Abstract
The habitat-forming Endangered ‘cauliflower’ soft coral Dendronephthya australis, endemic to South-east Australia, is in rapid decline. To aid future conservation strategies, it is vital to understand the fundamental biological processes of this species, particularly reproduction. This study describes the first records of sexual reproduction and asexual clonal replication, with observations both in aquaria and in the wild. We used a combination of observations including histological analyses of fresh specimens, and images of colonies in situ taken over 19 years, to investigate the reproductive cycle of D. australis. Mature oocytes and spermaries were found to develop within colonies during February/March, 2022. We subsequently closed the life cycle of D. australis from colonies spawned in aquaria, documenting all stages of embryogenesis and larval development through to polyp metamorphosis, and successfully transplanted juvenile colonies back into the field and documented their growth over six months to a maximum 435 polyps in size. We also document autonomous fragmentation events to provide accounts of asexual clonal propagation. These records confirm that D. australis is gonochoric and likely a broadcast spawning species that is also capable of utilising asexual reproduction by clonal replication. Observations of mature gametes support the hypothesis that spawning activity coincides with the seasonal increase in water temperature, and is likely to be a continuous phenomenon over 5 months of the year (November–March). These observations not only contribute to the knowledge base for this species, but also provide invaluable information on reproductive strategies that will support conservation efforts to assist the recovery of D. australis populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Gaining foundational knowledge of reproduction and early life stages is vital for understanding population dynamics of any species (Gaines and Roughgarden 1985). These processes are particularly important for the establishment and proliferation of sessile benthic marine invertebrates, as the larval phase is the only stage where dispersal occurs (Grantham et al. 2003; Cecino and Treml 2021). Larvae require appropriate environmental conditions and suitable substrate for dispersal and settlement (Müller and Leitz 2002; Treml et al. 2012). Behavioural data can indicate settlement preferences (Doropoulos et al. 2016), and competency time frames can indicate likely dispersion tendencies (Richmond 1987; Cowen and Sponaugle 2009; Figueiredo et al. 2013; Underwood et al. 2020). More generally, identifying modes of propagation allows for a greater understanding of threats to species and extinction risk (Mace et al. 2008).
Corals and sea anemones (Class Anthozoa) typically utilise both sexual and asexual reproductive strategies (Hughes et al. 1992; Bocharova and Kozevich 2011). Sexually, the majority of hard coral species (Order: Scleractinia) are hermaphroditic and are broadcast spawners (Harrison and Wallace 1990; Kerr et al. 2011). By contrast, Octocorallia (which includes the soft coral Order Malacalcyonacea (McFadden et al. 2022)) are primarily gonochoric, with rare instances of hermaphroditism (Kahng et al. 2011). Similar numbers of species in this family utilise brooding (via internal and external fertilisation) and broadcasting spawning strategies (Kahng et al. 2011). However, on rare occasions, corals within the same genus, such as Dendronephthya, can employ differing reproductive strategies; with two species (D. castanea and D. gigantea) found to be internal brooders (Hwang and Song 2007, 2012), and two others (D. hemprichi and D. suensoni) broadcast spawners (Dahan and Benayahu 1997a; Choi and Song 2007). Consequently, modes of sexual propagation are challenging to predict without direct observations of individual species, with increased research needed, particularly for threatened species (Pukazhenthi et al. 2005).
Within asexual reproductive strategies, clones of soft corals are known to disperse opportunistically, often taking advantage of available space (Fabricius 2011). These asexual processes take a number of forms, and include: autonomous fragmentation which entails the separation of a group of polyps as a daughter colony (Dahan and Benayahu 1997b; Kramarsky-Winter et al. 1997); polyp expulsion or budding, which describes the detachment of single polyps from the mother colony (Kramarsky-Winter et al. 1997); fission, where colonies split in two; and translocation, where corals produce runners away from the parent to form a new daughter colony (Fabricius and Alderslade 2001; Fabricius 2011; Kahng et al. 2011). Individual species can employ a number of these strategies, and species-specific research is required to ascertain which modes of asexual reproduction are most commonly utilised. This information provides a greater understanding of how aggregations of soft coral colonies form and can increase knowledge about genetic dynamics.
Dendronephthya (Cnidaria: Malacalcyonacea) is a genus of soft coral found on both tropical and temperate reefs. Despite the fact that > 400 Dendronephthya species have been described worldwide (WoRMS Editorial Board 2022), we found published accounts of sexual reproductive processes for only four species within the genus: D. hemprichi (Dahan and Benayahu 1997b,a, 1998); D. castanea (Hwang and Song 2012); D. suensoni (Choi and Song 2007) and D. gigantea (Hwang and Song 2007). Only one of these studies provided details of asexual clonal propagation (for D. hemprichi) (Dahan and Benayahu 1997b). With such limited research to date, it is unclear whether reproductive similarities occur among members of the genus, or within the order Malacalcyonacea more generally.
Dendronephthya australis (family Nephtheidae) is an endemic soft coral with a distribution that is restricted to the temperate waters of south-eastern Australia (Davis et al. 2015a; Poulos et al. 2016). In recent years, the total area inhabited by this species has rapidly declined (Harasti 2016; Larkin et al. 2021b), and it is now listed as Endangered (NSW Fisheries Scientific Committee 2021) and a priority species within the Australian Commonwealth’s Threatened Species Action Plan (Department of Climate Change, Energy, the Environment and Water 2022). Dendronephthya australis colonies comprise a hydroskeleton supported by small calcite sclerites (Verseveldt and Alderslade 1982), with umbellate colonies growing up to ~ 1 m in height. Prior to the recent decline of this species (Larkin et al. 2021b), aggregations of D. australis provided habitat for a range of species, including juveniles of the commercially and recreationally fished Australasian Snapper (Chrysophrys auratus) (Poulos et al. 2013), the Endangered White’s seahorse (Hippocampus whitei) (Harasti et al. 2014), and a diverse array of invertebrates (Corry et al. 2018; Davis et al. 2018; Finlay-Jones et al. 2021). Dendronephthya australis was first described in 1905 with specimens from the Sydney region (Kükenthal 1905), and later redescribed from specimens from Port Stephens (Verseveldt and Alderslade 1982). The species has been documented within the Port Stephens estuary, NSW, for over 50 years (Verseveldt and Alderslade 1982; Poulos 2011; Harasti et al. 2014), but its rapid decline over the past decade (Larkin et al. 2021b) has provided a strong impetus for targeted research to provide critical information about its biology and ecology.
To date, there are no published accounts of reproductive processes for D. australis. However, improved understanding of reproductive processes is clearly an important step in supporting conservation efforts that aim to promote restoration of populations (Comizzoli et al. 2019). This study therefore examines both the sexual and asexual reproductive strategies of D. australis based on ex situ observations in aquaria, and in situ observations in the coral’s natural environment. The aim of this paper was ultimately to complete the life cycle of D. australis to facilitate recovery and restoration of this rare and Endangered species.
Materials and methods
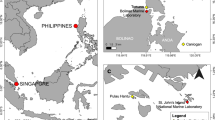
In situ field observations, and installation trials of juvenile corals, took place at two sites (Sandy Point and Little Beach) in the Port Stephens estuary, NSW, on shallow (8–10 m depth) subtidal reefs along the southern shoreline of the marine-dominated eastern basin (Fig. 1). These sites were the primary locations of remaining D. australis aggregations when this study took place in January–March, 2022. Ex situ sexual reproduction observations were conducted in aquaria at the Port Stephens Fisheries Institute, Taylors Beach, NSW, during investigations of restoration feasibility in January–March, 2022. Observations of asexual reproduction took place at the National Marine Science Centre, Coffs Harbour, NSW, during experiments on the survival of transplant fragments in August, 2020 (Larkin et al. 2023). Aquarium-based work utilised small branches of D. australis removed with sterile, sharp scissors, from wild adult colonies in the Port Stephens estuary. Images were taken using Olympus TG4 and TG6 cameras both in situ and ex situ throughout this study. All polyp counts were conducted with the DotDotGoose v1.5.3 counting software (Ersts 2022).
