Abstract
Crustose coralline algae (CCA) play a key role in invertebrate recruitment, yet their influence on the settlement of patellid limpets is under discussion. This study is aimed at resolving the role of CCA as a settlement inducer for patellid limpets, providing insight into the influence of different CCA-related factors. The larvae of the economically valuable limpet Patella candei were used as a model. Six assays were performed: (1) different CCA assemblages, (2) exposure time, (3) artificial removal of epibionts, (4) substrate area, (5) soluble cues (CCA-conditioned seawater), and (6) substrate selection in a choice experiment. Settlers were identified by velum loss and teleoconch development. Species composition of the CCA assemblages significantly influenced settlement, with a preference for Titanoderma pustulatum and combined Neogoniolithon sp. and Hydrolithon farinosum crusts. The substrates dominated by Agissea inamoena, marginal presence of CCA or which epibionts were artificially removed, were statistically similar to those in the negative control. The ratio of settlers increased until 4 days of exposure, after which it remained stable over time. The results support that CCA releases soluble cues with settlement-inducing effect on P. candei larvae, explaining why the ratio of settlers increased with substrate area. The choice experiment suggests that P. candei larvae have limited selectivity with respect to the substrate surface. In conclusion, the present study points to the relevance of CCA assemblages as settlement substrates for limpet larvae, with an impact on limpet recruitment in the wild as well as on the production of post-larvae for limpet aquaculture.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Settlement and metamorphosis are key processes for marine invertebrates with planktonic larvae, as they must find a suitable site in the benthos for transitioning to a benthic post-larval lifestyle (Rodríguez et al. 1993; Hadfield and Paul 2001; Jenkins et al. 2009). An understanding of these processes is necessary in different biological fields, including developmental biology, marine benthic community ecology, aquaculture, and biofouling (Hadfield and Paul 2001). The identification of settlement inducers for different taxonomical groups has been a productive research field in recent decades (Rodríguez et al. 1993; Hadfield and Paul 2001; Jenkins et al. 2009; Hadfield 2011; Espinel-Velasco et al. 2018).
Limpets (order Patellogastropoda) are one of the most ubiquitous groups of marine gastropods found on rocky shores worldwide and are a subject of interest in different biological disciplines (Lindberg 2008; Espinosa and Rivera-Ingraham 2017; Firth 2021). However, the study of their settlement and recruitment has been hampered by the lack of consistent settlement cues and laboratory methodologies to induce settlement, limiting the production of post-larvae and the understanding of their ecological requirements (Ferranti et al. 2018, 2022; Mau and Jha 2018; Seabra et al. 2019; Guallart et al. 2020). Some publications have proposed that crustose coralline algae (CCA) might be suitable substrates for inducing settlement in different limpet species (Mau and Jha 2018; Castejón et al. 2021). CCA represent a worldwide group of red algae found from intertidal pools to the greatest depths recorded for marine algae, being a dominant group along rocky shorelines (Steneck 1986; Dethier 1994; Littler and Littler 2013; McCoy and Kamenos 2015). Coralline algae also represent major reef builders with an important role in carbon sequestration, invertebrate recruitment and fish nursery grounds (Steneck 1986; Littler and Littler 2013; McCoy and Kamenos 2015; Terradas-Fernández et al. 2019; Lugilde Yáñez et al. 2022). CCA assemblages promote settlement in diverse marine invertebrates, e.g., cnidarians (Kenkel et al. 2011; Tebben et al. 2015; Zelli et al. 2020), echinoderms (Keesing et al. 1997; Lambert and Harris 2000), and mollusks (Barnes and Gonor 1973; Roberts et al. 2004; Williams et al. 2008; Spotorno-Oliveira et al. 2015), including species of aquacultural interest such as abalone species of the genus Haliotis (Roberts and Nicholson 1997; Daume et al. 1999; Courtois de Vicose et al. 2010, 2012; Roberts et al. 2010).
Moreover, CCA form important grazing fields for several limpet species (Steneck et al. 1991; Pueschel and Miller 1996; Maneveldt et al. 2006); and limpet juveniles have been found naturally in CCA assemblages, e.g., Patella depressa (Seabra et al. 2020), Patella pellucida (McGrath 1992; McGrath and Foley 2005), Patella ulyssiponensis (Seabra et al. 2019, 2020) and Patelloida mufria (Fletcher 1988). Nevertheless, experiments performed under controlled conditions were not conclusive regarding the role of CCA in limpet settlement; i.e., some studies failed to trigger metamorphosis using rock chips covered by coralline algal crusts in Lottia digitalis (Kay 2002) and P. ulyssiponensis (Seabra et al. 2019). In contrast, other studies triggered the metamorphosis of different patellids when using natural substrates covered by CCA (Ribeiro 2008; Castejón et al. 2021). Later, Castejón et al. (2022) showed CCA as a potential settlement substrate for Patella candei, but the influence of CCA was not significantly different from that of the negative control.
Castejón et al. (2021, 2022) proposed such discrepancies as a consequence of different CCA-related factors influencing limpet settlement, such as the CCA species, substrate area and algal health status. The present study was designed to test these hypotheses using the limpet Patella candei d’Orbigny 1840 (Patellogastropoda: Patellidae). This limpet taxon represents a species complex with morphological and molecular divergence among the Macaronesian archipelagos (Hawkins et al. 2000; Sá‐Pinto et al. 2008, Carreira et al. 2017, Faria et al. 2017, Titselaar 2019). This limpet species is an important fishery resource in Macaronesia (Ferraz et al. 2001; Diogo et al. 2016; Riera et al. 2016; Sousa et al. 2020), and it is protected by different conservation measures, including minimum landing sizes, personal catch limitations, seasonal closures and Marine Protected Areas (Sousa et al. 2019a, b, 2020).
Six assays were performed to study the influence of different CCA-related factors on the settlement of P. candei larvae, namely, (1) species composition of the CCA assemblages, (2) exposure time, (3) presence/absence of epibionts in the substrate, (4) substrate area, (5) soluble settlement cues, such as CCA-conditioned seawater, and (6) substrate selection in a choice experiment. Altogether, these assays have been designed to resolve the discrepancies reported in previous studies focused on limpet settlement. The results obtained here will shed light on the recruitment of limpets in the wild and facilitate the development of hatchery protocols.
Material and methods
Patella candei represents a species complex currently under discussion. In the present study, the name Patella candei is used to refer to the Madeira populations following recent publications (Sousa et al. 2020; Cañizares et al. 2021; Nunes et al. 2021; Castejón et al. 2022), as the scientific consensus is yet to be established: Patella candei d’Orbigni (Côrte-Real et al. 1996; Sá‐Pinto et al. 2008), Patella candei ordinaria Mabille (Weber and Hawkins 2002; Carreira et al. 2017), Patella ordinaria Mabille (Faria et al. 2017) and Patella tenuis Gmelin (Titselaar 2019).
Adult limpets were captured by professional fishers on the Madeira coast from February 2022 to April 2022 within the reproductive period of the species (Henriques et al. 2012), transported to the Mariculture Centre of Calheta (Madeira, Portugal) and kept in 200 l PVC cylinderconical tanks following previous studies (Castejón et al. 2022): flow-through system (1–4 l·min−1), natural temperature (20 ± 1 °C), salinity (36 ± 1 g·l−1), photoperiod (12 h light: 12 h dark), and aeration for high oxygen saturation. Adults were characterized by measuring the shell length, total mass and visual gonadal maturation index (Orton et al. 1956) in males; and the shell length, total mass and gonadosomatic index (GSI) in females, the latter was calculated following Sousa et al. (2017). The information regarding the number of adult specimens used in each batch and their corresponding morphological data are summarized in Table 1.
Protocol for the production of competent pediveliger larvae. All the experimental procedures and assays were performed with UV-treated filtered (5 µm) seawater (FSS). The protocols employed to obtain the gametes by dissection, artificial maturation of the oocytes, fertilization, incubation, larval culture, and management of the larvae followed the methods described by Castejón et al. (2023). Artificial maturation of the oocytes was performed using NH4OH at pH 9 for 10–30 min. Fertilization, incubation and larval culture were performed in beakers filled with 500–1200 ml FSS. Fertilization was carried out using 80–200 oocytes·ml−1 and 105 sperm cells·ml−1. The incubation lasted 24 h and was performed at 16.5 ± 1.5 °C to obtain trochophores. Larval culture was performed using 4–10 trochophores·ml−1 and lasted 48 additional hours at 16 ± 1 °C. Then, the pediveligers (72 h post-fertilization) were collected and pooled for settlement assays. Larvae were not fed, as feeding is not required for larval development (Castejón et al. 2021, 2022).
