Abstract
Sex-specific foraging behaviour may lead to differences between the sexes in both resource acquisition and exposure to threats and thereby contribute to sex-specific reproductive roles or mortality. As such, it is important to identify in which species sex-specific foraging behaviour occurs. We deployed GPS devices to incubating common terns (Sterna hirundo) from a German breeding population to study how sex and spatial or temporal extrinsic factors influence the daily activity budget, foraging distribution, and trip characteristics of this slightly sexually size dimorphic seabird. Birds of both sexes only foraged during the day, showing peaks of activity after sunrise and before sunset, perhaps in response to temporal variation in prey availability and/or as a strategy to overcome or prepare for nocturnal fasting. Furthermore, foraging was more frequent around low tide and at the beginning of the flood tide and mainly occurred in shallow (< 5 m depth) and coastal waters (< 2 km from coastline) up to 20 km from the colony. Females rested less, foraged closer to the colony in more coastal waters, and showed a lower maximum flight speed than males. Males foraged more outside protected areas than females and showed higher variability in their foraging distribution throughout the tide cycle. As such, our study provides evidence for sex-specific aspects of foraging behaviour in common terns and underlines the importance of considering sex-specific foraging distributions when assessing the impact of at-sea threats on seabirds, knowledge of which should be incorporated when developing conservation management strategies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Variation in life-history traits and trajectories is shaped by between- and within-individual variation in resource acquisition and resource allocation (van Noordwijk and de Jong 1986; Descamps et al. 2016). Foraging, as one of the main components of resource acquisition, therefore is an important behaviour with implications for reproductive performance and survival (Lemon 1991; Jeanniard-Du-dot et al. 2017; Weimerskirch 2018), as well as the resolution of trade-offs between the two (e.g., Erikstad et al. 1998). In addition, knowledge of when and where individuals forage is required to assess their level of exposure to specific threats and risks to develop or improve conservation management strategies (Allen and Singh 2016; Sahri et al. 2021).
As foraging is energetically demanding, animals should adopt foraging strategies that allow them to gain the maximum energy possible while minimizing their energy expenditure (Ydenberg et al. 1994). Across temporal scales (e.g., day, month, season, and year), animals should match their peak foraging effort to peak food abundance and availability, although this may be constrained by biological adaptations (e.g., night foraging depends on nocturnal vision) and predation risk (Moore et al. 1989; Phalan et al. 2007; Owen-Smith 2008). Similarly, from a spatial perspective, the foraging distribution of an animal can be constrained by its locomotion capacity and breeding stage, or by the environmental characteristics of its foraging areas (Huey et al. 1984; Shaffer et al. 2003; Heithaus et al. 2009). Therefore, when studying aspects of foraging behaviour, it is important to assess the potential influence of temporal and spatial factors.
Foraging behaviour not only varies across time and space but can also differ between the sexes (Morehouse et al. 2010). Sex-specific foraging behaviour has, indeed, been detected in many animal groups (Ruckstuhl and Neuhaus 2005; Wearmouth and Sims 2008) and several non-exclusive hypotheses have been proposed to explain such sexual segregation (Ruckstuhl and Neuhaus 2005; Ruckstuhl 2007; Wearmouth and Sims 2008). The competitive exclusion hypothesis suggests that the competitively dominant sex excludes the less-dominant one from the preferred foraging resource or habitat (Peters and Grubb 1983; González‐Solís et al. 2000). This tends to occur in species with large sexual size dimorphism, in which the larger sex is usually dominant (e.g., González‐Solís et al. 2000). In contrast, the niche specialization hypothesis postulates that each sex can be morphologically and/or physiologically adapted to forage in a different habitat or on a different resource (Selander 1966; Phillips et al. 2004; Catry et al. 2005; Cleasby et al. 2015). During reproduction, sexual segregation in foraging behaviour may also result from sex-specific reproductive roles, as suggested by the reproductive role specialization hypothesis (Paredes et al. 2006; Burke et al. 2015; Hernández-Pliego et al. 2017). This occurs, for example, when females carry and give birth to offspring in mammals, or produce and lay eggs in insects, reptiles, and birds, or when only one of the parents is responsible for providing the offspring with resources. Finally, sex-specific foraging behaviour may occur even when parents share reproductive roles, but their energetic investment in those roles is not equal, as described by the energetic constraint hypothesis (Welcker et al. 2009; Elliott et al. 2010; Ludynia et al. 2013). Independent of its underlying driver(s), sex-specific foraging behaviour may lead to differences between the sexes in resource acquisition and exposure to threats and thereby contribute to sex-specific mortality rates (e.g., Kienle et al. 2022).