Sexual reproduction
As documented within histological work by Choi and Song (2007), maturing oocytes and spermaries in another species of Dendronephthya (D. suensoni) were found to increase in size, and migrate to the gastrovascular cavity and detach from the mesenteries. For this study, it was therefore anticipated that only mature oocytes and spermaries in the gastrovascular cavity would be visible with the naked eye, thus indicating that spawning was imminent. As our initial observations of oocytes and spermaries were the first for this species, they triggered the close monitoring of fragments for gamete release in aquaria, as well as retrospective analyses of images containing D. australis colonies.
Aquarium-based observations of sexual reproduction
Observations in aquaria utilised 33 individual branches with a mean crown width of 6.7 ± 1.2 cm from six donor colonies at Sandy Point in Port Stephens (Fig. 1). Branches from two of the colonies were collected on the 23rd of January 2022, and a collection from a further four colonies took place on the 20th of February 2022. Branches were placed in separate 700 ml containers to enable observation of individuals, and to ensure consistent feeding and water flows (Larkin et al. 2023). Branches were maintained in 600 L circulating filtered seawater, and fed daily with Reef Revolution Polyp Feast Coral Food (powdered zooplankton), live rotifers, and live microalgae Tisochrysis lutea, with complete water exchanges every 48 h.
Aquarium-based observations were conducted daily between 08:00 and 18:00 over a 10-week period. Photographs of all branches were taken every two days, to enable detailed, post hoc observations where required. Details of gamete movements within branches, release events, and subsequent larval progression were documented daily. If oocytes had been visible in a fragment at 18:00 one day, but were no longer were visible at 08:00 the next, an overnight release event was recorded. Gametes and planulae were observed with an AmScope T720Q compound microscope, and photographed with an AmScope MU1000-HS microscope digital camera. Naturally released oocytes (N = 13) were measured using a graticule, under 10 × magnification. Swimming speeds of mobile, six-day-old larvae (~ 1 mm length, N = 4) were observed under an AmScope T20Q compound microscope and a Nikon SMZ800N dissecting microscope, on a 1 mm grid Sedgewick Rafter slide for scale.
Histological processing
Samples ~ 1.5 cm in diameter were taken from ten coral fragments (both January 2022 and February 2022 collections), preserved in unbuffered 4% formalin and seawater for 48 h, and stored in 70% ethanol until tissue processing for histology. Tissues were washed in EDTA disodium salt (pH 7.4) for removal of calcium carbonate spicules from the tissues, following which samples were prepared for paraffin embedding, tissue sectioning and staining at the Katharina Gaus Light Microscopy Faculty at the Mark Wainwright Analytical Centre, University of New South Wales. Tissues were processed through successive washes of ethanol, xylene and paraffin for paraffin embedding, sectioned at 10 µm thickness and stained with hematoxylin and eosin. Stained tissue sections were permanently mounted on slides and scanned using an Olympus VS200 scanner. The images were viewed in the QuPath v0.3.2 software (Bankhead et al. 2017). Tissue sections can be viewed in Supplementary Material S1. The average diameters of 30 large oocytes and spermaries were measured using Image J (Rasband 1997–2018). Only large spermaries and oocytes as visible in the histology images were selected for these measurements, as they were considered to be the closest to central sectioning and therefore most representative of true mature gamete diameter; gametes that appeared smaller in the images were likely to have been sectioned through edges of the oocytes and spermaries.
Fertilisation and settlement
Following observations of the commencement of oocyte release in aquaria on the 25th of February 2022, ~ 1200 oocytes were distributed between ten separate 1 L containers. One small branchlet from a male branch (comprising 20 polyps) was macerated and sperm was filtered through a 180 μm mesh and diluted in 100 ml filtered seawater. Five to seven drops of the sperm mix were added to each container with the oocytes. Fertilisation success was monitored for the following 3 h, and determined by evidence of cell division. Subsequently, all embryos were combined in 4 × 20 L containers, with growth of planulae recorded daily thereafter. Water was exchanged daily for all containers containing larvae.
To provide opportunities for larval settlement, small pebbles (~ 1–3 cm width) coated with patchy coralline algae, and small fragments of conditioned ‘live rock’ (skeleton of scleractinian coral) were placed in containers with larvae. The pebbles with coralline algae were collected from the Port Stephens estuary and, to minimise the risk of bacterial impact on the larvae, were cleaned in fresh water for 4 h prior to being introduced to the aquaria. Live rock was conditioned in filtered seawater for one month. A subsample of 18 larvae was kept in a separate container, to determine the maximum time of competency without the presence of any substrate.
A microcosm experiment was concurrently conducted to determine if larval settlement could be induced by a chemical cue. Methods were adopted from a study by (Yang et al. 2022), that showed some promise with Pocillopora damicornis larvae. A concentration of 40 mM CaCl2 was added to 3 × 70 ml ‘treatment’ and threecontrol vessels each containing 5 × 14-day-old larvae in filtered seawater. Small quartz-based tiles conditioned in filtered seawater for 14 days were placed in the base of each container as a non-reactive substrate. Daily water exchanges took place for all replicates (and replacement of the equivalent 40 mM CaCl2 solution in the treatment vessels) for seven days.
Post-settlement larval survival and growth
Post-settlement larval survival and growth was investigated using a field trial. For this trial, fragments of live rock (N = 11) and coralline algae-covered pebbles (N = 29) were added to four shallow containers, with light aeration in each. An estimated 280 larvae were haphazardly distributed among the containers. The majority of D. australis larvae settled on these fragments within 21 days post-fertilisation. Subsequently, the rock fragments and pebbles bearing settled polyps were secured with a polymer adhesive (Gorilla Super Glue Gel) to small plastic bases to allow handling without contacting the rocks (Fig. 2A, B). These plastic bases were placed inside hydroponic net pot ‘pods’, with three to six pods fixed onto stainless steel poles at varied heights above the seafloor, ranging from 0 to 1.1 m height (Fig. 2C). Four poles were installed at each of the Sandy Point and Little Beach sites, with a total of 18 pods per site, at a depth of 9 m. The poles were installed within 10 m of adult colonies, to ensure that surrounding conditions were suitable for survival. Survival and growth rates were recorded every 4–6 weeks during SCUBA dives conducted over the six-month period post-installation, and photographs were taken of each juvenile colony. Growth rates were measured as the number of polyps in each colony.
On the left and centre, two Dendronephthya australis larval settlement substrates glued onto plastic bases: coralline algae-covered pebble (A); live rock with juvenile individuals attached (B). On the right, steel posts with pods containing juvenile Dendronephthya australis colonies in the field (C)
Field-based observations of gametes
Following preliminary observations of reproductive activity in aquaria, a total of three 40-min roaming dive surveys was conducted to document the presence of gametes visible with the naked eye at the Sandy Point and Little Beach sites (Fig. 1) during February and March, 2022. After a freshwater flood event, follow-up surveys were conducted at the same sites in April to determine if gametes were still visible. The number of colonies with visible oocytes and spermaries were recorded on each occasion. Average diameters (two perpendicular lengths) of gravid colonies were also recorded. As D. australis colonies expand and contract multiple times per day (Davis et al. 2015b), determining a basis for size measurements is a challenge. Polyp counts provide an effective method for estimating size of small Dendronephthya colonies (Fabricius et al. 1995). Therefore, the size of gravid D. australis colonies was based on polyp counts. Counts were conducted for small colonies (< 50 mm average diameter) and for portions of larger colonies (> 50 mm average diameter). The total polyp count for larger colonies was estimated by scaling the counts for the measured area by the total surface area of the colony calculated from the measured colony diameters. To do this, polyps were counted on a single cluster at the end of a branch on a D. australis colony, and multiplied by the number of clusters on the colony. The total surface area of a colony was assumed to be a half sphere. The total number of polyps per colony was divided by the surface area, to provide a number of polyps per cm2. This value was multiplied by the surface area of larger colonies, providing an estimate of total polyps (2πr2 × polyps per cm2).
Timing of spawning
Tidal data were examined to explore potential relationships among the aquarium-based spawning observations and the lunar cycle. Additionally, the historical presence of mature gametes was determined from images of D. australis colonies, from the period 2004–2022, obtained from images taken in all months. A summary of the number of colonies surveyed each year is provided in Table 1.