Identification of P. candei life stages. Identification was performed using a dissecting microscope (Leica M165, Leica Microsystems, Wetzlar, Germany). Pediveliger larvae were identified by the velum as an oval-shaped head organ responsible for swimming (Fig. 1A–B). Metamorphics were the first settled stage; they were identified by velum loss and were commonly found resting over different surfaces (Fig. 1C–E). The loss of the velum defines the onset of the settlement and metamorphosis in limpets (Dodd 1957; Guallart et al. 2020; Castejón et al. 2021, 2022; Ferranti et al. 2022). Post-larvae were the next settled stage; they were identified by the development of the teleoconch and active grazing behavior (Fig. 1F–H) (Castejón et al. 2021, 2022; Ferranti et al. 2022). Finally, “deformed” specimens corresponded to nonviable specimens identified by the absence of shell (Fig. 1I).
Life stages of P. candei identified in the present study. Scale bar = 100 µm. Crawling pediveliger larvae (A–B): over the bottom of the culture wells (10 days post-fertilization) (A), and over crustose coralline algae (4 days post-fertilization) (B). Metamorphic stage (C–E): group resting over the bottom of the culture well (7 days post-fertilization) (C), group resting over crustose coralline algae (8 days post-fertilization) (D), and group resting over a shell break (10 days post-fertilization) (E). Post-larval stage (F–H): specimen crawling over the bottom of the culture well (13 days post-fertilization) (F), specimen crawling over coralline algal community composed by Neogoniolithon sp. and Hydrolithon farinosum (13 days post-fertilization) (G), and specimen grazing over the same algal community (14 days post-fertilization) (H). Post-larvae showing shell alterations such as white protoconch and orange teleoconch (G–H). Specimen deformed defined by the lack of shell (7 days post-fertilization) (I). Abbreviations: F feces, GM grazing marks, P protoconch, T teleoconch, V velum
Preparation of the CCA as experimental substrates. The CCA were obtained from limpet shells with natural occurrence of encrusting species, a topic already covered in the literature (Martins et al. 2014; Pereira et al. 2022). In the present study, shell pieces rich in CCA were obtained from two sympatric and congeneric limpet species from Madeira, Patella aspera and Patella candei. The methodology presented here is an improved version of that described by Castejón et al. (2021) that removes any potential interference of soft tissue remains on settlement. First, the soft body was removed using a scalpel and the shell aperture was wiped with paper and washed with FSS. The shells were then placed in the culture tanks for several days (2 to 10 days) under the same conditions used for the adults; the purpose was to macerate and soften the remaining tissues, which were removed by again cleaning the shell aperture with paper and FSS. Next, the limpet shells were broken into pieces using a hammer. Repeated pressure washes were applied using laboratory wash bottles, first with distilled water and then with FSS, to remove remaining debris and sediments. As a final step, the shell pieces were examined under a dissecting microscope to ensure that naturally settled specimens were not present. Finally, the shell pieces were used as settlement substrates.
Additional cleaned and air-dried limpet shells (five whole shells and two pieces) were sent to the Canary Islands Harmful Algal Observatory (OCHABS; University of Las Palmas de Gran Canaria, Spain) for the identification of the encrusting algae. The samples were dried using an oven (40 °C for 24 h) and the identification was performed with optical and scanning electron microscopy. The main crusts corresponded to the following taxa (Fig. 2; Suppl. Files 1–2): (1) Agissea inamoena (Pilger) Pestana, Lyra, Cassano & J.M.C. Nunes (Peyssonneliales: Peyssonneliaceae), formerly Peyssonnelia inamoena according to Pestana et al. (2021); (2) Neogoniolithon sp. Setchell & L. R. Mason (Corallinales: Spongitaceae); (3) Pneophyllum sp. Kützing (Corallinales: Mastoporaceae); (4) Titanoderma pustulatum (J. Lamouroux) Nägeli (Corallinales: Lithophylloideae) and (5) Hydrolithon farinosum (J. Lamouroux) Penrose & Y. M. Chamberlain (Corallinales: Hydrolithaceae). The identification report was used to perform approximate visual identification of the major crusts used in each assay (Figs. 2, 3; Suppl. File 1). Unidentified algal crusts were not discarded.
Natural algal assemblages from limpet shell pieces and used as settlement substrates. A–B NCC, shell piece with negligible CCA coverage (0.36 ± 0.25% piece surface). C–D CLN, “cleaned limpet shell”, shell piece which epibionts were artificially removed. E–F AIN, algal assemblage dominated by Agissea inamoena, where other encrusting species were absent or marginal, i.e., Neogoniolithon sp., Pneophyllum sp. and Hydrolithon farinosum. G–H NEO, assemblage formed by crusts of Neogoniolithon sp. and H. farinosum. I–J PNE, assemblage formed by Pneophyllum sp. and H. farinosum and the minor presence of Neogoniolithon sp. and A. inamoena. K–L TTA, assemblage dominated by Titanoderma pustulatum and other crusts were occasionally present, including A. inamoena, Neogoniolithon sp., Pneophyllum sp. and H. farinosum. M–N MIX, assemblage with several encrusting species without clear dominance, e.g., A. inamoena, Neogoniolithon sp., Pneophyllum sp. and H. farinosum. O–P NPH, assemblage with several encrusting species without clear dominance, e.g., Neogoniolithon sp., Pneophyllum sp., H. farinosum, Titanoderma pustulatum and other unidentified crusts, being A. inamoena generally scarce
Resume of the experiments performed in the present study. A Six-well culture plates used as culture containers in Assays 1–5. B Assay 1, study of the influence of different CCA assemblages on settlement, being used a negative control, biofilms of N. salinicola (NAV), and six different limpet shell substrates. C Assay 2, study of the influence of time of exposure to coralline algae, being used three treatments combined with four exposure times. D Assay 3, study of the influence of shell pieces in the absence of epibionts, being used a negative control, biofilms of N. salinicola, shell pieces which epibionts were artificially removed (CLN) and shell pieces with mixed coralline algae (NPH). E Assay 4, study of the influence of the substrate area, being used a negative control and three sizes of shell pieces. F Assay 5, test of CCA-conditioned seawater (CSW) as settlement inducer, being the CSW prepared by conditioning the FSS during four days, four treatments were applied combining FSS/CSW with absence/presence of CCA. G Assay 6, choice of substrate experiment, glass bowls were used as experimental containers and four substrates of choice
Assays 3 and 6 were performed using shell pieces from which epibionts had been artificially removed. For this purpose, shell pieces with negligible CCA coverage (0.5 ± 0.3 mm2, representing 0.36 ± 0.25% of the piece surface) were selected (Fig. 2A–B), placed for two weeks in the freezer ( – 21 °C) and air-dried in darkness at room temperature for two more weeks. Next, the surface of the limpet shells was brushed to remove epibionts with a scouring pad and pressure washed with distilled water repeatedly. The shell pieces were air-dried in the dark for additional days. Finally, the shell pieces were brushed and pressure washed again with distilled water and FSS (Fig. 2C–D) and used as settlement substrates.
The surface area of the limpet shell pieces was measured by taking pictures of each shell piece with a dissecting microscope connected to a camera and image analysis software (LAS V4.12; Leica Microsystems, Wetzlar, Germany). Pictures were manually merged and converted to 8-bit pictures using the image editing software GIMP 2.10.22, before being measured using the image analysis software ImageJ 1.53 k.
Assays 1–3 tested biofilms of the benthic diatom Navicula salinicola, formerly Navicula incerta following Kociolek et al. (2022), to replicate previous results about its potential as an effective settlement inducer for P. candei larvae (Castejón et al. 2022). This algal strain was originally provided by the University of Las Palmas de Gran Canaria (Gran Canaria, Spain). Then, it was cultured at the Mariculture Center of Calheta (Madeira, Portugal) using culture media f/2 enriched with silica. The diatoms were added to the settlement wells by taking the volume of culture media with algae required for a final density of ca. 3,000 cells/mm2 in the bottom of the culture wells (Fig. 3A–D).
General procedures for the larval settlement assays. Assays 1–5 were performed in sterile, six-well culture plates (SIAL0516 Sigma ‒ Aldrich, USA; Ref. 657160 Cellstar® Greiner Bio-One, Austria) (Fig. 3A). The distribution of treatments among the wells followed the patterns obtained by a random number generator (stattrek.com). For assays 1–4, the pediveligers were kept in the culture wells with 5 ml FSS for the first 3 days. Then, on the third day of the assay (equivalent to Day 6 post-fertilization), after monitoring the culture plates, 3 ml FSS (for a total of 8 ml FSS) and the corresponding substrate were added. This strategy was followed to ensure the presence of settlement-competent larvae before applying the treatments (Castejón et al. 2021, 2022). Assays 5 and 6 followed a different approach detailed in their corresponding sections. All assays were performed at 16 ± 1 °C.
