Abstract
Senescence is the irreversible decline in physiological functioning and survival with age. While this phenomenon has been studied in a range of different taxa, including seabirds, it has seldom been assessed for both sexes of monomorphic species, and in conservation contexts. Here, we studied the effect of age and sex on the foraging trip characteristics and energetics of the monomorphic Cape gannet (Morus capensis). Between 2017 and 2020, we used GPS recorders and miniaturised three-dimensional accelerometers to obtain data on the foraging trip characteristics and energy expenditure of 39 Cape gannets rearing chicks on Malgas Island, South Africa. This sample included 11 females and 28 males between the ages of 4 and 23 years. No difference in foraging trip characteristics was apparent between sexes or individuals of different ages. The energy expenditure of aging females (> 17 years) was higher than that of aging males. Aging females spent both more energy flying and less energy resting than males, despite similar foraging trip durations and distances. Males spent more energy diving and taking off from the water than females. The age-related sexual differences in energy expenditure presented in our study might reflect niche and/or risk partitioning strategies to ensure adequate provisioning to the chick, or a possible earlier onset of senescence in females relative to males. The higher energy expenditure of aging females, which presumably requires a concomitantly higher energy intake, likely reduces their resilience to environmental change.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Organismal survival rates and reproductive performance often increase with age until the onset of senescence (Wunderle 1991; Forslund and Pärt 1995), the progressive deterioration of physiological functioning with age (Monaghan et al. 2008). This notably includes a decline in immune response (Palacios et al. 2011), muscular function (Hindle et al. 2009), and visual acuity over time (Schmolesky et al. 2000). Due to their exceptionally long lifespans and slow aging rates (Holmes and Austad 1995), birds, and in particular seabirds, are powerful study systems for researching age-related questions (Holmes and Austad 1995). Multiple studies have investigated the influence of seabird age on factors such as reproductive success (Anderson and Apanius 2003), survival (Ramírez et al. 2021), and physiology (Palacios et al. 2011). Of the few studies that have investigated behavioural changes in aging seabirds, conflicting results have been found (Pelletier et al. 2014; Elliott et al. 2015). For example, no foraging behavioural changes with age were found in Thick-billed murres (Uria lomvia), possibly due to their ability to make physiological changes (by reducing the rate of oxygen utilization with age following the reduced oxygen storage; Elliott et al. 2015). In contrast, spatial segregation was found between middle-aged and old Little penguins (Eudyptula minor) with older individuals foraging closer to shore in shallower waters as a result of diet preferences which differ among age classes (Pelletier et al. 2014). This calls for more research across different species especially focused on understanding the proximate causes of senescence, such as behaviour. In particular, foraging behaviour and success determine the resources an individual can allocate to maintenance and reproduction, and therefore play a key role in determining fitness. Previous studies have found that reduced physiological condition in older breeders affects trip duration, total distance travelled and habitat use (Catry et al. 2011; Jaeger et al. 2014). Thus, studying foraging performance may inform on mechanisms behind senescence. Behavioural adaptability to environmental change (which is crucial for survival; e.g. Jaeger et al. 2014) thus may differ between age classes as birds in each age class experience age-related foraging restrictions differently.
In addition to age, both sexually mono- and dimorphic seabird species can show sex-specific differences in aspects of their foraging behaviour e.g. at sea distribution, diving behaviour, or diet (Lewis et al. 2002; Phillips et al. 2004; Botha and Pistorius 2018). In sexually size-dimorphic species, differences in foraging behaviour may arise from size-related dominance at prey sites (González‐Solís et al. 2000) which ultimately reduces intersexual competition for food resources (De Pascalis et al. 2020). Within-sexes of dimorphic species, age can influence birds’ foraging. Foraging behaviour may change over the lifespans of specific sexes as seen in the sexually dimorphic Wandering albatrosses (Diomedea exulans) from the Crozet Islands (Weimerskirch et al. 2014) with older males increasing their foraging distance from the colony. In size-monomorphic species, it is unlikely for one sex to have a morphological advantage over another as a result of no or slight differences in size (Rishworth et al. 2014). Foraging differences between sexes in monomorphic species may instead be driven by divergent dietary requirements (Botha et al. 2017), intraspecific competition, or differential parental investment in chick provisioning (Elliot et al. 2010), with potential implications for energy expenditure, and in turn, aging trajectories.
The Cape gannet (Morus capensis) is a monomorphic seabird species, whose longevity makes it a useful species for investigating differences in aging between sexes of similar morphology. Endemic to Southern Africa (Crawford 2005), it is considered a useful indicator species of the ecological resilience of the Benguela upwelling ecosystem (Distiller et al. 2012). While age and sex both influence the foraging behaviour of various seabird species, the potential synergetic effects of these traits remain unclear (Fay et al. 2018), especially in monomorphic species. Therefore, in this study, we aimed to investigate age- and sex-related foraging behaviour in this monomorphic seabird.