All images with clear, side-on views of D. australis colonies were closely examined for the presence of mature gametes (N = 142). When present, visible oocytes and spermaries were noted together with the date of the image. These data were analysed to determine whether reproduction events showed a seasonal periodicity. Temperature data from 2015 to 2022, collected using Onset Hobo U22-001 temperature loggers at 30-min intervals (www.onsetcomp.com), were averaged by day and examined to explore the potential relationships between water temperature and the historical presence of visible, mature gametes, visible to the naked eye.
Asexual reproduction
Aquarium-based observations of asexual reproduction
Observations of asexual reproduction were conducted in aquaria at Coffs Harbour using branches from four large donor colonies which were sectioned to provide 48 branches (with a mean crown width 6.3 ± 1.1 cm) in August–September, 2020. Branches were maintained in aquaria for a total of 4 weeks, using methods as per Larkin et al. (2023), with inspections conducted daily. Photographs were used to document instances of asexual reproduction over the study period.
Field-based observations of asexual reproduction
Instances of asexual reproduction occurring in the field were recorded during 28 surveys in Port Stephens in 2020–2021. Photographs were obtained during all field observations. The date of each observation was noted, as well as any other visible reproductive activity. Polyp counts were conducted using images of reproductive branches, for both aquarium-based and the field-based specimens. Based on the 250-degree visibility of the colonies in photographic images, polyp counts were scaled by a factor of 1.3 to account for polyps outside the field of view.
Results
Observations of sexual reproduction in aquaria
Histological H&E-stained sections demonstrated the presence of developed oocytes (with visible yolks) in the gastrovascular canals of female Dendronephthya australis samples (Fig. 3B, C), and spermaries (with dense sperm aggregations) in the gastrovascular canals of the male samples (Fig. 3E, F). This confirmed that D. australis is gonochoric and likely a broadcast spawner, as had been anticipated from the preliminary observations in aquaria and the field. The average diameter of large oocytes was 313.5 ± 3.0 (SE) μm, and of spermaries was 196.3 ± 2.5 (SE) μm. No fertilised oocytes were present in the samples analysed. Large oocytes and spermaries were free in the gastrovascular canals of the polyps, and detached from the mesenteries, demonstrating that gametes were consistently well developed between samples, and therefore maturing synchronously in both male and female colonies.
Female oocyte-bearing Dendronephthya australis branch (orange oocytes visible with the naked eye) (A) and H&E-stained histological image (B, C); and male sperm-bearing Dendronephthya australis branch (D), with yellow arrows highlighting locations of creamy-white coloured sperm sacs, visible with the naked eye; and H&E-stained histological image (E, F)
Dendronephthya australis oocytes were first observed in the gastrovascular canals of coral fragments in aquaria on the 3rd of February, 2022 (Fig. 3A), 11 days after the branches were collected from the field. Although oocytes were present and visible in retrospective analyses of fragment images by day two after collection, these went unnoticed as the colonies were originally collected for transplantation experiments (Larkin et al. 2023). Oocytes were orange in colour, and too dense for microscope lighting to penetrate. Subsequent inspections identified that all other branches that originated from the same colony also contained oocytes. Based on the observed density of oocytes, it was estimated that these branches, with ~ 1500 polyps, each contained ~ 5000 oocytes. Further inspection revealed that branches collected from a second colony were likely to be sperm bearing, with creamy-white coloured sperm sacs visible without the aid of magnification (Fig. 3D). The presence of sperm was confirmed under a microscope, and H&E-stained histology images (Fig. 3E, F). Samples from four additional colonies confirmed that either oocytes or spermaries were visible in separate individuals, thus confirming gonochorism.
Branches in aquaria released batches of oocytes during the observation period. Oocytes progressed up the gastrovascular cavities of each branch and appeared to cluster near the polyps over the ~ 7–14 days preceding release. During this period, there were no visible cues to help predict the exact timing of spawning prior to the event; oocytes appeared clustered for many days prior to release. During spawning, female fragments were expanded, with all polyps open. Some events saw the release of more numerous oocytes than others and ceased when no oocytes were visible in the branches. The average size of released oocytes was 371 ± 5.5 (SE) μm (Table 2). All female branches released oocytes on at least two occasions, with the timing of most release events occurring concurrently with other branches. Oocytes were neutrally buoyant in the water column (see video in Supplementary Material S2) and were distributed at varying depths after one hour of being released (after which sperm retrieved via maceration of male polyp bundles was added, and fertilisation commenced). No natural sperm release was observed during this time; however, it may have occurred unnoticed.
Mass oocyte release in aquaria occurred on multiple occasions during the observation period. These events occurred on the 10th of February, 25th of February, and between the 9th and 10th of March. Minor oocyte release events occurred on two occasions within the 6-day period prior to these mass releases. When plotted against the tidal cycle, all three mass oocyte release events coincided with, or were close to, neap tides (Fig. 4).
Field observations of gametes
Field-based surveys confirmed that the majority of wild colonies also contained mature gametes during February and early March 2022, coinciding with the observations of mature gametes, and spawning in aquaria. Of the 47 colonies inspected during field surveys, 34% were oocyte bearing, 30% were sperm bearing and the remaining 36% did not contain gametes large enough to be visible to the naked eye. The smallest colony with visible gamete production had an average diameter of 11.5 cm when retracted, with an estimated 12,300 polyps. The largest gravid colony had an average diameter of 37.5 cm when retracted, with an estimated 143,000 polyps. Follow-up surveys at the same sites in April 2022 found that all colonies that survived a freshwater flooding event in March 2022 had ceased visible gamete production.
Of the 142 historical images analysed, visible gametes were present in 16 of the 19 summers represented by the dataset time frame (2004–2022, Table 1). A total of 35 images with oocytes (25% of images analysed), and 28 (20%) with spermaries were observed (images were not included in this count if there was any uncertainty). The earliest record of visible oocytes or spermaries in any year was in October (in 2015), and the latest was in April (in 2010). Overall, the majority of mature gametes were evident in photos from November to March. Figure 5 presents an example of colonies with no visible gametes. For the 2015–2021 period, for which temperature data were available, oocytes and spermaries were only visible in images when water temperatures were above 19.1 °C (Fig. 6). The number of colonies surveyed within each month and the outcomes of both field-based and image-based observations are shown in Table 3.
Fertilisation and settlement in aquaria
The first observation of cell division occurred 1 h after oocytes (Fig. 7A) and sperm were combined, and there was a high (~ 80%) fertilisation success (hereafter t = 0 is referred to as the time when the gametes were mixed). Division to eight cells was observed in embryos after 2–3 h (Fig. 7B), and 32 cells after 4–5 h. Approximately half of the embryos were negatively buoyant immediately after fertilisation, the other half remained suspended in the water column. Mortality rates of ~ 15% occurred within the first 24 h, with minimal mortality thereafter. After 24 h, embryos took the form of slightly irregular spheres. Transition to dense, spherical planulae occurred during the first 40–50 h, after which cilia and motility were first observed. Ciliated, pear-shaped larvae were highly mobile after three days (Fig. 7C), reaching their maximum length (~ 1 mm) and vermiform morphology (Fig. 7D) by day five. Throughout their embryonic and larval stages, planulae were observed in the water column and on the base of the aerated containers. In the absence of aeration, most were negatively buoyant. Preliminary microscope-based measurements showed average six-day-old larval maximum swimming speeds were 0.85 ± 0.06 mm s−1.
Four developmental stages of Dendronephthya australis (A–E): A oocyte; B cell division (eight cells) within 3 h of sperm and oocytes combined; C ciliated planula (three days); D fully grown larva (although most reached full length after five days, this individual was eight days old when photographed); E two juvenile Dendronephthya australis attached to coralline algae-covered substrate (25 days old). Images A–D were taking using a dissecting microscope, E with a handheld camera
Larval settlement and metamorphosis to polyps (Fig. 7E) first occurred eight days post-fertilisation. When larvae were placed in containers with a choice between pebbles with coralline algae, or live rock, 60% settled on pebbles with coralline algae, and 40% settled on live rock; all settlement occurred within 18 days of fertilisation. Settlement occurred on all conditioned aspects of the calcareous substrates, with no apparent preference with respect to light exposure or orientation. The time to competency in the control treatment (with no settlement substrates present), was between 22 and 30 days (median 25 days, N = 18 larvae). By day 30, 30% of the larvae had metamorphosed (N = 18). No larval settlement occurred within the treatment and control replicates during the CaCl2 chemical cue experiment.