Assay 1: influence of different CCA assemblages on settlement. The objective was to study the influence of different assemblages of naturally occurring epibionts on limpet shells (Fig. 3B; Suppl. File 1). Eight treatments were employed: (1) negative control with no substrate (CNT); (2) cultured biofilms of the benthic diatom N. salinicola (NAV); (3) shell pieces 144 ± 21 mm2 with negligible CCA coverage (0.5 ± 0.3 mm2) as shell piece controls (NCC: Fig. 2A–B); (4) shell pieces 163 ± 33 mm2 dominated by Agissea inamoena, where other crustose species were absent or marginal, i.e., Neogoniolithon sp., Pneophyllum sp. and H. farinosum (AIN: Fig. 2E–F); (5) shell pieces 160 ± 18 mm2 with an assemblage formed by crusts of Neogoniolithon sp. and H. farinosum (NEO: Fig. 2G–H); (6) shell pieces 228 ± 43 mm2 with an assemblage formed by Pneophyllum sp. and H. farinosum and the minor presence of Neogoniolithon sp. and A. inamoena (PNE: Fig. 2I–J); (7) shell pieces 168 ± 53 mm2 with an assemblage dominated by Titanoderma pustulatum, the presence of other crust species was minimal, including A. inamoena, Neogoniolithon sp., Pneophyllum sp. and H. farinosum (TTA: Fig. 2K–L); and (8) shell pieces 169 ± 35 mm2 covered by diverse crusts of A. inamoena, Neogoniolithon sp., Pneophyllum sp. and H. farinosum (MIX: Fig. 2M–N). Five replicates were used in each treatment, except for TTA, for which four replicates employed because of its rarity. Larval density was 5 ± 1 larvae·ml−1. Assay 1 lasted 11 days (equivalent to 14 days post-fertilization).
Assay 2: influence of time of exposure to coralline substrates. Assay 2 aimed to study the occurrence of settled specimens over time when using similar CCA assemblages (Fig. 3C). Three substrates were tested: (1) negative control with no substrate (CNT); (2) cultured biofilms of the benthic diatom N. salinicola (NAV); and (3) shell pieces 104 ± 29 mm2 with an assemblage formed by crusts of Neogoniolithon sp. and H. farinosum (NEO: Fig. 2G–H). The NEO assemblage was selected for three main reasons: (1) it showed an apparent homogeneous structure, (2) it was relatively common, and (3) Assay 1 showed both a significant settlement rate and the potential to induce malformations later on.
Each substrate treatment was subjected to four exposure time treatments: 2, 4, 6 and 8 days of exposure (equivalent to 8, 10, 12 and 14 days post-fertilization, respectively), resulting in a total of 12 combined treatments (substrate x time). Four replicates per combined treatment were employed. The larval density was 5 ± 1 larvae·ml−1.
Assay 3: influence of shell pieces in the absence of epibionts. Limpet shell pieces were the natural source of CCA used in this study. Assay 3 was designed to study the influence of the shell pieces themselves on the settlement of P. candei (Fig. 3D). For this purpose, selected limpet shell pieces (Fig. 2A–B) were processed to obtain shell pieces from which epibionts were artificially removed, now named “cleaned limpet shells” (Fig. 2C–D). The procedure used to obtain “cleaned limpet shells” is described in the section “Preparation of the substrates used in the assays”. Four treatments with five replicates each were performed in this study: (1) negative control with no substrate; (2) biofilm of the benthic diatom N. salinicola (NAV); (3) “cleaned limpet shell” pieces 137 ± 10 mm2 (CLN; Fig. 2C–D); and (4) shell pieces 166 ± 30 mm2 with an assemblage containing crusts of Neogoniolithon sp., Pneophyllum sp., H. farinosum, A. inamoena (scarce) and other unidentified crusts (NPH; Fig. 2O–P). Larval density was 3 ± 1 larvae·ml−1. Assay 3 lasted 10 days (equivalent to 13 days post-fertilization).
Assay 4: influence of the substrate area. Castejón et al. (2021) reported that the substrate area influenced the settlement rate in the limpet P. aspera. This assay was carried out to replicate this result by performing a similar assay in the congeneric species P. candei (Fig. 3E). The CCA assemblage formed by crusts of Neogoniolithon sp. and H. farinosum was selected (NEO; Fig. 2G–H) due to its apparent homogeneous structure, which facilitated the preparation of different substrate areas. Four treatments with four replicates each were used: (1) negative control with no substrate; (2) small shell pieces 10 ± 2 mm2 (small); (3) medium shell pieces 121 ± 17 mm2 (medium); and (4) large shell pieces 341 ± 72 mm2 (large). The larval density was 3 ± 1 larvae·ml−1. Assay 4 lasted 8 days (equivalent to 11 days post-fertilization).
Assay 5: CCA-conditioned seawater as a settlement inducer. This assay tested whether CCA release soluble cues able to trigger settlement of P. candei larvae (Fig. 3F). Four treatments were used: (1) negative control treatment, i.e., no substrate in clean, filtered, sterilized seawater (NNS + FSS); (2) no substrate in conditioned seawater (NNS + CSW); (3) CCA in clean, filtered, sterilized seawater (CCA + FSS); and (4) CCA in conditioned seawater (CCA + CSW). Four replicates were used for each treatment.
The CCA corresponded to shell pieces of 286 ± 68 mm2 containing crusts of Titanoderma pustulatum, Neogoniolithon sp., Pneophyllum sp., H. farinosum, A. inamoena and potentially other unidentified species (Fig. 2O–P). Larvae were kept in the culture wells with 4 ml of FSS until Day 5 of the assay (equivalent to Day 8 post-fertilization). Conditioned seawater was obtained by placing the CCA in other culture wells with 8 ml FSS during the four days prior to the application of the treatments. Treatments were applied by adding 4 ml of FSS or 4 ml of CSW and the CCA if required according to the treatment (Fig. 3F). The larval density was 3 ± 1 larvae·ml−1. Assay 5 lasted 10 days (equivalent to 13 days post-fertilization).
Assay 6: choice of substrate. This assay was designed as a choice experiment for competent P. candei larvae exposed to different substrates (Fig. 3G). The assay was carried out in glass bowls with a 500 ml capacity filled with 225 ml FSS and ca. 4 pediveligers·ml−1. Pediveligers were kept in the glass bowls without any substrate for five days (equivalent to Day 8 post-fertilization) to ensure an abundance of settlement-competent larvae, following the Assay 2 results. Five glass bowls were used as replicates. Each glass bowl contained four substrates: (1) “cleaned limpet shell” pieces 3.8 ± 0.5 cm2 (CLN; Fig. 2C–D); (2) shell pieces 3.5 ± 0.5 cm2 dominated by A. inamoena and sparse Neogoniolithon sp., Pneophyllum sp. and H. farinosum (AIN: Fig. 2E–F); (3) shell pieces 4.4 ± 1.0 cm2 covered by crusts of Neogoniolithon sp. and H. farinosum (NEO: Fig. 2G–H); and (4) shell pieces 3.9 ± 0.8 cm2 dominated by Titanoderma pustulatum and with sparse A. inamoena, Neogoniolithon sp., Pneophyllum sp. and H. farinosum (TTA: Fig. 2K–L). The bottom of the glass bowls, with no shell pieces, was considered the negative control (average 98 ± 2 cm2). These substrates were selected because showed different settlement responses according to Assays 1 and 3: low in AIN and CLN and high in NEO and TTA.
Metamorphics were used as markers for substrate selection, as this settled stage has been generally observed resting over surfaces (Fig. 1C–E) (Castejón et al. 2021, 2022). This assay was finished two days after substrate placement to fit the short duration of the metamorphic stage (ca. 72 h) (Castejón et al. 2021, 2022). The specimens settled on the shell pieces were identified separately on the upper (treatment) and lower (mother-of-pearl) shell surfaces.
Daily monitoring and finalization of the assays. The Assays 1–5 were finished by identifying all specimens following the steps described by Castejón et al. (2021): (1) the shell pieces were placed in a Petri dish with FSS to identify the specimens located on the upper and lower surfaces of the pieces; (2) the seawater was pipetted out of the wells, and the collected specimens were identified; and (3) the last specimens to be identified were those located at the bottom and walls of the wells. Life stages were as described in the section “Identification of P. candei life stages”. The “total specimens” included the total number of pediveliger larvae, metamorphics, post-larvae, deformed specimens and empty shells.
In Assays 1 and 3–5, daily monitoring was performed to identify the specimens visible in the field of view of the dissecting microscope. Pediveligers were classified according to their behavior as swimming or crawling following Castejón et al. (2021). “Total specimens” was used to calculate four daily ratios: (1) daily ratio of swimming pediveligers = number swimming pediveligers x total specimens−1; (2) daily ratio of crawling pediveligers = number crawling pediveligers x total specimens−1; (3) daily ratio of settlers = (number of metamorphics + number of post-larvae) × total specimens−1; and 4) daily ratio of mortality = number of empty shells × total specimens−1.
At the end of each assay, different ratios were calculated. In all the assays, the final ratio of post-larvae = number of post-larvae x total specimens−1. In Assays 2–5, the final ratio of settlers = (number of metamorphics + number of post-larvae) x total specimens−1. In Assay 2, the ratio of deformed specimens = number of deformed specimens x total specimens−1. In assay 6, the final density of settlers was equal to the number of early metamorphics per cm2 of substrate; the different substrates were control (glass bowl bottom), each upper shell surface (as treatments) and each lower shell surface (mother-of-pearl).