Cape gannets mainly feed on sardines (Sardinops sagax) and anchovies (Engraulis capensis; Batchelor and Ross 1984; Adams and Klages 1999). In the southern Benguela upwelling system, the spatial distribution of these two small pelagic fish species has shifted since the early 1990s while their stock dwindled as a result of climate change and overfishing (Coetzee et al. 2008). This has led to a spatiotemporal mismatch between the traditional feeding areas of Cape gannets breeding on the west coast of South Africa and their eastward-shifting prey base, with detrimental effects for adult body condition, chick growth rates, individual fitness and ultimately population dynamics (Cohen et al. 2014; Grémillet et al. 2016).
Previous studies have demonstrated the impact of unprofitable foraging conditions on Cape gannet fitness, aligning with a regional population decline (Cohen et al. 2014; Grémillet et al. 2016). Foraging efficiency plays a key role in influencing the survival rates of gannets, and assessing how this behaviour varies across individuals of varying sex and age is necessary to estimate the vulnerability of Cape gannet populations to a changing environment. We use GPS and accelerometer data to determine age-related and sex-specific resource utilisation based on energy-related costs. We predict foraging performances to follow a bell-shaped curve (e.g. Monaghan et al. 2020; Fay et al. 2022; Saraux and Chiaradia 2022) with performance increasing to a certain age whereafter it decreases. We predict that middle-aged birds, having gained foraging experience (Fig. 1a) and have increased fitness, will forage more efficiently (Fig. 1c) than younger birds (with less experience) and older birds (potentially experiencing foraging-related senescence; Fig. 1b). With breeding females having generally longer foraging trips than males (Rishworth et al. 2014), we would also expect them to display earlier foraging senescence than males since life-history theory predicts that individuals which invest more energy into reproduction are likely to undergo faster and earlier senescence (Lemaître et al. 2015).
Methodology
Study area
Fieldwork was conducted on Malgas Island (33.0528° S, 17.9254° E, Saldanha Bay, South Africa. The Cape gannet population on Malgas Island has decreased from 52,000 breeding pairs in 1996/1997 to 22,000 breeding pairs in 2018/2019 (Sherley et al. 2019), and continues to decline (BirdLife International 2018). At this site, gannet chicks have been fitted with metal rings since 1990 (Distiller et al. 2012), and resighting data are still being collected and collated by the South African Bird Ringing Unit (SAFRING). This allowed us to study the at sea movements and energetics of known age, breeding adult Cape gannets during October and November in 2017, 2019, and 2020. In total, we gathered data from 12 birds between 4 and 10 years old, 14 between 11 and 17, and 13 between 18 and 23. The Cape gannet generational length is estimated to be 18.3 years (Sherley et al. 2019). Although the Cape gannet’s life expectancy is unclear, that of their relatives, the Australasian gannet (Morus serrator) and Northern gannet (Morus bassanus), are 15.1 (Norman 2001) and 16.2 years (Nelson 1966), respectively. For this reason, we can assume that the sample used in this study, aged 4–23 years, appropriately captures the life expectancy of Cape gannets. These 39 birds comprised 11 females and 28 males (Table 1).
Foraging trip characteristics
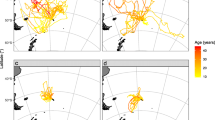
Prior to being fitted with data loggers, 39 birds of known age rearing a one to six-week-old chick were caught on the nest and handled in the shade with their heads covered by a cotton cloth to minimize handling stress. Handling during deployment lasted less than 10 minutes. The birds were released as close to their nest as possible after logger deployment and retrieval. The birds were fitted with loggers for one foraging trip, lasting between 3 and 60 hours. A three-dimensional accelerometer recorder (Axy-4, TechnoSmArt, 9 × 15 × 2 mm, 16 g) was securely mounted on top of a GPS device (CatTrack1, Catnip Technologies, 4.7 × 3.0 × 1.3cm, 20 g) after which both were waterproofed using heat-shrink tubing. Loggers were attached to the lower back of the 39 birds using waterproof Tesa® tape (Wilson and Wilson 1989). This ensured that surging acceleration was measured along the longitudinal body axis of the birds, and heaving acceleration was measured dorsoventrally at 50 Hz (Fig. 2). The combined weight of the GPS device, accelerometer, and heat-shrink tubing was less than 2% of the average body mass of adult gannets (Grémillet et al. 2004). We monitored nests every second hour during daylight to retrieve the loggers soon after the birds returned to the colony. The Tesa® tape strips were removed completely. We used the software X Manager to configure the loggers and retrieve the data from the accelerometers, and @trip PC for the GPS devices. From the GPS data, we extracted information on the following foraging trip characteristics: trip duration (time between departure from and return to the colony, in hours), maximum distance from the nest (farthest point from the colony before returning, in km), and total distance travelled (distance covered between departure and return, in km; see Grémillet et al. 2004 for details). We mapped the core foraging ranges (50% fixed kernel density distributions in QGIS with HRef as a reference bandwidth; Kertson and Marzluff 2011; Signer and Balkenhol 2015) of birds of different age categories to visually determine if there was spatial segregation observed between sexes of different ages (Fig. 2).