Survival and growth rates of juvenile polyps
In situ observations identified that 46% of the juvenile polyps installed at Sandy Point survived the first month; however, none survived at Little Beach. After 190 days (~ 6 months), 22% of the juvenile colonies were still alive at Sandy Point. Growth rates accelerated over time (Fig. 8), with an average colony size of 141 polyps after 190 days. There was considerable variation in the size of colonies, with the smallest comprising 16 polyps, and the largest 435 polyps. The slower-growing juveniles (with fewer polyps) tended to be on more densely populated pebbles.
Asexual reproduction
Autonomous fragmentation, or clonal propagation, was observed after 26 days in the aquarium, with one branch dropping a small polyp bundle (comprising 15 polyps). A second fragmentation event, comprising 32 polyps, was observed from a second branch two days later (Fig. 9A). No instances of clonal propagation were observed from the other 46 branches being monitored. Other physical signs of stress (such as sclerite shedding) were observed, confirming that clonal propagation strategies were likely the result of stress from branches being maintained in aquaria for a substantial period (> 25 days). Within seven days of release from the parent branches, both daughter polyp bundles had formed root-like-processes (RLPs) (Fig. 9B). This indicates that the fragmented polyp bundles were capable of attachment to a substrate and forming new colonies.
Dendronephthya australis branch and fragment (inset) (A); two D. australis fragments seven days after bailout showing root-like-processes (indicated the arrows) (B); in-field observation of a D. australis colony in poor condition releasing a polyp bundle (C); root-like processes were visible at the base of the fragment being released (circled) (D)
During field-based observations, 14 instances of autonomous fragmentation were recorded, seven during the cooler months (May–September, 2019–2021), and seven during warmer months (November–March, 2019–2021). The clonal propagation process was particularly noticeable when D. australis colonies were retracted with all polyps closed in the parent colony, but open in the bundle preparing to separate from its parent (Fig. 9C). Some fragments detached without RLPs, and some appeared to grow RLPs prior to the release of the bundle (Fig. 9D). The smallest, fragmented bundle comprised 64 polyps, while the largest had 1500 polyps. Fragmentation was recorded from both male and female colonies when spermaries and oocytes were visible during the summer months (two of each).
Discussion
This study confirms that the Endangered soft coral Dendronephthya australis is gonochoric and utilises broadcast spawning as its sexual reproductive strategy. Histological images show that oocytes and sperm were synchronously developing in the stems of separate colonies. Mature oocytes were released in aquaria and were subsequently fertilised with laboratory-extracted sperm. As gametes were at the final developmental stages within colonies of both sexes during these observations in January–March 2022, and historical images demonstrated that gametes were large enough to be visible over historical summers, it is likely that the spawning events witnessed in this study occurred at a time they are likely to naturally spawn (not as a result of stress, as is possible with some corals (Fadlallah 1983)). Our observations suggest that D. australis is a broadcast spawner, as no embryos were present within sectioned female branches sampled from the field. There was no evidence to support the suggestion that this species utilises brooding, as previously proposed (Williamson et al. 2022). These observations align with the gonochoric broadcast strategies employed by two other Dendronephthya species, D. hemprichi (Dahan and Benayahu 1997a), and D. suensoni (Choi and Song 2007). However, this mode is not consistent across all Dendronephthya species, as D. castanea and D. gigantea have been shown to be gonochoric brooders (Hwang and Song 2007, 2012). Previous studies have identified that timing and frequency of gametogenesis between species are varied (Dahan and Benayahu 1997a; Choi and Song 2007; Hwang and Song 2007, 2012), and only two studies have previously detailed embryonic developmental stages (Dahan and Benayahu 1998; Hwang and Song 2007).
The evidence presented here suggests that D. australis colonies likely spawn between October and April, with most instances of sexual reproduction occurring during the Austral summer months, when the water temperature in Port Stephens is generally > 19 °C. Although timing of spawning within the Order Malacalcyonacea is highly variable, it is common for species to spawn during the warmer months of the year (Beiring and Lasker 2000; Fan et al. 2005; Hwang and Song 2007). Field-based observations and historical images confirmed the presence of mature gametes during multiple summers in most of the years of observation. Three of the four previous studies of reproduction in Dendronephthya spp. also demonstrated that most spawning occurs during the summer months (Choi and Song 2007; Hwang and Song 2007, 2012), with D. hemprichi the only species demonstrating year-round gamete release (Dahan and Benayahu 1997a).
The ~ 1:1 sex ratio in the Port Stephens population provides preliminary data for future population fecundity work. As the sample size was relatively small, these data are not necessarily representative of broader population parameters. Approximately one-third of the colonies observed did not contain gametes visible to the naked eye. These could potentially have been male colonies with less visible spermaries (Bloomberg and Holstein 2021), immature colonies, not yet capable of sexual reproduction (Fan et al. 2005; Waller and Tyler 2011), stressed colonies putting energy into survival instead of reproduction (Bloomberg and Holstein 2021), or recently spawned colonies with reduced gamete density in the tips of the gastrovascular cavities (Hwang and Song 2009).
Oocyte release in aquaria was observed at times that were close to, or coinciding with, the neap tide lunar phases in the Port Stephens estuary (Fig. 4). Ecologically, this strategy is advantageous for broadcast spawners in environments with strong tidal flow, as neap tides result in slower water flow, maximising the opportunity for oocyte and sperm interaction (Van Woesik 2010), and retention of larvae within the same habitat (Ellien et al. 2004). Neap tide spawning has previously been recorded as a strategy utilised by other cnidarian species (Monteiro et al. 2016; Wolstenholme et al. 2018). Only two studies have previously observed Dendronephthya gamete release: the brooding species D. gigantea spawned on or close to the full or new moon (Hwang and Song 2007); and D. hemprichi spawned on several successive nights, without apparent correlation with the lunar cycle (Dahan and Benayahu 1997a). The inconsistency between these strategies suggests that timing of gamete release is likely an adaptation to specific environments for Dendronephthya soft corals.
This study provides the first record of larval development, settlement, and early life colony growth rates for D. australis. The larvae grew to their full length within five days, and settled and metamorphosed to polyps from eight days of age. Settlement occurred on both coralline algae-covered pebbles, and the conditioned surfaces of the coral rubble. Larval settlement on coralline algae-covered substrate is typical for many coral species, including octocorals (Sebens 1983), and Scleractinia (Morse et al. 1988; Harrington et al. 2004). These interactions between coral larvae and coralline algae are complex, and not entirely understood (Ritson-Williams et al. 2009; Gómez-Lemos et al. 2018). It is generally accepted that settlement induction occurs due to chemical signals from specific algal species (Slattery et al. 1999; Tebben et al. 2015), or due to signals from associated biofilm bacterium (Sneed et al. 2014) (or a possible combination of these) (Suzuki and Hayashibara 2011; Gómez-Lemos et al. 2018). Chemical cues from alternative conditioned calcareous substrates such as coral skeletons or coral rubble have also been found to induce coral larval settlement (Dahan and Benayahu 1997a; Heyward and Negri 1999; Golbuu and Richmond 2007). Benthic topography (Doropoulos et al. 2016; Levenstein et al. 2022), conditions associated with habitat depth (Ritson-Williams et al. 2009; Shlesinger and Loya 2021), and algal or bacterial inhibitors (Kuffner et al. 2006) are other factors that may influence larval substrate selection. Although our observations of larval settlement in D. australis indicate that substrate preferences are likely similar to many coral species, more work is required to gain a true understanding of the settlement ecology of this soft coral. With an observed competency period of 8–18 days in the presence of suitable substrates, and a longevity of up to 30 days, D. australis larvae have the potential to disperse a moderate distance away from the point of fertilisation. Although modelling shows coral communities primarily self-recruit (Figueiredo et al. 2013), the Port Stephens estuary’s strong tidal currents may result in the relocation of larvae away from parent populations. Further observations to confirm the robustness of competency and longevity time frames, coupled with detailed tidal modelling, would facilitate calculations of the probable dispersal range for the larval stages of this species.