Statistical analyses. Statistical analyses were performed using the statistical software R version 4.1.0 (R Development Core Team 2021). For all the assays, the parametric assumptions were tested by applying Levene’s test (package “car 3.0–12”) for the homogeneity of the variances and the Shapiro‒Wilk test for normality. One-way analysis of variance (ANOVA) (Type II) (package “car 3.0–12”) was used to analyze the final ratio of post-larvae in Assays 1 and 3, the final ratio of settlers in Assays 3 and 5, and the density of settlers in Assay 6. Tukey’s HSD was the post hoc test applied when differences were significant. The final ratio of settlers in Assay 4 and the final ratio of post-larvae in Assay 5 did not meet the assumption of homogeneity of variances, so they were analyzed using the nonparametric robust ANOVA based on trimmed means (package “WRS2 1.1–3”) followed by the Games–Howell test (package “rstatix 0.7.0”) as a post hoc approach. The final ratio of post-larvae in Assay 4 had zero values in all the control replicates, so it was analyzed using the Kruskal–Wallis test followed by Dunn’s test (package “dunn.test 1.3.5”).
In Assay 2, the final ratios of settlers, post-larvae and deformed specimens were analyzed using the nonparametric robust one-way ANOVA (package “WRS2 1.1–3”), followed by the ‘lincon’ formula of the package “WRS2 1.1–3” for each substrate x time subset, and using the robust two-way ANOVA (‘t2way’ formula) for the interaction between substrate and time.
The null hypothesis was rejected when p < 0.05.
Results
Assay 1: influence of different CCA assemblages on settlement. The daily ratio of swimming pediveligers was lower in all treatments involving shell pieces (Fig. 4A). The daily ratio of crawling pediveligers peaked on Day 7 (one day after substrate placement) in the treatments with Titanoderma pustulatum (TTA) and A. inamoena crusts (AIN) (Fig. 4B). The daily ratio of settlers increased beginning on Day 7 in all the treatments, except in the negative control (Fig. 4C). The greater daily ratio of settlers occurred with Titanoderma pustulatum (TTA), Neogoniolithon sp. plus H. farinosum crusts (NEO) and Pneophyllum sp. plus H. farinosum crusts (PNE) (Fig. 4C). The daily ratio of mortality increased in treatments with shell pieces, especially in those with either negligible CCA coverage (NCC) or A. inamoena crusts (AIN) (Fig. 4D).
Assay 1: influence of different CCA assemblages on settlement. A Daily ratio of swimming pediveligers. B Daily ratio of crawling pediveligers. C Daily ratio of settled specimens. D Daily ratio of mortality. E Final ratio of post-larvae. Bars indicate average ± SD. Different letters (a–d) indicate significant differences among treatments (Tukey’s HSD, p < 0.05). Abbreviations: AIN algal assemblage dominated by A. inamoena, Control, negative control with no substrate, MIX mixed crusts of A. inamoena, Pneophyllum sp., Neogoniolithon sp. and H. farinosum, NAV biofilms of the benthic diatom N. salinicola, NCC shell pieces with negligible CCA coverage, NEO dominance of Neogoniolithon sp. and H. farinosum, PNE dominance of Pneophyllum sp. and H. farinosum, TTA dominance of Titanoderma pustulatum
The final ratio of post-larvae varied significantly among the treatments (ANOVA, F7,31 = 18.72, p < 0.001). Significant differences were found between the CCA assemblages and the negative control, except for A. inamoena crusts (AIN; Figs. 2E–F, 4E). Moreover, the CCA assemblages showed significant differences; i.e., the ratio of post-larvae was greater in assemblages dominated by Titanoderma pustulatum (TTA; Figs. 2K–L, 4E) and Neogoniolithon sp. plus H. farinosum crusts (NEO; Figs. 2G–H, 4E), while intermediate values occurred with the assemblages dominated by Pneophyllum sp. plus H. farinosum crusts (PNE; Figs. 2I–J, 4E) and a variety of crusts (MIX; Figs. 2M–N, 4E). Shell pieces with negligible CCA coverage (NCC; Fig. 2A–B) were not significantly different from the negative control (Fig. 4E). Settlement with biofilms of the benthic diatom N. salinicola was significantly different from that in the negative control showing intermediate values (Fig. 4E).
In Assay 1, the treatment with Neogoniolithon sp. and H. farinosum crusts affected shell development, which was initially noted as a change in coloration: white protoconch and orange teleoconch (Fig. 1G–H). At the end of the assay some of these post-larvae lost their shells and were classified as “deformed” (Fig. 1I). Post-larvae were observed grazing on shed epithallium and depositing white feces (Fig. 1H).
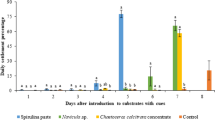
Assay 2: influence of time of exposure to coralline substrates. Settlers appeared after 2 days of exposure (Fig. 5A), and post-larvae appeared after 4 days of exposure (Fig. 5B). The ratios of settlers and post-larvae reached higher values and stabilized after 4 days of exposure (Fig. 5A–B). The interaction between substrates and exposure time was not significant for the ratio of settlers (2 to 8 days; p = 0.12), the ratio of post-larvae (4 to 8 days; p = 0.79) or the ratio of deformed specimens (2 to 8 days; p = 0.26). Shell pieces colonized by Neogoniolithon sp. and H. farinosum crusts (Fig. 2G–H) showed the greatest ratios of settlers and post-larvae for all exposure times (Fig. 5A–B), while biofilms of the benthic diatom N. salinicola induced lower ratios similar to those in the negative control in several of the substrate x time combinations (Fig. 5A–B). The ratio of deformed specimens was marginal in all treatments, except for shell pieces colonized by Neogoniolithon sp. and H. farinosum crusts at 8 days of exposure (Fig. 5C).
Assay 2: influence of time of exposure to coralline substrates. A Final ratio of settlers (metamorphics + post-larvae). B Final ratio of post-larvae. C Final ratio of deformed specimens. Bars indicate average ± SD. Different letters (a–c) indicate significant differences (‘lincon’ formula, p < 0.05) and the letters ns indicate non-significant differences among treatments within the same time (exposure days). Abbreviations: Control, negative control with no substrate, NAV biofilm of the benthic diatom N. salinicola, NEO Neogoniolithon sp. and H. farinosum crusts
Assay 3: influence of shell pieces in the absence of epibionts. The coralline algal treatment (NPH) showed the lowest daily ratio of swimming pediveligers (Fig. 6A) and the highest daily ratio of settled specimens (Fig. 6C). The daily ratio of swimming pediveligers was similar among the control (CNT), the “cleaned limpet shell” treatment (CLN) and the treatment with biofilms of the benthic diatom N. salinicola (NAV) (Fig. 6A). The daily ratios of crawling pediveligers (Fig. 6B) and mortality (Fig. 6D) were low.
Assay 3: influence of shell pieces in the absence of epibionts. A Daily ratio of swimming pediveligers. B Daily ratio of crawling pediveligers. C Daily ratio of settled specimens. D Daily ratio of mortality. E Final ratio of settlers (metamorphics + post-larvae). F Final ratio of post-larvae. Bars indicate average ± SD. Different letters (a–c) indicate significant differences among treatments (Tukey’s HSD, p < 0.05). Abbreviations: CLN “cleaned limpet shell” pieces, Control, negative control with no substrate, NAV biofilms of the benthic diatom N. salinicola; NPH, shell pieces with a diversity of coralline algae crusts
Significant differences were found in the final ratio of settlers (ANOVA, F3,16 = 41.29, p < 0.001; Fig. 6E) and the final ratio of post-larvae (ANOVA, F3,16 = 49.93, p < 0.001; Fig. 6F). The final ratios of settlers and post-larvae were significantly higher in the coralline algal treatment, while those in the “cleaned limpet shell” treatment were statistically similar to those in the negative control (Fig. 6E–F). Biofilms of the benthic diatom N. salinicola had a modest influence; i.e., the final ratio of settlers was statistically higher than that in the negative control (Fig. 6E), while the final ratio of post-larvae was statistically similar than that in the negative control (Fig. 6F).
Assay 4: influence of the substrate area. The daily ratio of swimming pediveligers decreased abruptly in the treatments with medium and large shell pieces (Fig. 7A). The first settlers were reported beginning on Day 7 (one day after substrate placement) in the medium and large shell piece treatments and beginning on Day 8 in the negative control and small shell piece treatments (Fig. 7C). The daily ratio of settled specimens increased markedly in all the shell piece treatments during the experiment (Fig. 7C). The daily ratios of crawling pediveligers (Fig. 7B) and mortality (Fig. 7D) were low.