Energy expenditure
At-sea energy expenditure was estimated using acceleration data. We used Igor Pro 9.0 (WaveMetrics, OR, USA) to process data and classify behaviors (Grunst et al. 2023). In brief, acceleration in all dimensions was smoothed using a rolling algorithm (2 s sliding window) to extract static acceleration. Static acceleration was derived from the body angle with respect to gravity, and the values ranged from + 1 to − 1 g for a non-moving accelerometer. We calculated body pitch using the formula: atan(SX/(sqrt(SY^2 + SZ^2)) × (180/pi)), where SX, SY, and SZ are smoothed acceleration. Minimum specific acceleration (MSA) was calculated using the formula: abs(sqrt(X^2+Y^2+Z^2) − 1), where X, Y and Z are acceleration, as an indication of the bird’s activities (Simon et al. 2012). We reconstructed accurate Cape gannet time budgets (± 1 s) using activity specific acceleration (Fig. S1), whereby four activity categories and their associated energy requirements were determined (following Grémillet et al. 2016 and Green et al. 2009). Flying required 42.0 W.s−1, resting on the water surface required 26.5 W.s−1, diving required 55.2 W.s−1, taking off from the water surface required 85.9 W.s−1, as determined by Green et al. (2009) to determine the activity-specific rates of energy expenditure of Australasian gannets from their average heart rate per minute.
Genetic sexing
Chest feathers were collected from each of the 39 birds of known age on Malgas Island of which 11 were female and 28 were male. In the genetics laboratory, whole genomic DNA was isolated from the feathers using a Zymo Research Quick DNA extraction kit for feathers (Ryan et al. 2022). One chest feather per bird was submerged in a mixture of water, solid tissue buffer, dithiothreitol and Proteinase K, which was vortexed and incubated at 55 °C for 100 minutes in a dry block. The mixture was subsequently extensively washed, vortexed, and centrifuged (following Appendix D of the Zymo Research Quick DNA Plus extraction kit instructions) to extract the DNA. The supernatant was stored at − 20 ºC. The sex-linked CHD-1 genes were amplified using the forward primer 2550F (5′-GTTACTGATTC GTCTACGAGA-3′) and reverse primer 2718R (5′-ATTGAAA TGATCCAGTGCTTG-3′; Fridolfsson and Ellegren 1999). These primers detect males as ZZ (electrophoresis results display one band) and females as ZW (electrophoresis results displaying two bands; Rishworth et al. 2014). Polymerase chain reactions (PCRs) using 15 µl per sample (7.5 µl GoTaq Green Master Mix hot start, 0.6 µl of both primers, 4.3 µl nuclease-free water, and 2 µl of the DNA template) were performed in a C1000 Touch Thermal Cycler BioRad. Initial denaturation occurred at 94 °C for 2 minutes, followed by denaturation at 94 °C for 30 s, annealing for 30 s, and extension at 72 °C for 1 min (Rishworth et al. 2014). This process was repeated 42 times. A final extension at 72 °C for 5 min was subsequently initiated for the primer to form a double helix DNA strand (Connan et al. 2017), which was stored at 4 °C. PCR products were individually separated on a 1.8% agarose gel using 2.5 µl Prohasafe nucleic acid staining solution in 1X TAE buffer. Electrophoresis was then performed at 100 V for 30 minutes, and bands were visualized using ultraviolet radiation light (Connan et al. 2018).
Statistical analysis of foraging trip characteristics
To test for effects of age-related changes on the foraging trip characteristics (foraging trip duration, maximum distance from the nest, and total distance travelled) of tracked birds, we included age as well as age2 (representing a potential non-linear relationship, which was calculated by squaring the age of each bird; Lescroël et al. 2019; Debeffe et al. 2017) as predictor variables in Gaussian Generalized Linear Models fitted using the MASS Package (Venables and Ripley 2002) in R version 3.5.3 (R Core Team 2017). To normalize data spread we applied log10 transformation to maximum distance from the nest and total distance travelled, and log transformation to energy expenditure. Sex (and an interaction with age) was also included as a predictor variable to test for differences in aging trajectories between males and females. The full set of explanatory variables used in our models were age (continuous), age2 (continuous), and sex (categorical). An interaction between age and sex, and age2 and sex was run to test sexual differences in behavioural senescence. In each iteration, the variable with the highest p value was removed in a backward stepwise selection (Zhuang et al. 2018) to identify the best subset model (with and without interactions).
The year of study had no impact on the analysis of trip duration, maximum distance from the nest, total distance travelled or energy expended (year * age p > 0.05 in all models, Table S1) and was subsequently removed from further analysis.
Statistical analysis of activity-related energy expenditure
To determine the effect of age, sex, and their interaction on the amount of energy expended while flying, resting on the water, diving and taking off, we ran four separate General Linear Models with a Gaussian distribution using backwards stepwise selection in the MASS Package (Venables and Ripley 2002) in R version 3.5.3 (R Core Team 2017). The full set of explanatory variables used in our models were age (continuous), age2 (continuous), and sex (categorical), with an interaction between age and sex, and age2 and sex. A backward stepwise selection (Zhuang et al. 2018) was applied to identify the best subset model (with and without interactions).