The single-polyp D. australis juveniles were found to grow rapidly over the first six months in their natural habitat, with the largest surviving colony growing to 435 polyps, and the average trajectory of growth for all not yet plateauing by the end of the monitoring period (Fig. 8). Field observations showed that colonies can be sexually mature when they are still relatively small, with the smallest gravid colony observed having an average diameter of 11 cm, with an estimated ~ 12,300 polyps. This is comparable to D. hemprichi, in which gonads were found in colonies ≥ 9 cm in length (Dahan and Benayahu 1997a). Due to the constrained population size during the observations within this study, further work is required to determine the size and potential age at which D. australis colonies start to become sexually mature.
We were able to confirm that D. australis also reproduces asexually through autonomous fragmentation. The rapid development of RLPs in these fragments allows for attachment to the substratum and the establishment of colonies in new locations, mirroring the process described for D. hemprichi by Dahan and Benayahu (1997b). The size of detached fragments was found to vary greatly, ranging from 15 to 1500 polyps. This indicates that a single large colony, such as the one estimated to have ~ 143,000 polyps (estimate for a large, gravid colony), has the potential to produce many fragmented offspring, through asexual reproduction. We found no seasonal variation in the occurrence of fragmentation, and consequently it is unlikely that water temperature was a primary trigger for these events, with fragmentation therefore providing a year-round means for D. australis to reproduce. As shown in Fig. 9, some colonies releasing fragments were in poor condition, suggesting that D. australis may employ asexual reproduction in an attempt to persist and relocate during times of stress (Sammarco 1982; Fordyce et al. 2017). Ecologically, asexual reproduction can assist species populations by, for example: spreading mortality risk (Smith and Hughes 1999); increasing population; promoting the ability to colonise space (Dahl et al. 2012); avoiding the cost of breeding; and, avoiding the process of deterioration with age (Hughes 1989). This process also allows strong genotypes to proliferate (Ayre 1983; Hughes 1989; Pirog et al. 2019). However, there are also disadvantages of clonal propagation which include: high mortality risk due to loss of tissue during the physical process of fragmentation; relocation of ramets to unsuitable habitat; and reduced fecundity (Smith and Hughes 1999).
Propagation of malacalcyonacean soft coral colonies via the exploitation of clonal, asexual reproductive processes is a common approach for transplantation programmes (Oren and Benayahu 1997; Perkol-Finkel and Benayahu 2009; Ng et al. 2015; Larkin et al. 2021a). While using sexual reproduction for restoration is becoming more common for scleractinian corals (Cameron and Harrison 2020; Dela Cruz and Harrison 2020; Miller et al. 2022), there are no known studies exploring the potential for this approach to aid the recovery of soft coral populations. Methodologies employed with scleractinian corals (Cameron and Harrison 2020) could be considered in future attempts to restore populations of D. australis. As a strategy to revolutionise restoration for this species, utilisation of sexual reproduction has the potential to generate a million larvae from the collection of just 20 female branches 1500 polyps in size, and a fewer number of male branches due to the large quantities of sperm found within each branch. With the high rates of larval survival demonstrated in this study, and the potential for high post-settlement survival in the field, this approach has strong potential for generating new colonies to aid future restoration efforts. Juvenile placement at multiple sites can reduce the risks associated with deployment in the field. Sand mobilisation during a severe weather event was the likely cause of the mortality at Little Beach; however, a high percentage of juveniles survived at Sandy Point, suggesting that the risks from various stressors are spatially variable and a multi-site restoration approach is likely to promote success. Understanding the critical risks facing colonies post-deployment, and their spatial and temporal patterns, is clearly an important avenue for further research to ensure that our findings can be successfully applied to broader restoration efforts.
Understanding that D. australis uses both sexual and asexual propagation strategies provides some important ecological perspective for populations in Port Stephens. The presence of mature gametes suggests that D. australis likely spawns during approximately five months of the year. Repetitive and synchronous spawning events during neap tides over this period mean that colonies have the potential to produce millions of progeny during a single summer. Multiple gamete release events are a common phenomenon within Octocorallia (Gutiérrez-Rodríguez and Lasker 2004; Gori et al. 2007; Kahng et al. 2008; Hellström et al. 2010) that can increase the chance of appropriate oceanic conditions for larval survival and settlement (Vermeij et al. 2006), and reduce the risk of polyspermy (Ritson-Williams et al. 2009). Conversely, smaller gamete release events such as these have also been associated with a reduction in fertilisation success in the wild when compared to single mass broadcast spawning events (Oliver and Babcock 1992). Furthermore, fertilisation is reliant on synchronous spawning of male and female colonies within proximity to one another (Levitan and Petersen 1995). The synchronicity of spawning times for broadcasters can also be susceptible to disruption by environmental changes such as rapid temperature fluctuations, and anthropogenic pollution (Shlesinger and Loya 2019). Due to the recent rapid decline of D. australis aggregations in their historically most abundant location, Port Stephens (Larkin et al. 2021b), the findings within this study are important, albeit concerning, for this Endangered species, as they indicate that any rapid recovery will likely be reliant on the persistence of populous aggregations, and suitable environmental conditions. Seasonal sexual reproduction, combined with year-round asexual clonal propagation, have the potential to create substantial aggregations comprising many thousands of D. australis colonies, an ecological status that was once found in the estuary (pers. obs. back to the mid-1980s).
Ultimately, working with an Endangered species inherently comes with limitations. Following recent declines in abundance, very few D. australis colonies remain in the wild, particularly in the Port Stephens estuary (Larkin et al. In Review). Consequently, conventional large-scale sampling using multiple colonies is not feasible and any form of destructive sampling should be minimised. By using image analyses and field observations, we eliminated the need for physical impact on this Endangered species through extensive sampling, the methodology used to determine the timing of gamete production for other Dendronephthya spp. (Dahan and Benayahu 1997a; Choi and Song 2007). To continue monitoring sexual reproduction in the wild, photographs and analyses by trained personnel could continue to be used to determine likely reproductive activity. However, sampling of whole colonies will be required to determine more detailed processes of gametogenesis and colony fecundity (as in Tsounis et al. 2006; Gori et al. 2007) if such data are deemed important.
To better understand the population dynamics of this species, future studies can build upon our findings that D. australis is a seasonal gonochoric broadcast spawner that also employs autonomous fragmentation to asexually reproduce. Knowledge of these reproductive processes provides insight into how historical populations prospered. Consequently, these discoveries should aid restoration efforts to create insurance populations and assist the recovery of this ecologically important habitat-forming coral: this work therefore represents an important step in efforts to prevent its further decline towards extinction.
Data and/or code availability
Data and documentary evidence are available from the corresponding author upon reasonable request.