Assay 4: influence of the substrate area. The treatments corresponded to absence of substrate as negative control (Control), shell pieces 10 ± 2 mm2 (small), shell pieces 121 ± 17 mm2 (medium) and shell pieces 341 ± 72 mm2 (large). A Daily ratio of swimming pediveligers. B Daily ratio of crawling pediveligers. C Daily ratio of settled specimens. D Daily ratio of mortality. E Final ratio of settlers (metamorphics + post-larvae). F Final ratio of post-larvae. Bars indicate average ± SD. Different letters indicate significant differences among treatments (x–y, Games Howell, p < 0.05; r–s, Dunn’s Test, p < 0.05)
There were similarities between treatments regarding the final ratios of settlers and post-larvae (Fig. 7E–F). Significant differences among treatments were found in the final ratios of settlers (robust ANOVA, F = 17.25, p < 0.01; Fig. 7E) and post-larvae (Kruskal‒Wallis, χ2 = 11.58, df = 3, p < 0.01; Fig. 7F). The ratios of settlers and post-larvae increased with substrate area, with larger values in the treatments with medium and large shell pieces than in the negative control (Fig. 7E–F).
Assay 5: CCA conditioned seawater as settlement inducer. The daily ratio of swimming pediveligers decreased sharply in the treatments based on CCA substrates and moderately in the treatment based on conditioned seawater without substrate (Fig. 8A). The daily ratio of crawling pediveligers was low (Fig. 8B). Spontaneous settlement was reported on Day 8 post-fertilization (Fig. 8C). The daily ratio of settled specimens increased abruptly from day 9 (one day after substrate and conditioned seawater placement) in all the treatments, except the negative control (Fig. 8C). The daily ratio of mortality was greater in those treatments based on CCA substrates and/or conditioned seawater (Fig. 8D).
Assay 5: CCA conditioned seawater as settlement inducer. The treatments resulted from the combination of substrate and seawater. The substrates were absence of substrate (NNS) or crustose coralline algae (CCA). The seawater was either clean, sterilized seawater (FSS) or FSS conditioned with CCA (CSW). A Daily ratio of swimming pediveligers. B Daily ratio of crawling pediveligers. C Daily ratio of settled specimens. D Daily ratio of mortality. E Final ratio of settlers (metamorphics + post-larvae). F Final ratio of post-larvae. Bars indicate average ± SD. Different letters indicate significant differences among treatments (a–c, Tukey’s HSD, p < 0.05; x–y, Games Howell, p < 0.05)
Assay 5 also showed similar trends between treatments regarding the final ratios of settlers and post-larvae (Fig. 8E–F). Significant differences were found in the final ratios of settlers (ANOVA, F3,12 = 23.09, p < 0.001; Fig. 8E) and post-larvae (robust ANOVA, F = 104.58, p < 0.001; Fig. 8F). The treatments based on CCA substrates showed the highest final ratios of settlers and post-larvae (Fig. 8E–F). The final ratios of settlers and post-larvae obtained when using conditioned seawater without substrate were significantly higher than those in the negative control and moderately lower than those obtained in the presence of CCA (Fig. 8E–F).
Assay 6: choice of substrate. The upper shell surfaces (treatments) showed marginally significant differences (ANOVA, F4,20 = 38.79, p = 0.017; Fig. 9A), while the lower shell surfaces (mother-of-pearl) did not show significant differences (ANOVA, F4,20 = 0.33, p = 0.85; Fig. 9B). Regarding the upper shell surfaces, the treatment based on Neogoniolithon sp. and H. farinosum crusts showed a significantly higher density of settlers than the treatment based on “cleaned limpet shell” pieces (Fig. 9A). The density of settlers in the former was on the statistical limit of being significantly higher than in the treatment based on A. inamoena crusts (Fig. 9A; Tukey’s HSD, p = 0.052).
Assay 6: choice of substrate. Density of settlers indicated as metamorphics per cm2 of substrate A–B: upper surface of the substrates (treatments) A and lower surface of the substrates (mother-of-pearl) B Bars indicate average ± SD. Different letters (a–b) indicate significant differences (Tukey’s HSD, p < 0.05), and the letters ns indicate non-significant differences. The bottom of the glass bowls, with no shell pieces, was considered the negative control (Control). Abbreviations: CLN “cleaned limpet shell” pieces, AIN A. inamoena, NEO Neogoniolithon sp. and H. farinosum, TTA Titanoderma pustulatum
Discussion
The present results show that natural crustose coralline algae (CCA) are strong settlement inducers for P. candei competent larvae, supporting a previous suggestion for this limpet species (Castejón et al. 2022), as well as previous reports of successful use of natural CCA as settlement inducers for other patellid species (Ribeiro 2008; Castejón et al. 2021). These experimental results are congruent with the natural occurrence of juveniles of different limpet species in rocky environments covered by CCA assemblages (McGrath and Foley 2005; Seabra et al. 2019, 2020), which synergizes with the role of limpets in controlling the expansion and growth of different macroalgal canopies (Benedetti-Cecchi 2000; Coleman et al. 2006; Lorenzen 2007). Moreover, the present observations of post-larvae of P. candei grazing on shed CCA epithallium (Fig. 1H), together with similar observations made in post-larvae of P. aspera (Castejón et al. 2021) and the fact that CCA are important grazing fields for numerous limpet species (Steneck et al. 1991; Pueschel and Miller 1996; Maneveldt et al. 2006), support the hypothesis that natural CCA assemblages from rocky shorelines are a key environment for limpet recruitment, feeding, and development.
Influence of the different settlement substrates
The CCA assemblages are composed of diverse crusts, often including different species, which compete for the same space (Padilla 1984; Steneck 1986; McCoy and Pfister 2014). The same has been observed for CCA assemblages growing as epibionts over limpet shells, in the present as well as previous studies (Castejón et al. 2021). Natural CCA assemblages have specific zonation patterns dependent on numerous factors, including location, stage of succession, and influence of physical and biological disturbances (Padilla 1984; Steneck 1986; McCoy and Kamenos 2015). As different limpet species also tend to be associated with ecological constraints and consistent zonation patterns (Branch 1971; Della Santina and Chelazzi 1991; Della Santina et al. 1993; Bazterrica et al. 2007), it is congruent to assume that the different CCA assemblages represent different quality indicators of suitable ecological spaces. Similarly, Jorissen et al. (2021) proposed that each CCA species has a key chemical signature that could serve as an environmental indicator for coral larvae seeking a suitable habitat and substrate.
Our results support this hypothesis, as the CCA assemblages differently influenced settlement; i.e., the ratio of post-larvae was high in the treatments with Titanoderma pustulatum and Neogoniolithon sp. plus H. farinosum; moderate in those with Pneophyllum sp. plus H. farinosum and assemblages composed of mixed crusts; and low in those with assemblages dominated by A. inamoena. One limitation of this study was the difficulty in discerning the contributions of the encrusting species richness to the settlement, as the habitat’s heterogeneity may contribute to the settlement. However, the CCA assemblages with a higher ratio of post-larvae seemed to be dominated by specific crusts rather than mixed crusts, so the larvae of P. candei are probably able to differentiate among different encrusting species. In this sense, the competent larvae of the congeneric limpet species P. aspera showed a differential settlement response to different CCA substrates (Castejón et al. 2021). The same has been reported in different abalone species (genus Haliotis), whose larvae preferred certain coralline species for settlement; e.g., the Haliotis laevigata ratio of settlement was higher with Sporolithon durum than with Mesophyllum engelhartii and Hydrolithon rupestre (Daume et al. 1999), Haliotis iris settled faster with Pneophyllum coronatum and Hydrolithon rupestris than with other encrusting algae (Roberts et al. 2004), and the settlement success of Haliotis asinina was greater with the genera Amphiroa, Neogoniolithon and Lithophyllum than with other Corallinales (Williams et al. 2008). Further studies are required to gain insight into the influence of habitat heterogeneity on limpet settlement and the contribution of the different encrusting species.
In P. candei, the settlement success in the negative control treatments was similar than in those treatments with shell pieces almost devoid of CCA (Assay 1) and with shell pieces which epibionts were artificially removed (Assay 3), supporting the key importance of CCA assemblages for settlement. A previous experiment also showed that alive CCA promote a significantly greater settlement than dead CCA (by air-drying) in the congeneric species P. aspera (Castejón et al. 2021). Abalone (Haliotis asinina) larvae also settled less with the dead CCA than with the live CCA (Williams et al. 2009).
The P. candei larvae settled modestly to weakly when exposed to biofilms of the benthic diatom Navicula salinicola. This study used monocultures of N. salinicola with enriched media, but in the wild, diatom biofilms generally include several diatom species and other microbial taxa (Molino and Wetherbee 2008; Salta et al. 2013). The differences in the diatom community structure are considered a factor for the settlement of higher organisms (Patil and Anil 2005). In future studies, it would be interesting to test the influence of multispecies biofilms resembling natural conditions. In addition, the different limpet species might vary in their settlement requirements, implying the necessity of performing the settlement studies at specific scales in limpets.
Influence of exposure time and potential allelopathic effects
The present study used 6 days post-fertilization at 16 ± 1 °C as the minimum age to ensure several P. candei larvae at a settlement-competent age, which is within the range of 5 to 7 days post-fertilization established for other patellid species (Castejón et al. 2021; Ferranti et al. 2022). Under these conditions, the CCA elicited fast metamorphosis with post-larvae present as early as 24 h after substrate placement. A fast settlement response was also reported in abalone; i.e., settled specimens were present after competent larvae were exposed to coralline algae from a few hours (Moss and Tong 1992; Stewart et al. 2008) to 2–3 days (Daume et al. 1999; Roberts et al. 2004, 2010; Stewart et al. 2008; Courtois de Vicose et al. 2010).