We examined the effect of age and sex on (1) total energy expended, and (2) energy expenditure of each of the four at-sea activities. Total energy expenditure was calculated by summing energy expended per activity for a given foraging trip and dividing this number by foraging trip duration to calculate the average of energy expenditure rate during the trip (J/h). To normalize data spread, we log transformed energy spent diving and energy spent taking off from the water.
Results
Sex and age-related spatial distribution
In total, we analysed foraging trip characteristics data of 39 birds between the ages of 4 and 23 years with 11 females and 28 males (Table 1). There was a marginal degree of sex-related segregation at sea in relation with age in Cape gannet, as assessed visually (Fig. 2). Older birds tended to forage farther north than younger birds, and farther south than middle-aged birds (Fig. 3). Middle-aged birds generally tended to forage closest to the colony compared to other age classes. Older females travelled farther away from the colony than males irrespective of age, and young and middle-aged females.
Sex and age-related differences in foraging trip characteristics
There was no significant effect of the variable ‘age2’ in any of our models, so it was removed from the subsequent analyses (Table S2–5). Therefore, the following results are based only on the best (linear) subset model (Table 2).
Neither age nor sex influenced trip duration, maximum distance from the nest, or total distance travelled (Table 2).
Sex and age-related differences in energy expenditure
There was no significant effect of the variable ‘age2’ in any of our models, so it was removed from the subsequent analyses (Table S5–9). Therefore, the following results are based only on the best (linear) subset model (Table 3).
Females’ energy expenditure was positively related with age (p = 0.007; Fig. 4a) whereas overall, energy expenditure was not related to age in males. Females older than 17 years spent more energy per hour (mean = 423242 J/h; standard deviation = 611009) than males irrespective of age (mean = 120017 J/h; standard deviation = 7730; Fig. 4a; Table S5). Females, especially aging females (> 17 years), spent more energy in flight than males (p = 0.007; Table 3; Fig. 4b). Males spent similar amounts of energy resting irrespective of age, whereas females spent less energy resting with age (p = 0.005; Table S7; Fig. 4c). Males spent more energy on diving and taking off from the water than females (p = 0.005, Fig. 5a; p = 0.019, Fig 5b; Table S8–9).
Relation between age (in years) and both a total energy expenditure (flying, resting, diving and taking off from the water; J/h, log), b energy spent flying (J/h), and c energy spent resting on the water (J/h) of 11 female Cape gannets rearing chicks on Malgas Island, South Africa, between 2017 and 2020. The effect of age was not significant for males hence it is not included in the graph
Discussion
While no differences were apparent in Cape gannets across age or sex in foraging trip characteristics, aging females (> 17 years) had higher energy expenditure than that of aging males, rested less and spent more energy in flight.
Despite there being no differences between age or sex in trip duration, maximum distance from the nest, or total distance travelled, older females (> 17 years) tended to spend more energy during a foraging trip than males of the same age, and generally expended increasingly more energy with age, unlike males. Aging females spent more time in flight than males, despite showing similar foraging trip durations and distances.
In a monomorphic species like the Cape gannet, there is no apparent difference in body size (Rishworth et al. 2014) and therefore no physical difference that might result in a competitive advantage of one sex over another while feeding at sea. We found that the energy expenditure of aging females (> 17 years) was higher than that of aging males, possibly as a result of the energetic cost of egg-laying (Lewis et al. 2002). Aging females also seemed to travel both farther north and south compared to young females (Fig. 3a) and old males (Fig. 3c). This could be a result of different dietary preferences (Lewis et al. 2002), and/or avoiding competition with younger, fitter individuals (Pettex et al. 2019). Young females expended the least amount of energy per hour, which suggests higher foraging efficiency (compared to middle-aged or old females) and/or different dietary preferences (compared to males; Lewis et al. 2002). However, younger gannets rested more than middle- or old-aged gannets, which may suggest an increased need to recuperate (Zango et al. 2020). Future research is required to determine which of the aforementioned explanations are more likely to reduce energy expenditure in young females. This could include the study of daily mass gains and/or foraging success (e.g., Fayet et al. 2015) to aid an understanding of the foraging behaviour and success of young gannets.
