrzejaczek for providing guidance on the preparation of one of the figures.
Funding
The project was funded by a National Geographic Society Explorer Grant (NGS-59743R-19).
Author information
Authors and Affiliations
Contributions
TAC and MdeLB led the study conception and design. Data collection was conducted by MdeLB, and data processing, analysis and visualization were conducted by TAC. A first draft was written by TAC with major contributions from MdeLB.
Corresponding author
Ethics declarations
Conflict of interest
TAC is an Associate Editor for Marine Biology.
Ethical approval
All applicable institutional guidelines for the care and use of animals were followed. All capture, handling, and tagging procedures were in accordance with permits provided by Servicio Agrícola y Ganadero, Chile (permit no. 9793/2019). Ethical approval was provided by the Corporación Nacional Forestal (CONAF; certificate 009/2019) that authorized work in the Archipélago de Juan Fernández National Park.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Clay, T.A., Brooke, M.d. Trans-equatorial migration links oceanic frontal habitats across the Pacific Ocean: year-round movements and foraging activity of a small gadfly petrel. Mar Biol 171, 60 (2024). https://doi.org/10.1007/s00227-023-04373-3
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04373-3