In Assay 2, the ratio of settlers increased until 4 days of exposure, becoming stable over time. Assay 1 yielded a similar pattern for the daily ratio of settled specimens, while Assays 4 and 5 confirmed the increasing daily ratio of settled specimens until 4 days of exposure. Altogether, this information suggests that most limpet larvae were settlement-competent within 10 days post-fertilization.
Assay 2 also showed that the ratio of settlers was low and stable over time when using suboptimal settlement cues such as biofilms of the benthic diatom N. salinicola, rather than increasing with time. This result suggests that each P. candei larva may have a specific and stable sensitivity to a set of cues. In the abalone Haliotis iris, the settlement rate was also stable over time when larvae were exposed to some cues (Alfaro et al. 2014). On the other hand, laboratory experiments revealed that limpet larvae can remain in the pediveliger stage for approximately one month before settling successfully (Castejón et al. 2022; Ferranti et al. 2022). The ability of some larvae to delay settlement raises different hypotheses to be tested in future studies; i.e., some larvae may mature significantly slower than other, some larvae could gain additional sensitivity to settlement cues over time, and/or the settlement could occur spontaneously after a given time threshold.
Coralline algae, in addition to being strong settlement substrates for several marine invertebrates, also release secondary metabolites with inhibitory or allelopathic effects (McCoy and Kamenos 2015; Young et al. 2015), including germination inhibitors against most algal species (Kim et al. 2004; Gomez-Lemos and Diaz-Pulido 2017) and toxicity to ascidian larvae (Degnan & Johnson 1999). In this context, the shell changes reported in limpet post-larvae (Fig. 1G–H) might result from secondary metabolites released by the CCA assemblage formed by Neogoniolithon sp. and Hydrolithon farinosum crusts. Previously, Castejón et al. (2021) reported the death and degradation of the same CCA assemblage under laboratory conditions, ultimately killing the limpet larvae. The larvae of Haliotis also exhibited toxic effects of high concentrations CCA extracts (Morse and Morse 1984; Roberts and Nicholson 1997). Further studies are required to elucidate the effects of the secondary metabolites from the different CCA assemblages on limpet larvae and post-larvae.
Influence of area, soluble cues and substrate surface
In P. candei, the ratios of settlers and post-larvae increased with CCA substrate area (Assay 4). A similar result was reported for the congeneric species P. aspera (Castejón et al. 2021). These results suggest an increasing chance of settling in parallel with increasing substrate surface availability. However, other results from our experiments seem to point to a major role of chemical factors released by most CCA assemblages in P. candei settlement when compared to physical factors such as the size of the available substrate surface.
First, P. candei metamorphics were found over different types of surfaces, including plastic, shell fractures and CCA (Fig. 1C–E). Second, when given the choice, the early post-larvae were found at similar densities over surfaces as diverse as glass, mother-of-pearl and some CCA assemblages (Assay 6). Third, CCA-conditioned seawater induced an important settlement response nearly as strong as that induced by CCA substrates themselves (Assay 5). Therefore, increasing quantities of CCA resulted in higher cue concentrations, explaining the influence of CCA substrate area (Assay 4). This idea is supported by Suenaga et al. (2004) work, in which the settlement rate of Haliotis larvae increased with the concentration of commercial δ-aminovaleric acid, a compound isolated from the coralline alga Hydrolithon samoense. Crude extracts of CCA also triggered the settlement and metamorphosis of different Haliotis species (Morse and Morse 1984; Roberts and Nicholson 1997), and the biochemical surface profile of different coralline species (Hydrolithon onkodes, Lithophyllum moluccense and Amphiroa ephedraea) partially correlated with the settlement rate in the abalone Haliotis asinina (Williams et al. 2009).
The significant differences in settler density between “cleaned shell pieces” and Neogoniolithon sp. and Hydrolithon farinosum crusts (Assay 6) suggests that limpet larvae can discern certain surfaces, despite the general capacity to settle over a wide variety of surfaces. In P. candei, settlement seemed to be a single-step process induced by soluble cues rather than a two-step process involving soluble cues and substrate-attached cues, as proposed for P. aspera (Castejón et al. 2021).
In conclusion, it is important to consider the potential of CCA as an enhancement substrate for the production of post-larvae in limpet aquaculture and the importance of CCA assemblages for limpet recruitment in the wild. This is particularly relevant for P. candei. This study demonstrates that different factors associated with CCA affect their capacity as settlement inducers, including CCA species, exposure time, substrate size and the presence of soluble cues. There seems to be synergy between CCA and limpets for the success of both communities. CCA favor the settlement of limpet larvae and support juvenile feeding. Simultaneously, grazing limpets maintain control of macroalgal development, securing the grounds for the CCA and the limpet themselves. The potential of CCA soluble cues as a tool for limpet aquaculture is an interesting topic to explore in future studies.
Availability of data and material
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Alfaro AC, Young T, Bowden K (2014) Neurophysiological control of swimming behaviour, attachment and metamorphosis in black-footed abalone (Haliotis iris) larvae. NZ J Mar Freshwat Res 48:314–334
Barnes J, Gonor J (1973) The larval settling response of the lined chiton Tonicella lineata. Mar Biol 20:259–264
Bazterrica MC, Silliman BR, Hidalgo FJ, Crain CM, Bertness MD (2007) Limpet grazing on a physically stressful Patagonian rocky shore. J Exp Mar Biol Ecol 353:22–34
Benedetti-Cecchi L (2000) Predicting direct and indirect interactions during succession in a mid-littoral rocky shore assemblage. Ecol Monogr 70:45–72
Branch GM (1971) The ecology of Patella Linnaeus from the Cape Peninsula, South Africa I. Zonation, movements and feeding. African Zoology 6:1–38
Cañizares JM, Castejón D, Haroun R, Nogueira N, Andrade CAP (2021) Enhancing oocyte maturation and fertilisation in the black-foot limpet Patella candei d′Orbigny, 1840 (Patellidae, Mollusca). Aquaculture Reports 21:100856
Carreira GP, Shaw PW, Gonçalves JM, McKeown NJ (2017) Congruent molecular and morphological diversity of Macaronesian limpets: insights into eco-evolutionary forces and tools for conservation. Front Mar Sci 4:75
Castejón D, Nogueira N, Andrade CAP (2021) Limpet larvae (Patella aspera Röding, 1798), obtained by gonad dissection and fecundation in vitro, settled and metamorphosed on crustose coralline algae. J Mar Biol Assoc UK 101:991–1002
Castejón D, García L, Cañizares JM, De Girolamo M, Nunes C, Isidro E, Courtois de Viçose G, Nogueira N, Andrade CAP (2022) Methodologies for Patellid Limpets’ Aquaculture: From Broodstock Management to Juveniles. Front Mar Sci 9:884262
Castejón D, García L, Nogueira N, Andrade CAP (2023) Improving efficiency of methods for hatchery production of the limpet Patella candei (Patellogastropoda: Patellidae). Journal of the World Aquaculture Society 54(4):945–964
Coleman RA, Underwood AJ, Benedetti-Cecchi L, Åberg P, Arenas F, Arrontes J, Castro J, Hartnoll RG, Jenkins SR, Paula J (2006) A continental scale evaluation of the role of limpet grazing on rocky shores. Oecologia 147:556–564
Côrte-Real H, Hawkins S, Thorpe J (1996) Population differentiation and taxonomic status of the exploited limpet Patella candei in the Macaronesian islands (Azores, Madeira, Canaries). Mar Biol 125:141–152
Courtois de Vicose G, Viera M, Bilbao A, Izquierdo M (2010) Larval settlement of Haliotis tuberculata coccinea in response to different inductive cues and the effect of larval density on settlement, early growth, and survival. J Shellfish Res 29:587–591
Courtois de Vicose G, Viera MP, Huchette S, Izquierdo MS (2012) Larval settlement, early growth and survival of Haliotis tuberculata coccinea using several algal cues. J Shellfish Res 31:1189–1198
Daume S, Brand-Gardner S, Woelkerling WJ (1999) Settlement of abalone larvae (Haliotis laevigata Donovan) in response to non-geniculate coralline red algae (Corallinales, Rhodophyta). J Exp Mar Biol Ecol 234:125–143