References
Ayre D (1983) The effects of asexual reproduction and inter-genotypic aggression on the genotypic structure of populations of the sea anemone Actinia tenebrosa. Oecologia 57:158–165. https://doi.org/10.1007/BF00379575
Bankhead P, Loughrey MB, Fernández JA, Dombrowski Y, McArt DG, Dunne PD et al (2017) QuPath: open source software for digital pathology image analysis. Sci Rep 7:1–7. https://doi.org/10.1038/s41598-017-17204-5
Beiring EA, Lasker HR (2000) Egg production by colonies of a gorgonian coral. Mar Ecol Prog Ser 196:169–177. https://doi.org/10.3354/meps196169
Bloomberg J, Holstein DM (2021) Mesophotic coral refuges following multiple disturbances. Coral Reefs 40:821–834. https://doi.org/10.1007/s00338-021-02087-w
Bocharova ES, Kozevich IA (2011) Modes of reproduction in sea anemones (Cnidaria, Anthozoa). Biol Bull 38:849–860. https://doi.org/10.1134/S1062359011090020
Cameron KA, Harrison PL (2020) Density of coral larvae can influence settlement, post-settlement colony abundance and coral cover in larval restoration. Sci Rep 10:1–11. https://doi.org/10.1038/s41598-020-62366-4
Cecino G, Treml EA (2021) Local connections and the larval competency strongly influence marine metapopulation persistence. Ecol Appl 31:e02302. https://doi.org/10.1002/eap.2302
Choi EJ, Song JI (2007) Reproductive biology of the temperate soft coral Dendronephthya suensoni (Alcyonacea: Nephtheidae). Integrat Biosci 11:215–225. https://doi.org/10.1080/17386357.2007.9647338
Comizzoli P, Brown JL, Holt WV (2019) Reproductive science as an essential component of conservation biology: new edition. In: Comizzoli P, Brown JL, Holt WV (eds) Reproductive sciences in animal conservation. Springer International Publishing, Cham, pp 1–10
Corry M, Harasti D, Gaston T, Mazumder D, Cresswell T, Moltschaniwskyj N (2018) Functional role of the soft coral Dendronephthya australis in the benthic food web of temperate estuaries. Mar Ecol Prog Ser 593:61–72. https://doi.org/10.3354/meps12498
Cowen RK, Sponaugle S (2009) Larval dispersal and marine population connectivity. Ann Rev Mar Sci 1:443–466. https://doi.org/10.1146/annurev.marine.010908.163757
Dahan M, Benayahu Y (1997a) Reproduction of Dendronephthya hemprichi (Cnidaria: Octocorallia): year-round spawning in an azooxanthellate soft coral. Mar Biol 129:573–579. https://doi.org/10.1007/s002270050198
Dahan M, Benayahu Y (1997b) Clonal propagation by the azooxanthellate octocoral Dendronephthya hemprichi. Coral Reefs 16:5–12. https://doi.org/10.1007/s003380050053
Dahan M, Benayahu Y (1998) Embryogenesis, planulae longevity, and competence in the octocoral Dendronephthya hemprichi. Invertebr Biol 117:271–280
Dahl M, Pereyra R, Lundälv T, André C (2012) Fine-scale spatial genetic structure and clonal distribution of the cold-water coral Lophelia pertusa. Coral Reefs 31:1135–1148. https://doi.org/10.1007/s00338-012-0937-5
Davis T, Harasti D, Smith S (2015a) Developing a habitat classification typology for subtidal habitats in a temperate estuary in New South Wales, Australia. Mar Freshw Res 67:1186–1195. https://doi.org/10.1071/MF15123
Davis T, Harasti D, Smith SD (2015b) Extension of Dendronephthya australis soft corals in tidal current flows. Mar Biol 162:2155–2159. https://doi.org/10.1007/s00227-015-2732-7
Davis T, Harasti D, Smith S (2018) Responses of Dendronephthya australis to predation by Dermatobranchus sp. nudibranchs. Mar Freshw Res 69:186–190. https://doi.org/10.1071/MF17040
Dela Cruz DW, Harrison PL (2020) Enhancing coral recruitment through assisted mass settlement of cultured coral larvae. PLoS ONE 15:e0242847. https://doi.org/10.1371/journal.pone.0242847
Department of Climate Change, Energy, the Environment and Water (2022) Threatened species strategy action plan 2022–2032, Australian Government, Canberra, Australia. This publication is available at:https://www.dcceew.gov.au/sites/default/files/documents/threatened-species-action-plan-2022-2032.pdf. Accessed 06 Oct 2022
Doropoulos C, Roff G, Bozec YM, Zupan M, Werminghausen J, Mumby PJ (2016) Characterizing the ecological trade-offs throughout the early ontogeny of coral recruitment. Ecol Monogr 86:20–44. https://doi.org/10.1890/15-0668.1
Ellien C, Thiébaut E, Dumas F, Salomon J-C, Nival P (2004) A modelling study of the respective role of hydrodynamic processes and larval mortality on larval dispersal and. J Plankton Res 26:117–132. https://doi.org/10.1093/plankt/fbh018
Ersts PJ (2022) DotDotGoose (version 1.5.3). American Museum of Natural History, Center for Biodiversity and Conservation. https://biodiversityinformatics.amnh.org/open_source/dotdotgoose. Accessed 01 June 2022
Fabricius KE (2011) Octocorallia. In: Hopley D (ed) Encyclopedia of modern coral reefs: structure, form and process encyclopedia of earth sciences. Springer, Dordrecht, The Netherlands, pp 740–745
Fabricius KE, Alderslade PP (2001) Soft corals and sea fans: a comprehensive guide to the tropical shallow water genera of the central-west Pacific, the Indian Ocean and the Red Sea. Australian Institute of Marine Science (AIMS), Australia
Fabricius KE, Genin A, Benayahu Y (1995) Flow-dependent herbivory and growth in zooxanthellae-free soft corals. Limnol Oceanogr 40:1290–1301. https://doi.org/10.4319/lo.1995.40.7.1290
Fadlallah YH (1983) Sexual reproduction, development and larval biology in scleractinian corals. Coral Reefs 2:129–150. https://doi.org/10.1007/BF00336720
Fan T-Y, Chou Y-H, Dai C-F (2005) Sexual reproduction of the alcyonacean coral Lobophytum pauciflorum in southern Taiwan. Bull Mar Sci 76:143–154
Figueiredo J, Baird AH, Connolly SR (2013) Synthesizing larval competence dynamics and reef-scale retention reveals a high potential for self-recruitment in corals. Ecology 94:650–659. https://doi.org/10.1890/12-0767.1
Finlay-Jones H, Raoult V, Harasti D, Gaston T (2021) What eats a cauliflower coral? An assessment of predation on the Endangered temperate soft coral, Dendronepthya australis. Mar Freshw Res 73:307–318. https://doi.org/10.1071/MF21155
Fordyce AJ, Camp EF, Ainsworth TD (2017) Polyp bailout in Pocillopora damicornis following thermal stress. F1000Research 6:687. https://doi.org/10.12688/f1000research.11522.2
Gaines S, Roughgarden J (1985) Larval settlement rate: a leading determinant of structure in an ecological community of the marine intertidal zone. Proc Natl Acad Sci USA 82:3707–3711. https://doi.org/10.1073/pnas.82.11.370
Golbuu Y, Richmond RH (2007) Substratum preferences in planula larvae of two species of scleractinian corals, Goniastrea retiformis and Stylaraea punctata. Mar Biol 152:639–644. https://doi.org/10.1007/s00227-007-0717-x
Gómez-Lemos LA, Doropoulos C, Bayraktarov E, Diaz-Pulido G (2018) Coralline algal metabolites induce settlement and mediate the inductive effect of epiphytic microbes on coral larvae. Sci Rep 8:1–11. https://doi.org/10.1038/s41598-018-35206-9
Gori A, Linares C, Rossi S, Coma R, Gili J-M (2007) Spatial variability in reproductive cycle of the gorgonians Paramuricea clavata and Eunicella singularis (Anthozoa, Octocorallia) in the Western Mediterranean Sea. Mar Biol 151:1571–1584. https://doi.org/10.1007/s00227-006-0595-7
Grantham BA, Eckert GL, Shanks AL (2003) Dispersal potential of marine invertebrates in diverse habitats: ecological archives A013–001-A1. Ecol Appl 13:108–116. https://doi.org/10.1890/1051-0761(2003)013[0108:DPOMII]2.0.CO;2
Gutiérrez-Rodríguez C, Lasker HR (2004) Reproductive biology, development, and planula behavior in the Caribbean gorgonian Pseudopterogorgia elisabethae. Invertebr Biol 123:54–67. https://doi.org/10.1111/j.1744-7410.2004.tb00141.x
Harasti D (2016) Declining seahorse populations linked to loss of essential marine habitats. Mar Ecol Prog Ser 546:173–181. https://doi.org/10.3354/meps11619
Harasti D, Martin-Smith K, Gladstone W (2014) Ontogenetic and sex-based differences in habitat preferences and site fidelity of White’s seahorse Hippocampus whitei. J Fish Biol 85:1413–1428. https://doi.org/10.1111/jfb.12492
Harrington L, Fabricius K, De’ath G, Negri A (2004) Recognition and selection of settlement substrata determine post-settlement survival in corals. Ecology 85:3428–3437. https://doi.org/10.1890/04-0298
Harrison PL, Wallace C (1990) Reproduction, dispersal and recruitment of scleractinian corals. Ecosyst World 25:133–207
Hellström M, Kavanagh KD, Benzie JAH (2010) Multiple spawning events and sexual reproduction in the octocoral Sarcophyton elegans (Cnidaria: Alcyonacea) on Lizard Island, Great Barrier Reef. Mar Biol 157:383–392. https://doi.org/10.1007/s00227-009-1325-8
Heyward AJ, Negri AP (1999) Natural inducers for coral larval metamorphosis. Coral Reefs 18:273–279. https://doi.org/10.1007/s003380050193
Hughes RN (1989) Functional biology of clonal animals. Springer, Dordrecht, U.K.