We also found that females, especially older females, expended more energy in flight than males irrespective of age, despite them having similar travelling duration and distance to males. This could be as a result of the early onset of senescence in females. Another explanation of this could be because adults of different sexes do not invest the same amount of time or energy into feeding the chick (Elliot et al. 2010). In our study, we speculate that aging females spent more time in flight, relative to males (marginal spatial segregation between sexes with age; Fig. 3), as a result of female nutritional requirements for egg production (Monaghan and Nager 1997). This could be driving them to search more actively for food than males (since females also rested less with age), and while doing so, spends energy more conservatively on diving and taking off from the water compared to males. Mortality and breeding costs may arise from the higher energetic expense of breeding in females (through egg production and laying), relative to males. This could lead to a decrease in population fitness through decreased female body condition and subsequent breeding attempts and success (Bijleveld and Mullers 2009; Froy et al. 2017) as foraging energetic constraints could lead to the onset of senescence and/or sex-biased mortality. With regards to males’ aging trends, we found that they expend the same amount of energy irrespective of their age. This could mean that they maintain a similar foraging strategy throughout their lifespan and might be experiencing senescence in a different trait that is not measured in our study e.g. foraging success, hence expending more energy on diving than females. Energy-related risks during foraging could influence foraging decisions, as individuals may either choose a food source that is less nutritious, but closer and more consistently available or a more nutritious, but less consistently available food source at greater distances. Central place foraging forms part of the life-history traits of Cape gannets whereby they are spatially constrained in the breeding season. A change in food distribution may result in longer foraging trips leading to irregular feeding of their chicks, and/or prey being beyond the foraging range leading to selection of poor-quality prey. As a life-history trait, gannets have slow-growing chicks which reduces the energetic risk of irregular food supply, however, during periods of low food availability, adults may prioritise their own survival above that of the chick (Bijleveld and Mullers 2009). Energy-related risks and trade-offs may influence foraging success (Wu and Giraldeau 2005), breeding success (Grémillet et al. 2016) as well as sex-biased mortality (Pichegru and Parsons 2014).
Conservation implications
The Cape gannet, having experienced a dramatic population decline since the late 1990s (Sherley et al. 2019), has already lost some genetic diversity (Reed and Frankham 2003), with consequences for population resilience to disruptions (Booy et al. 2000; Bradshaw and Holzapfel 2008; Vandewoestijne et al. 2008). Our results suggest that aging female gannets expend more energy than males to obtain food to sustain themselves and their chick while replenishing the resources lost from earlier investment in reproduction i.e. egg-laying. We speculate that older females might be physically incapable of further increasing energy they spend searching for food. If conditions of food availability deteriorate beyond a certain threshold, they may abandon the breeding attempt (Bijleveld and Mullers 2009). The already low breeding success on Malgas Island (Makhado et al. 2006) could have affected the population demography and could have increased the proportion of older birds in the population, which might further exacerbate the risk of increased breeding failure and lessen the resilience of this colony to environmental changes. Because of a shortage of food, the Cape gannet colony on Malgas Island appears to have entered an extinction vortex, defined as the reinforcement of processes that drive population extinction (Brook et al. 2008; Kovalenko 2019). Predation of Cape gannet offspring by both Cape fur seals (Arctocephalus pusillus pusillus) and Kelp gulls (Larus dominicanus vetula; Strydom et al. 2022a, b) further enhances pressure on that species. An additional stress factor could be that aging females are struggling in terms of energetic costs; therefore, Cape gannets might experience a further decrease in reproductive output. This could include a reduction in older females’ breeding attempts and food provision to chicks (Bijleveld and Mullers 2009), which influences population dynamics. It could be valuable in future to investigate body condition of females over time to establish if their body condition is deteriorating with age.
In conclusion, our study demonstrated the influence of age and sex on energy expenditure in foraging Cape gannets from Malgas Island. With the nature of our study being cross-sectional, future longitudinal research is required to better understand if either senescence or selective mortality is occurring in old Cape gannet females, as age-dependent trait variation can be caused by within-individual change (Zhang et al. 2015). This could further aid an understanding of intrinsic factors influencing the foraging behaviour of older females which is necessary to estimate the future population trends of this species more accurately. Our results underline the need to consider individual ages when investigating differences in foraging behaviour on a species level as these differences have potential consequences for the fitness of a threatened population of seabirds. Our study provided a new understanding of behavioural heterogeneity inherent to the Cape gannet population, which is necessary to estimate their vulnerability to a changing marine environment.
Data availability
The dataset analysed during the current study is available in the “Data” repository, https://github.com/Seabirdlady/Data.git.
Code availability
The code for the data in the current study is available in the “Code” repository, https://github.com/Seabirdlady/Code.git.