Degnan BM, Johnson CR (1999) Inhibition of settlement and metamorphosis of the ascidian Herdmania curvata by non-geniculate coralline algae. Biol Bull 197:332–340
Della Santina P, Chelazzi G (1991) Temporal organization of foraging in two Mediterranean limpets, Patella rustica L. and P. coerulea L. J Exp Mar Biol Ecol 153:75–85
Della Santina P, Sonni C, Sartoni G, Chelazzi G (1993) Food availability and diet composition of three coexisting Mediterranean limpets (Patella spp.). Mar Biol 116:87–95
Dethier MN (1994) The ecology of intertidal algal crusts: variation within a functional group. J Exp Mar Biol Ecol 177:37–71
Diogo H, Pereira JG, Schmiing M (2016) Catch me if you can: non-compliance of limpet protection in the Azores. Mar Policy 63:92–99
Dodd JM (1957) Artificial fertilisation, larval development and metamorphosis in Patella vulgata L. and Patella caerulea L. Pubblicazioni Della Stazione Zoologica Di Napoli 29:172–185
Espinel-Velasco N, Hoffmann L, Agüera A, Byrne M, Dupont S, Uthicke S, Webster NS, Lamare M (2018) Effects of ocean acidification on the settlement and metamorphosis of marine invertebrate and fish larvae: a review. Mar Ecol Prog Ser 606:237–257
Espinosa F, Rivera-Ingraham GA (2017) Chapter Three - Biological conservation of giant limpets: the implications of large size. In: Curry BE (Ed) Advances in Marine Biology, Book 76. Academic Press, United Kingdom & United States
Faria J, Martins GM, Pita A, Ribeiro PA, Hawkins SJ, Presa P, Neto AI (2017) Disentangling the Genetic and Morphological Structure of Patella Candei Complex in Macaronesia (NE Atlantic) 7:6125–6140
Ferranti MP, Monteggia D, Asnaghi V, Chiantore M (2018) Artificial reproduction protocol, from spawning to metamorphosis, through noninvasive methods in Patella caerulea Linnaeus, 1758. Aquac Res 49:3386–3391
Ferranti MP, Guallart J, Fanciulli G, Panzalis PA, Chiantore M (2022) Advancements towards restoration of the endangered limpet Patella ferruginea Gmelin, 1791 through controlled reproduction. Aquac Res 53:782–798
Ferraz RR, Menezes GM, Santos RS (2001) Limpet (Patella spp.) (Mollusca: Gastropoda) exploitation in the Azores, during the period 1993–1998. Arquipélago Life Marine Sci 59–65
Firth LB (2021) What have limpets ever done for us?: On the past and present provisioning and cultural services of limpets. Int Rev Environ History 7:5–45
Fletcher W (1988) Intraspecific interactions between adults and juveniles of the subtidal limpet, Patelloida mufria. Oecologia 75:272–277
Gomez-Lemos LA, Diaz-Pulido G (2017) Crustose coralline algae and associated microbial biofilms deter seaweed settlement on coral reefs. Coral Reefs 36:453–462
Guallart J, Peña J, Pérez-Larruscaín J, Luque Á, Templado J (2020) Filling gaps: closing the life cycle of the endangered Mediterranean limpet Patella ferruginea Gmelin, 1791 (Gastropoda, Patellidae). Mediterr Mar Sci 21:400–419
Hadfield MG (2011) Biofilms and marine invertebrate larvae: what bacteria produce that larvae use to choose settlement sites. Ann Rev Mar Sci 3:453–470
Hadfield MG, Paul VJ (2001) Natural chemical cues for settlement and metamorphosis of marine invertebrate larvae. Marine Chem Ecol 13:431–461
Hawkins SJ, Corte-Real HBSM, Pannacciulli FG, Weber LC, Bishop JDD (2000) Thoughts on the ecology and evolution of the intertidal biota of the Azores and other Atlantic islands. Hydrobiologia 440:3–17
Henriques P, Sousa R, Pinto A, Delgado J, Faria G, Alves A, Khadem M (2012) Life history traits of the exploited limpet Patella candei (Mollusca: Patellogastropoda) of the north-eastern Atlantic. J Mar Biol Assoc UK 92:1379–1387
Jenkins SR, Marshall D, Fraschetti S (2009) Settlement and Recruitment. In: Wahl M (Ed) Marine Hard Bottom Communities: Patterns, Dynamics, Diversity, and Change. Springer, Berlin
Jorissen H, Galand PE, Bonnard I, Meiling S, Raviglione D, Meistertzheim A-L, Hédouin L, Banaigs B, Payri CE, Nugues MM (2021) Coral larval settlement preferences linked to crustose coralline algae with distinct chemical and microbial signatures. Sci Rep 11:14610
Kay M (2002) Recruitment in the intertidal limpet Lottia digitalis (Patellogastropoda: Lottiidae) may be driven by settlement cues associated with adult habitat. Mar Biol 141:467–477
Keesing JK, Halford AR, Hall KC, Cartwright CM (1997) Large-scale laboratory culture of the crown-of-thorns starfish Acanthaster planci (L.) (Echinodermata: Asteroidea). Aquaculture 157:215–226
Kenkel C, Traylor M, Wiedenmann J, Salih A, Matz MV (2011) Fluorescence of coral larvae predicts their settlement response to crustose coralline algae and reflects stress. Proc Royal Soc B Biol Sci 278:2691–2697
Kim M-J, Choi J-S, Kang S-E, Cho J-Y, Jin H-J, Chun B-S, Hong Y-K (2004) Multiple allelopathic activity of the crustose coralline alga Lithophyllum yessoense against settlement and germination of seaweed spores. J Appl Phycol 16:175–179
Kociolek JP, Blanco S, Coste M, Ector L, Liu Y, Karthick B, Kulikovskiy M, Lundholm N, Ludwig T, Potapova M, Rimet F, Sabbe K, Sala S, Sar E, Taylor J, Van de Vijver B, Wetzel CE, Williams DM, Witkowski A, Witkowski J (2022) DiatomBase. Navicula incerta Grunow, 1880. Accessed 2022–05–18
Lambert DM, Harris LG (2000) Larval settlement of the green sea urchin, Strongylocentrotus droebachiensis, in the southern Gulf of Maine. Invertebr Biol 119:403–409
Lindberg D (2008) Patellogastropoda, Neritimorpha, and Cocculinoidea. In: Ponder DR, Lindberg DR (Eds) Phylogeny and evolution of the Mollusca
Littler MM, Littler DS (2013) The nature of crustose coralline algae and their interactions on reefs. Research and Discoveries: The Revolution of Science through Scuba
Lorenzen S (2007) The limpet Patella vulgata L. at night in air: effective feeding on Ascophyllum nodosum monocultures and stranded seaweeds. J Molluscan Stud 73:267–274
Lugilde Yáñez J, Bárbara I, Peña V (2022) Algas coralinas (Corallinophycidae, Rhodophyta) de Galicia y norte de Portugal. Universidade da Coruña, Servizo de Publicacións
Maneveldt GW, Wilby D, Potgieter M, Hendricks MG (2006) The role of encrusting coralline algae in the diets of selected intertidal herbivores. J Appl Phycol 18:619–627
Martins GM, Faria J, Furtado M, Neto AI (2014) Shells of Patella aspera as’ islands’ for epibionts. J Marine Biol Assoc UK 94:1027
Mau A, Jha R (2018) Aquaculture of two commercially important molluscs (abalone and limpet): existing knowledge and future prospects. Rev Aquac 10:611–625
McCoy SJ, Kamenos NA (2015) Coralline algae (Rhodophyta) in a changing world: integrating ecological, physiological, and geochemical responses to global change. J Phycol 51:6–24
McCoy S, Pfister C (2014) Historical comparisons reveal altered competitive interactions in a guild of crustose coralline algae. Ecol Lett 17:475–483
McGrath D (1992) Recruitment and growth of the blue-rayed limpet, Helcion pellucidum (L.), in south east Ireland. J Molluscan Stud 58:425–431
McGrath D, Foley H (2005) Settlement and recruitment of the blue-rayed limpet, Patella pellucida L. in Galway Bay, west coast of Ireland. In: Wilson JG (Ed) The Intertidal Ecosystem: The Value of Ireland’s Shores. Dublin: Royal Irish Academy, Dublin
Molino PJ, Wetherbee R (2008) The biology of biofouling diatoms and their role in the development of microbial slimes. Biofouling 24:365–379
Morse ANC, Morse DE (1984) Recruitment and metamorphosis of Haliotis larvae induced by molecules uniquely available at the surfaces of crustose red algae. J Exp Mar Biol Ecol 75:191–215
Moss GA, Tong LJ (1992) Effect of stage of larval development on the settlement of the abalone, Haliotis iris. NZ J Mar Freshwat Res 26:69–73
Nunes C, Ramirez A, Rodeia J, De Girolamo M, Isidro E (2021) Invasive vs. non-invasive methods of reproduction in Patella candei (D’Orbigny, 1840): from fertilization to settlement. Aquaculture Europe 2021 – Oceans of Opportunity. European Aquaculture Society, Funchal, Madeira, Portugal
Orton JH, Southward AJ, Dodd JM (1956) Studies on the biology of limpets: II. The breeding of Patella vulgata L. in Britain. J Mar Biol Assoc UK 35:149–176
Padilla DK (1984) The importance of form: Differences in competitive ability, resistance to consumers and environmental stress in an assemblage of coralline algae. J Exp Mar Biol Ecol 79:105–127
Patil JS, Anil AC (2005) Biofilm diatom community structure: Influence of temporal and substratum variability. Biofouling 21:189–206
Pereira F, Piló D, Carvalho AN, Rufino M, Moura P, Vasconcelos P, Gaspar MB (2022) Epibiont assemblages on limpet shells: Biodiversity drivers in intertidal rocky shores. Mar Environ Res 174:105556