Hughes TP, Ayre D, Connell JH (1992) The evolutionary ecology of corals. Trends Ecol Evol 7:292–295. https://doi.org/10.1016/0169-5347(92)90225-Z
Hwang S-J, Song J-I (2007) Reproductive biology and larval development of the temperate soft coral Dendronephthya gigantea (Alcyonacea: Nephtheidae). Mar Biol 152:273–284. https://doi.org/10.1007/s00227-007-0679-z
Hwang S-J, Song J-I (2009) Sexual reproduction of soft coral, Scleronephthya gracillimum, (Alcyonacea: Nephtheidae) based on long-term collection from Jejudo Island, Korea. Galaxea, J Coral Reef Stud 11:155–167. https://doi.org/10.3755/galaxea.11.155
Hwang S-J, Song J-I (2012) Sexual reproduction of the soft coral Dendronephthya castanea (Alcyonacea: Nephtheidae). Anim Cells Syst 16:135–144. https://doi.org/10.1080/19768354.2011.622486
Kahng SE, Benayahu Y, Wagner D, Rothe N (2008) Sexual reproduction in the invasive octocoral Carijoa riisei in Hawaii. Bull Mar Sci 82:1–17
Kahng SE, Benayahu Y, Lasker HR (2011) Sexual reproduction in octocorals. Mar Ecol Prog Ser 443:265–283. https://doi.org/10.3354/meps09414
Kerr AM, Baird AH, Hughes TP (2011) Correlated evolution of sex and reproductive mode in corals (Anthozoa: Scleractinia). Proc R Soc b: Biol Sci 278:75–81. https://doi.org/10.1098/rspb.2010.1196
Kramarsky-Winter E, Fine M, Loya Y (1997) Coral polyp expulsion. Nature 387:137–137
Kuffner IB, Walters LJ, Becerro MA, Paul VJ, Ritson-Williams R, Beach KS (2006) Inhibition of coral recruitment by macroalgae and cyanobacteria. Mar Ecol Prog Ser 323:107–117. https://doi.org/10.3354/meps323107
Kükenthal W (1905) Versuch einer Revision der Alcyonarien. II. Die Familie der Nephthyiden. 2. Teil. Die Gattungen Dendronephthya n.g. und Stereonephthya n.g. Zoologische Jahrbucher 21: 503–726, 631–632.
Larkin MF, Harasti D, Davis TR, Smith SDA (2021a) If you plant it, they will come: rapid recruitment of habitat-dependent marine invertebrates to transplanted fragments of an Endangered soft coral species. Diversity 13:79. https://doi.org/10.3390/d13020079
Larkin MF, Davis TR, Harasti D, Cadiou G, Poulos DE, Smith SD (2021b) The rapid decline of an Endangered temperate soft coral species. Estuar Coast Shelf Sci 255:107364. https://doi.org/10.1016/j.ecss.2021.107364
Larkin MF, Davis TR, Harasti D, Benkendorff K, Smith SDA (2023) Substantial advancement in aquaria rearing methods to assist recovery of an Endangered soft coral. Aquat Conserv: Mar Freshw 33:1–14. https://doi.org/10.1002/aqc.3895
Levenstein MA, Gysbers DJ, Marhaver KL, Kattom S, Tichy L, Quinlan Z et al (2022) Millimeter-scale topography facilitates coral larval settlement in wave-driven oscillatory flow. PLoS ONE 17:e0274088. https://doi.org/10.1371/journal.pone.0274088
Levitan DR, Petersen C (1995) Sperm limitation in the sea. Trends Ecol Evol 10:228–231. https://doi.org/10.1016/S0169-5347(00)89071-0
Mace GM, Collar NJ, Gaston KJ, Hilton-Taylor C, Akçakaya RH, Leader-Williams N et al (2008) Quantification of extinction risk: IUCN’s system for classifying threatened species. Conserv Biol 22:1424–1442. https://doi.org/10.1111/j.1523-1739.2008.01044.x
McFadden CS, van Ofwegen LP, Quattrini AM (2022) Revisionary systematics of Octocorallia (Cnidaria: Anthozoa) guided by phylogenomics. BSSB. https://doi.org/10.18061/bssb.v1i3.8735
Miller MW, Latijnhouwers KRW, Bickel A, Mendoza-Quiroz S, Schick M, Burton K et al (2022) Settlement yields in large-scale in situ culture of Caribbean coral larvae for restoration. Restor Ecol 30:e13512. https://doi.org/10.1111/rec.13512
Monteiro CA, Paulino C, Jacinto R, Serrão EA, Pearson GA (2016) Temporal windows of reproductive opportunity reinforce species barriers in a marine broadcast spawning assemblage. Sci Rep 6:29198. https://doi.org/10.1038/srep29198
Morse DE, Hooker N, Morse ANC, Jensen RA (1988) Control of larval metamorphosis and recruitment in sympatric agariciid corals. J Exp Mar Biol Ecol 116:193–217. https://doi.org/10.1016/0022-0981(88)90027-5
Müller WA, Leitz T (2002) Metamorphosis in the Cnidaria. Can J Zool 80:1755–1771. https://doi.org/10.1139/z02-130
Ng CSL, Lim SC, Ong JY, Teo LMS, Chou LM, Chua KE et al (2015) Enhancing the biodiversity of coastal defence structures: transplantation of nursery-reared reef biota onto intertidal seawalls. Ecol Eng 82:480–486. https://doi.org/10.1016/j.ecoleng.2015.05.016
NSW Fisheries Scientific Committee (2021). Fisheries Scientific Committee final determination for Cauliflower Soft Coral (Dendronephthya australis) Industries DoP. This publication is available at:https://www.dpi.nsw.gov.au/__data/assets/pdf_file/0007/1277782/Final-determination-D.australis-.pdf. Accessed 02 Apr 2022
Oliver J, Babcock R (1992) Aspects of the fertilization ecology of broadcast spawning corals: sperm dilution effects and in situ measurements of fertilization. Biol Bull 183:409–417
Oren U, Benayahu Y (1997) Transplantation of juvenile corals: a new approach for enhancing colonization of artificial reefs. Mar Biol. https://doi.org/10.1007/s002270050038
Perkol-Finkel S, Benayahu Y (2009) The role of differential survival patterns in shaping coral communities on neighboring artificial and natural reefs. J Exp Mar Biol Ecol 369:1–7. https://doi.org/10.1016/j.jembe.2008.09.016
Pirog A, Latreille AC, Madelaine C, Gélin P, Frouin P, Magalon H (2019) High clonal propagation and low population connectivity in the holothurian Stichopus chloronotus from the Indo-Pacific. Mar Biol 166:63. https://doi.org/10.1007/s00227-019-3512-6
Poulos DE, Harasti D, Gallen C, Booth DJ (2013) Biodiversity value of a geographically restricted soft coral species within a temperate estuary. Aquat Conserv: Mar Freshw 23:838–849. https://doi.org/10.1002/aqc.2362
Poulos DE, Gallen C, Davis TR, Booth DJ, Harasti D (2016) Distribution and spatial modelling of a soft coral habitat in the Port Stephens-Great Lakes Marine Park: implications for management. Mar Freshw Res 67:256–265. https://doi.org/10.1071/MF14059
Poulos DE (2011) Unique soft coral habitat in a temperate estuary: significance to biodiversity and marine park management. Honours Ph.D. thesis, University of Technology, Sydney
Pukazhenthi B, Comizzoli P, Travis AJ, Wildt DE (2005) Applications of emerging technologies to the study and conservation of threatened and endangered species. Reprod Fertil Dev 18:77–90. https://doi.org/10.1071/rd05117
Rasband WS (1997–2018) ImageJ. Bethesda, Maryland, USA. https://imagej.nih.gov/ij/. Accessed 15 June 2022
Richmond RH (1987) Energetics, competency, and long-distance dispersal of planula larvae of the coral Pocillopora damicornis. Mar Biol 93:527–533. https://doi.org/10.1007/BF00392790
Ritson-Williams R, Arnold SN, Fogarty ND, Steneck RS, Vermeij MJ, Paul VJ (2009) New perspectives on ecological mechanisms affecting coral recruitment on reefs. Smithson Contrib Mar Sci 38:437. https://doi.org/10.5479/si.01960768.38.437
Sammarco PW (1982) Polyp bail-out: an escape response to environmental stress and a new means of reproduction in corals. Mar Ecol Prog Ser 10:57–65
Sebens KP (1983) Settlement and metamorphosis of a temperate soft-coral larva (Alcyonium siderium Verrill): induction by crustose algae. Biol Bull 165:286–304. https://doi.org/10.2307/1541370