Reference
Adams NJ, Klages NTW (1999) Foraging effort and prey choice in Cape gannets. S Afr J Mar Sci 21:157–163
Anderson DJ, Apanius V (2003) Actuarial and reproductive senescence in a long-lived seabird: preliminary evidence. Exp Gerontol 38(7):757–760
Batchelor AL, Ross GJB (1984) The diet and implications of dietary change of Cape gannets on Bird Island, Algoa Bay. Ostrich 55(2):45–63
Bijleveld AI, Mullers RH (2009) Reproductive effort in biparental care: an experimental study in long-lived Cape gannets. Behav Ecol 20(4):736–744
BirdLife International (2018) Morus capensis. The IUCN Red List of Threatened Species 2018: e.T22696668A132587992. https://doi.org/10.2305/IUCN.UK.2018-2.RLTS.T22696668A132587992.en
Booy G, Hendriks RJJ, Smulders MJM, Van Groenendael JM, Vosman B (2000) Genetic diversity and the survival of populations. Plant Biol 2(04):379–395
Botha JA, Pistorius PA (2018) Variability in the foraging distribution and diet of Cape gannets between the guard and post-guard phases of the breeding cycle. Front Mar Sci 5:15
Botha JA, Rishworth GM, Thiebault A, Green DB, Pistorius PA (2017) Sex-specific foraging over space and time in Cape gannets during chick rearing. Mar Ecol Prog Ser 579:157–167
Bradshaw WE, Holzapfel CM (2008) Genetic response to rapid climate change: it’s seasonal timing that matters. Mol Ecol 17(1):157–166
Brook BW, Sodhi NS, Bradshaw CJ (2008) Synergies among extinction drivers under global change. Trends Ecol Evol 23(8):453–460
Catry P, Granadeiro JP, Ramos J, Phillips RA, Oliveira P (2011) Either taking it easy or feeling too tired: old Cory’s Shearwaters display reduced activity levels while at sea. J Ornithol 152:549–555
Coetzee JC, Van der Lingen CD, Hutchings L, Fairweather TP (2008) Has the fishery contributed to a major shift in the distribution of South African sardine? ICES J Mar Sci 65(9):1676–1688
Cohen LA, Pichegru L, Grémillet D, Coetzee J, Upfold L, Ryan PG (2014) Changes in prey availability impact the foraging behaviour and fitness of Cape gannets over a decade. Mar Ecol Prog Ser 505:281–293
Connan M, Bonnevie BT, Hagen C, van der Lingen CD, McQuaid C (2017) Diet specialization in a colonial seabird studied using three complementary dietary techniques: effects of intrinsic and extrinsic factors. Mar Biol 164(8):1–20
Connan M, Bonnevie B, McQuaid C (2018) Ontogeny, tissue, and species but not sex influence stable isotopic values of three albatross species. Polar Biol 41(6):1175–1186
Crawford RJM (2005) Cape gannet. Roberts’ birds of Southern Africa, 7th edn. John Voelcker Bird Book Fund, Cape Town, pp 565–567
De Pascalis F, Imperio S, Benvenuti A, Catoni C, Rubolini D, Cecere JG (2020) Sex-specific foraging behaviour is affected by wind conditions in a sexually size dimorphic seabird. Anim Behav 166:207–218
Debeffe L, Poissant J, McLoughlin PD (2017) Individual quality and age but not environmental or social conditions modulate costs of reproduction in a capital breeder. Ecol Evol 7(15):5580–5591
Distiller G, Altwegg R, Crawford RJ, Klages NT, Barham B (2012) Factors affecting adult survival and inter-colony movement at the three South African colonies of Cape gannet. Mar Ecol Prog Ser 461:245–255
Elliott KH, Gaston AJ, Crump D (2010) Sex-specific behavior by a monomorphic seabird represents risk partitioning. Behav Ecol 21(5):1024–1032
Elliott KH, Hare J, Le Vaillant M, Gaston AJ, Ropert-Coudert Y, Anderson WG (2015) Ageing gracefully: physiology but not behaviour declines with age in a diving seabird. Funct Ecol 29(2):219–228
Fay R, Barbraud C, Delord K, Weimerskirch H (2018) From early life to senescence: individual heterogeneity in a long-lived seabird. Ecol Monogr 88(1):60–73
Fay R, Martin J, Plard F (2022) Distinguishing within-from between-individual effects: How to use the within-individual centring method for quadratic patterns. J Anim Ecol 91(1):8–19
Fayet AL, Freeman R, Shoji A, Padget O, Perrins CM, Guilford T (2015) Lower foraging efficiency in immatures drives spatial segregation with breeding adults in a long-lived pelagic seabird. Anim Behav 110:79–89
Forslund P, Pärt T (1995) Age and reproduction in birds—hypotheses and tests. Trends Ecol Evol 10(9):374–378
Fridolfsson AK, Ellegren DH (1999) A simple and universal method for molecular sexing of non-ratite birds. J Avian Biol 30:116–121
Froy H, Lewis S, Nussey DH, Wood AG, Phillips RA (2017) Contrasting drivers of reproductive ageing in albatrosses. J Anim Ecol 86(5):1022
González-Solís J, Croxall JP, Wood AG (2000) Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels, Macronectes halli, during incubation. Oikos 90(2):390–398
Green JA, White CR, Bunce A, Frappell PB, Butler PJ (2009) Energetic consequences of plunge diving in gannets. Endanger Species Res 10:269–279