Pestana EMdS, Nunes JMdC, Cassano V, Lyra GdM (2021) Taxonomic revision of the Peyssonneliales (Rhodophyta): Circumscribing the authentic Peyssonnelia clade and proposing four new genera and seven new species. J Phycol 57:1749–1767
Pueschel CM, Miller TJ (1996) Reconsidering prey specializations in an algal-limpet mutualism: epithallial cell development in Clathromorphum circumscriptum (Rhodophyta, Corallinales) 1. J Phycol 32:28–36
R Development Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Ribeiro PMdA (2008) Dispersal and connectivity of northeastern Atlantic patellid limpets: a multidisciplinary approach. Thesis, University of Southampton, Southampton (United Kindgom)
Riera R, Pérez Ó, Álvarez O, Simón D, Díaz D, Monterroso Ó, Núñez J (2016) Clear regression of harvested intertidal mollusks. A 20-year (1994–2014) comparative study. Mar Environ Res 113:56–61
Roberts RD, Nicholson CM (1997) Variable response from abalone larvae (Haliotis iris, H. virginea) to a range of settlement cues. Molluscan Res 18:131–141
Roberts RD, Kaspar HF, Barker RJ (2004) Settlement of abalone (Haliotis iris) larvae in response to five species of coralline algae. J Shellfish Res 23:975–988
Roberts RD, Barker MF, Mladenov P (2010) Is settlement of Haliotis iris larvae on coralline algae triggered by the alga or its surface biofilm? J Shellfish Res 29:671–678
Rodríguez SR, Ojeda FP, Inestrosa NC (1993) Settlement of benthic marine invertebrates. Mar Ecol Prog Ser 97:193–207
Salta M, Wharton JA, Blache Y, Stokes KR, Briand J-F (2013) Marine biofilms on artificial surfaces: structure and dynamics. Environ Microbiol 15:2879–2893
Sá-Pinto A, Branco M, Sayanda D, Alexandrino P (2008) Patterns of colonization, evolution and gene flow in species of the genus Patella in the Macaronesian Islands. Mol Ecol 17:519–532
Seabra MI, Cruz T, Fernandes JN, Silva T, Hawkins S (2019) Recruitment of the limpet Patella ulyssiponensis and its relationship with crustose coralline algae: patterns of juvenile distribution and larval settlement. J Mar Biol Assoc UK 99:1787–1796
Seabra MI, Hawkins SJ, Espírito-Santo C, Castro JJ, Cruz T (2020) Rock-pools as nurseries for co-existing limpets: Spatial and temporal patterns of limpet recruitment. Region Stud Marine Sci 37:101339
Sousa R, Delgado J, Pinto AR, Henriques P (2017) Growth and reproduction of the north-eastern Atlantic keystone species Patella aspera (Mollusca: Patellogastropoda). Helgol Mar Res 71:8
Sousa R, Vasconcelos J, Henriques P, Pinto AR, Delgado J, Riera R (2019a) Long-term population status of two harvested intertidal grazers (Patella aspera and Patella candei), before (1996–2006) and after (2007–2017) the implementation of management measures. J Sea Res 144:33–38
Sousa R, Vasconcelos J, Riera R, Pinto AR, Delgado J, Henriques P (2019b) Potential impact of harvesting management measures on the reproductive parameters of the limpets Patella aspera and Patella candei from Madeira Island. Estuar Coast Shelf Sci 226:106264
Sousa R, Henriques P, Vasconcelos J, Pinto AR, Delgado J, Riera R (2020) The protection effects of marine protected areas on exploited molluscs from an oceanic archipelago. Aquat Conserv Mar Freshwat Ecosyst 30:717–729
Spotorno-Oliveira P, Figueiredo MA, Tâmega FT (2015) Coralline algae enhance the settlement of the vermetid gastropod Dendropoma irregulare (d’Orbigny, 1842) in the southwestern Atlantic. J Exp Mar Biol Ecol 471:137–145
Steneck RS (1986) The ecology of coralline algal crusts: convergent patterns and adaptative strategies. Annu Rev Ecol Syst 17:273–303
Steneck RS, Hacker SD, Dethier MN (1991) Mechanisms of competitive dominance between crustose coralline algae: an herbivore-mediated competitive reversal. Ecology 72:938–950
Stewart P, Soonklang N, Stewart MJ, Wanichanon C, Hanna PJ, Poomtong T, Sobhon P (2008) Larval settlement of the tropical abalone, Haliotis asinina Linnaeus, using natural and artificial chemical inducers. Aquac Res 39:1181–1189
Suenaga K, Hori H, Ishida H, Nukaya H, Roberts RD, Tsuji K (2004) Inducing substance for abalone larval metamorphosis from the crustose coralline alga Hydrolithon samoense. Fish Sci 70:342–344
Tebben J, Motti C, Siboni N, Tapiolas D, Negri A, Schupp P, Kitamura M, Hatta M, Steinberg PD, Harder T (2015) Chemical mediation of coral larval settlement by crustose coralline algae. Sci Rep 5:1–11
Terradas-Fernández M, Zubcoff J, Ramos-Esplá AA (2019) Early succession patterns in a Mediterranean vermetid reef. J Sea Res 152:101768
Titselaar FFLM (2019) Notes on the nomenclature of the Macaronesian Patella candei d’Orbigny complex, with special reference to Patella ordinaria Mabille and Patella crenata Gmelin (Patellogastropoda, Patellidae). Basteria 83:158–165
Weber LI, Hawkins SJ (2002) Evolution of the limpet Patella candei d’Orbigny (Mollusca, Patellidae) in Atlantic archipelagos: human intervention and natural processes. Biol J Lin Soc 77:341–353
Williams EA, Craigie A, Yeates A, Degnan SM (2008) Articulated coralline algae of the genus Amphiroa are highly effective natural inducers of settlement in the tropical abalone Haliotis asinina. Biol Bull 215:98–107
Williams EA, Cummins S, Degnan SM (2009) Settlement specifics: Effective induction of abalone settlement and metamorphosis corresponds to biomolecular composition of natural cues. Commun Integ Biol 2:347–349
Young RM, Schoenrock KM, von Salm JL, Amsler CD, Baker BJ (2015) Structure and function of macroalgal natural products. In: Stengel DB, Connan S (Eds) Natural Products From Marine Algae: Methods and Protocols. Springer, New York
Zelli E, Quéré G, Lago N, Di Franco G, Costantini F, Rossi S, Bramanti L (2020) Settlement dynamics and recruitment responses of Mediterranean gorgonians larvae to different crustose coralline algae species. J Exp Mar Biol Ecol 530–531:151427
Acknowledgements
The authors thank Gercende Courtois de Vicose for the donation of the benthic diatom Navicula salinicola strain. The authors thank Emilio Soler and the personnel of the “Observatorio Canario de Algas Nocivas” (OCHABS; Gran Canaria, Spain) for assisting in the identification of the main coralline crusts and the “Regional Agency for the Development of Research, Technology and Innovation” (ARDITI; Madeira, Portugal) for their financial support of this activity. The authors also would like to thank the technicians at Centro de Maricultura da Calheta for their assistance and local fishermen of Calheta for providing the specimens. The authors have no conflict of interest to declare.
Funding
Open access funding provided by FCT|FCCN (b-on). Animal management and experiments financed by the project “AQUAINVERT—Development of sustainable, integrated and innovative aquaculture in Macaronesia. Research and Development to promote the production of marine invertebrates of commercial interest (MAC/1. 1ª/282)” founded by FEDER under the INTERREG MAC 2014-2020. Coralline algae identification supported by ARDITI—Regional Agency for the Development of Research, Technology and Innovation (Madeira, Portugal). D.C. was supported by a grant under the project AQUAINVERT.
Author information
Authors and Affiliations
Contributions
CD: animal management, experimental design, experiment realization, monitoring and sampling, statistical analyses, pictures and graph design, drafted paper. GL: animal management, experiment realization, monitoring and sampling, drafted paper review. Andrade, CAP: drafted paper review, project elaboration, coordination and direction.
Corresponding author
Ethics declarations
Conflicts of interest
The authors declare not conflicts of interest.
Ethics approval
The sampling of limpets for scientific purposes during closed season was authorized by the Fisheries Directorate, Secretary for the Sea and Fisheries, Regional Government of Madeira (Portugal). The authors confirm that the ethical policies of the journal, as noted on the journal’s author guidelines page, have been adhered to. All applicable international, national, and/or institutional guidelines for the care and use of animals were followed.
Additional information
Responsible Editor: C. Pansch-Hattich.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Castejón, D., García, L. & Andrade, C.A.P. Crustose coralline algal factors determining the success of limpet (Patellogastropoda: Patellidae) settlement: species, exposure time, area and soluble cues. Mar Biol 170, 171 (2023). https://doi.org/10.1007/s00227-023-04321-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04321-1