Shlesinger T, Loya Y (2019) Breakdown in spawning synchrony: a silent threat to coral persistence. Science 365:1002–1007. https://doi.org/10.1126/science.aax0110
Shlesinger T, Loya Y (2021) Depth-dependent parental effects create invisible barriers to coral dispersal. Commun Biol 4:202. https://doi.org/10.1038/s42003-021-01727-9
Slattery M, Hines G, Starmer J, Paul V (1999) Chemical signals in gametogenesis, spawning, and larval settlement and defense of the soft coral Sinularia polydactyla. Coral Reefs 18:75–84. https://doi.org/10.1007/s003380050158
Smith L, Hughes T (1999) An experimental assessment of survival, re-attachment and fecundity of coral fragments. J Exp Mar Biol Ecol 235:147–164. https://doi.org/10.1016/S0022-0981(98)00178-6
Sneed JM, Sharp KH, Ritchie KB, Paul VJ (2014) The chemical cue tetrabromopyrrole from a biofilm bacterium induces settlement of multiple Caribbean corals. Proc R Soc b: Biol Sci 281:20133086. https://doi.org/10.1098/rspb.2013.3086
Suzuki G, Hayashibara T (2011) Do epibenthic algae induce species-specific settlement of coral larvae? J Mar Biol Assoc 91:677–683. https://doi.org/10.1017/S0025315410000573
Tebben J, Motti C, Siboni N, Tapiolas D, Negri A, Schupp P et al (2015) Chemical mediation of coral larval settlement by crustose coralline algae. Sci Rep 5:10803. https://doi.org/10.1038/srep10803
Treml EA, Roberts JJ, Chao Y, Halpin PN, Possingham HP, Riginos C (2012) Reproductive output and duration of the pelagic larval stage determine seascape-wide connectivity of marine populations. Integr Comp Biol 52:525–537. https://doi.org/10.1093/icb/ics101
Tsounis G, Rossi S, Aranguren M, Gili J-M, Arntz W (2006) Effects of spatial variability and colony size on the reproductive output and gonadal development cycle of the Mediterranean red coral (Corallium rubrum L.). Mar Biol 148:513–527. https://doi.org/10.1007/s00227-005-0100-8
Underwood JN, Richards Z, Berry O, Oades D, Howard A, Gilmour JP (2020) Extreme seascape drives local recruitment and genetic divergence in brooding and spawning corals in remote north-west Australia. Evol Appl 13:2404–2421. https://doi.org/10.1111/eva.13033
Van Woesik R (2010) Calm before the spawn: global coral spawning patterns are explained by regional wind fields. Proc R Soc b: Biol Sci 277:715–722. https://doi.org/10.1098/rspb.2009.1524
Vermeij M, Fogarty ND, Miller M (2006) Pelagic conditions affect larval behavior, survival, and settlement patterns in the Caribbean coral Montastraea faveolata. Mar Ecol Prog Ser 310:119–128. https://doi.org/10.3354/meps310119
Verseveldt J, Alderslade P (1982) Descriptions of types and other Alcyonacean material (Coelenterata: Octocorallia) in the Australian Museum, Sydney. Rec Aust Mus 34:619–647
Waller RG, Tyler PA (2011) Reproductive patterns in two deep-water solitary corals from the north-east Atlantic—Flabellum alabastrum and F. angulare (Cnidaria: Anthozoa: Scleractinia). J Mar Biol Assoc 91:669–675. https://doi.org/10.1017/S0025315410000822
Williamson JE, Gillings MR, Nevatte RJ, Harasti D, Raoult V, Ghaly TM et al (2022) Genetic differentiation in the threatened soft coral Dendronephthya australis in temperate eastern Australia. Austral Ecol 47:804–817. https://doi.org/10.1111/aec.13160
Wolstenholme J, Nozawa Y, Byrne M, Burke W (2018) Timing of mass spawning in corals: potential influence of the coincidence of lunar factors and associated changes in atmospheric pressure from northern and southern hemisphere case studies. Invertebr Reprod Dev 62:98–108. https://doi.org/10.1080/07924259.2018.1434245
WoRMS Editorial Board. (2022). World Register of Marine Species. Available at:https://www.marinespecies.org at VLIZ. Accessed 28 June 2022. https://doi.org/10.14284/170
Yang Q, Zhang W, Zhang Y, Tang X, Ling J, Zhang Y et al (2022) Promoting larval settlement of coral Pocillopora damicornis by calcium. Coral Reefs 41:223–235. https://doi.org/10.1007/s00338-021-02216-5
Acknowledgements
The authors are grateful for all the amazing people who assisted and provided advice along the journey of these discoveries, of whom there were many. We extend our special thanks to: Chris Westley who was always there to support this work both in the field and at home; the fantastic oyster hatchery team at PSFI (Wayne O’Connor, Mike Dove, Stephan O’Connor, Greg Kent, Brandt Archer, Kyle Johnston, and Emily Collier); the highly knowledgeable ‘coral spawning hotline’ team at AIMS (Katharina Fabricius, Andrew Heyward and Andrew Negri); Brooke McCartin, Nicola Fraser, Matt Nimbs, and Gwenael Cadiou, who were all there for support when needed. We would also like to express sincere thanks to the three anonymous reviewers whose comments and suggestions helped improve and clarify this manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was undertaken as part of a PhD project (MFL) titled ‘The biology, ecology and conservation of the Endangered soft coral Dendronephthya australis’. The project was supported through funding from NSW Department of Primary Industries, NSW Environmental Trust, Southern Cross University’s National Marine Science Centre and Marine Ecology Research Centre, and the Australian Government Research Training Program. Research was undertaken under NSW DPI scientific research permit P01/0059(A)-4 and Marine Parks permit MEAA19/138.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. The authors and their contributions were: MFL: material preparation, data collection, investigation, methodology, resources, project administration, visualisation, analysis, writing—original draft preparation; TRD: supervision, visualisation, analysis, validation, writing—review and editing; DH: supervision, funding acquisition, validation, writing—review and editing; SDAS: supervision, validation, writing—review and editing; TDA: methodology, analysis, validation, writing—review and editing; KB: supervision, methodology, visualisation, analysis, validation, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose. The authors have no conflicts of interest to declare that are relevant to the content of this article. All authors certify that they have no affiliations with or involvement in any organisation or entity with any financial interest or non-financial interest in the subject matter or materials discussed in this manuscript. The authors have no financial or proprietary interests in any material discussed in this article.
Ethical approval
All international, national, and/or institutional guidelines for sampling, care, and experimental use of organisms for the study were followed, and all necessary approvals were obtained.
Informed consent
No humans formed the basis of this study; therefore, no informed consent was required.
Additional information
Responsible Editor: A. Gori.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 21556 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Larkin, M.F., Davis, T.R., Harasti, D. et al. A glimmer of hope for an Endangered temperate soft coral: the first observations of reproductive strategies and early life cycle of Dendronephthya australis (Octocorallia: Malacalcyonacea). Mar Biol 170, 146 (2023). https://doi.org/10.1007/s00227-023-04298-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04298-x