Grémillet D, DellOmo G, Ryan PG, Peters G, Ropert-Coudert Y, Weeks SJ (2004) Offshore diplomacy, or how seabirds mitigate intra-specific competition: a case study based on GPS tracking of Cape gannets from neighbouring colonies. Mar Ecol Prog Ser 268:265–279
Grémillet D, Péron C, Kato A, Amélineau F, Ropert-Coudert Y, Ryan PG, Pichegru L (2016) Starving seabirds: unprofitable foraging and its fitness consequences in Cape gannets competing with fisheries in the Benguela upwelling ecosystem. Mar Biol 163(2):1–11
Grunst AS, Grunst ML, Grémillet D, Kato A, Bustamante P, Albert C, Brisson Curadeau É, Clairbaux M, Cruz-Flores M, Gentès S, Perret S, Ste-Marie E, Wojczulanis-Jakubas K, Fort J (2023) Mercury contamination challenges the behavioral response of a keystone species to arctic climate change. Environ Sci Technol 57:2054–2063
Hindle AG, Lawler JM, Campbell KL, Horning M (2009) Muscle senescence in short-lived wild mammals, the soricine shrews Blarina brevicauda and Sorex palustris. J Exp Zool A Ecol Genet Physiol 311(5):358–367
Holmes DJ, Austad SN (1995) Birds as animal models for the comparative biology of aging: a prospectus. J Gerontol A Biol Sci Med Sci 50:59–66
Jaeger A, Goutte A, Lecomte VJ, Richard P, Chastel O, Barbraud C, Weimerskirch H, Cherel Y (2014) Age, sex, and breeding status shape a complex foraging pattern in an extremely long-lived seabird. Ecology 95(8):2324–2333
Kertson BN, Marzluff JM (2011) Improving studies of resource selection by understanding resource use. Environ Conserv 38(1):18–27
Kovalenko KE (2019) Interactions among anthropogenic effects on aquatic food webs. Hydrobiologia 841(1):1–11
Lemaître JF, Berger V, Bonenfant C, Douhard M, Gamelon M, Plard F, Gaillard JM (2015) Early-late life trade-offs and the evolution of ageing in the wild. Proc R Soc B Biol Sci 282(1806):20150209
Lescroël A, Ballard G, Massaro M, Dugger K, Jennings S, Pollard A, Porzig E, Schmidt A, Varsani A, Grémillet D, Ainley D (2019) Evidence of age-related improvement in the foraging efficiency of Adélie penguins. Sci Rep 9(1):1–13
Lewis S, Benvenuti S, Dall-Antonia L, Griffiths R, Money L, Sherratt TN, Wanless S, Hamer KC (2002) Sex-specific foraging behaviour in a monomorphic seabird. Proc R Soc Biol Sci 269(1501):1687–1693
Makhado AB, Crawford RJ, Underhill LG (2006) Impact of predation by Cape fur seals Arctocephalus pusillus pusillus on Cape gannets Morus capensis at Malgas Island, Western Cape South Africa. Afr J Mar Sci 28(3–4):681–687
Monaghan P, Nager RG (1997) Why don’t birds lay more eggs? Trends Ecol Evol 12(7):270–274
Monaghan P, Charmantier A, Nussey DH, Ricklefs RE (2008) The evolutionary ecology of senescence. Funct Ecol 22:371–378
Monaghan P, Maklakov AA, Metcalfe NB (2020) Intergenerational transfer of ageing: parental age and offspring lifespan. Trends Ecol 35(10):927–937
Nelson JB (1966) Population dynamics of the Gannet (Sula bassana) at the Bass Rock, with comparative information from other Sulidae. J Anim Ecol 35:443–470
Norman FI (2001) Resights, recaptures and recoveries of Australian Gannets Morus serrator breeding in Port Phillip Bay Victoria. Corella 25(4):77–84
Palacios MG, Winkler DW, Klasing KC, Hasselquist D, Vleck CM (2011) Consequences of immune system aging in nature: a study of immunosenescence costs in free-living Tree Swallows. Ecology 92(4):952–966
Pelletier L, Chiaradia A, Kato A, Ropert-Coudert Y (2014) Fine-scale spatial age segregation in the limited foraging area of an inshore seabird species, the little penguin. Oecologia 176(2):399–408
Pettex E, Lambert C, Fort J, Dorémus G, Ridoux V (2019) Spatial segregation between immatures and adults in a pelagic seabird suggests age-related competition. J Avian Biol 50(5):01935
Phillips RA, Silk JRD, Phalan B, Catry P, Croxall JP (2004) Seasonal sexual segregation in two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc R Soc Biol Sci 271(1545):1283–1291
Pichegru L, Parsons NJ (2014) Female-biased mortality in African penguins. Afr J Mar Sci 36(2):279–282
R Core Team (2017) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. URL http://www.R-project.org/
Ramírez F, Chiaradia A, O’Leary DA, Reina RD (2021) Making the most of the old age: autumn breeding as an extra reproductive investment in older seabirds. Ecol Evol 11(10):5393–5401
Reed DH, Frankham R (2003) Correlation between fitness and genetic diversity. Conserv Biol 17(1):230–237
Rishworth GM, Tremblay Y, Green DB, Connan M, Pistorius PA (2014) Drivers of time-activity budget variability during breeding in a pelagic seabird. PLoS ONE 9(12):116544
Ryan PG, Ward VL, Miller SM (2022) First record of a Common diving petrel Pelecanoides urinatrix from continental Africa, and a summary of diving petrel distribution in the Southern Ocean. Mar Ornithol 50(2):211–214
Saraux C, Chiaradia A (2022) Age-related breeding success in little penguins: a result of selection and ontogenetic changes in foraging and phenology. Ecol Monogr 92(1):e01495
Schmolesky MT, Wang Y, Pu M, Leventhal AG (2000) Degradation of stimulus selectivity of visual cortical cells in senescent rhesus monkeys. Nat Neurosci 3(4):384–390
Sherley RB, Crawford RJ, Dyer BM, Kemper J, Makhado AB, Masotla M, Pichegru L, Pistorius PA, Roux JP, Ryan PG, Tom D (2019) The status and conservation of the Cape Gannet Morus capensis. Ostrich 90(4):335–346
Signer J, Balkenhol N (2015) Reproducible home ranges (RHR): a new, user-friendly R package for analyses of wildlife telemetry data. Wildl Soc Bull 39(2):358–363
Simon M, Johnson M, Madsen PT (2012) Keeping momentum with a mouthful of water: behavior and kinematics of humpback whale lunge feeding. J Exp Biol 215:3786–3798
Strydom Z, Waller L, Brown M, Fritz H, Shaw K, Venter JA (2022a) Factors that influence Cape Fur Seal predation on Cape Gannets at Lambert’s Bay. South Africa Peerj 10:e13416
Strydom Z, Waller LJ, Brown M, Fritz H, Venter JA (2022b) The influence of nest location and the effect of predator removal on Cape Gannet Morus capensis egg predation by Kelp Gulls Larus dominicanus vetula. Ostrich 93(2):120–128
Vandewoestijne S, Schtickzelle N, Baguette M (2008) Positive correlation between genetic diversity and fitness in a large, well-connected metapopulation. BMC Biol 6(1):1–11
Venables WN, Ripley BD (2002) Modern applied statistics with S, 4th edn. Springer, New York
Weimerskirch H, Cherel Y, Delord K, Jaeger A, Patrick SC, Riotte-Lambert L (2014) Lifetime foraging patterns of the wandering albatross: life on the move! J Exp Mar Biol Ecol 450:68–78
Wilson RP, Wilson MPT (1989) Tape: a package-attachment technique for penguins. Wildl Soc Bull 17:77–79
Wu GM, Giraldeau LA (2005) Risky decisions: a test of risk sensitivity in socially foraging flocks of Lonchura punctulata. Behav Ecol 16(1):8–14
Wunderle JM (1991) Age-specific foraging proficiency in birds. Curr Ornithol 8:273–324
Zango L, Navarro-Herrero L, García-Vendrell M, Safi K, González-Solís J (2020) Niche partitioning and individual specialization among age, breeding status and sex classes in a long-lived seabird. Anim Behav 170:1–14
Zhang H, Vedder O, Becker PH, Bouwhuis S (2015) Age-dependent trait variation: the relative contribution of within-individual change, selective appearance and disappearance in a long-lived seabird. J Anim Ecol 84:797–807
Zhuang J, Dvornek NC, Li X, Yang D, Ventola P, Duncan JS (2018) Prediction of pivotal response treatment outcome with task fMRI using random forest and variable selection. In: 2018 IEEE 15th International Symposium on Biomedical Imaging (ISBI 2018), pp 97–100
Acknowledgements
We thank Tiaan Strydom, Manon Amiguet, Laëtitia Vibert, Bénédicte Martin and Alexei Dyer for their time and effort assisting with the deployment of accelerometers and GPS devices on known-age Cape gannets on Malgas Island. This work is based on the research supported by the National Research Foundation of South Africa (NRF Grant Number: MND210426597498), The French National Centre for Scientific Research (CNRS), Ernst and Ethel Eriksen Trust, The REHABS Lab, The Wildlife Ecology Lab and the Nelson Mandela University Post-Graduate Research Scholarships.
Funding
Open access funding provided by Nelson Mandela University. This work is based on the research supported by the National Research Foundation of South Africa (NRF Grant Number: MND210426597498), The French National Centre for Scientific Research (CNRS), Ernst and Ethel Eriksen Trust, The REHABS Lab, The Wildlife Ecology Lab and the Nelson Mandela University Post-Graduate Research Scholarships.
Author information
Authors and Affiliations
Contributions
DG, JC and LP made substantial contributions to the conceptualization of the work. All authors contributed to the acquisition of data and their analysis. All authors revised the manuscript critically for intellectual content. All authors approved the version to be published.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
The work obtained ethics approval (ethics clearance reference number A20-SCI-NRM-003) issued by the Nelson Mandela University.
Permit
The work was conducted under a research permit (permit number CRC/2019-2020/001-2002/V1) issued by South African National Parks.
Additional information
Responsible Editor: T. Clay.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Strydom, Z., Grémillet, D., Fritz, H. et al. Age and sex-specific foraging movements and energetics in an endangered monomorphic seabird. Mar Biol 170, 138 (2023). https://doi.org/10.1007/s00227-023-04288-z
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04288-z