In monomorphic or slightly dimorphic species, it is impossible or extremely difficult to distinguish between the sexes, even at close range, hampering the study of sex-specific foraging behaviour. This is the case for many bird species, especially seabirds, for which the study of foraging behaviour is made even harder by the often remote and partly unpredictable foraging areas they use. In the past, foraging movements of seabirds were mainly detected by boat-based, on-land or aerial surveys and small telemetry devices, such as very high frequency (VHF) transmitters (Briggs et al. 1985; Wanless et al. 1990; Rock et al. 2007; Perrow et al. 2011). The recent miniaturization of tracking devices, such as global positioning systems (GPS), in combination with molecular sex determination, however, enables the investigation of the sex-specific foraging behaviour of an increasing number of seabird species at a finer spatiotemporal scale than ever before, including that of small seabird species (e.g. Mauck et al. 2023).
The common tern (Sterna hirundo) is a small seabird, well studied in several aspects, including its foraging distribution (Fasola et al. 1989; Becker et al. 1993; Schwemmer et al. 2009; Perrow et al. 2011; Lieber et al. 2021; Martinović et al. 2023). Its sex-specific foraging behaviour, however, has not been assessed before. Therefore, we aimed to (i) study how fine-scale temporal and spatial factors relate to the daily activity budget, foraging distribution, and trip characteristics (maximum distance from the colony, total distance travelled per trip, trip duration, and maximum speed) of male and female common terns, and (ii) assess the level of sexual segregation in foraging distribution and its overlap with protected marine areas. To do so, we deployed 6 male and 4 female common terns with GPS devices. Based on previous studies, we hypothesized that common terns will mostly forage during the day (Boecker 1967; Frank and Becker 1992), when the tide is receding (Frank 1992; Frank and Becker 1992; Becker et al. 1993) or low (Boecker 1967; Dunn 1972). As terns forage mainly by plunge-diving, diving-to-surface or dipping, and their diving capacity is extremely limited (~ 20–60 cm depth, Cabot and Nisbet 2013), we expected common terns to mainly forage in shallow areas. Finally, since common terns are only slightly sexually size dimorphic (with males being only slightly larger, Nisbet et al. 2007), and both parents share incubation duties (Becker and Ludwigs 2004), we did not expect competitive exclusion, niche preference, or reproductive role specialization to occur. Energetic constraints, however, we deemed potentially important, given that, despite shared incubation, males and females could differ in their energetic investments throughout the breeding period (Wiggins and Morris 1987). In common terns, males are responsible for female courtship feed during the pre-laying period and for providing more food to the chicks during the early chick-rearing period, while females allocate a great amount of energy to egg production during the laying period and spend more time brooding young chicks (González‐Solís et al. 2001; Becker and Ludwigs 2004). Hence, if common terns during the incubation period behave similarly to black-backed gulls (Larus fuscus, Camphuysen et al. 2015), males would perform less frequent but more distant foraging trips to areas with prey of better quality to prepare for the chick-rearing period, while females would allocate more time to foraging in areas closer to the colony to recover from egg production.
Materials and methods
Study species and area
The common tern is a colonially breeding seabird with a broad breeding distribution across the northern hemisphere (Arnold et al. 2020). It is a long-distance migratory species that, in Germany, reaches the breeding areas from early April (e.g., Kürten et al. 2022) and feeds mainly on small fish (< 150 mm long), although, in some colonies, common terns also rely on crustaceans and insects (Becker and Ludwigs 2004). During the pre-laying period, males provide prey for females to help them reach the nutritional state needed to lay eggs (Wendeln and Becker 1996; González‐Solís et al. 2001). Clutches contain 1–3 eggs, laid at intervals of 1–2 days, that are incubated by both parents for 21–23 days (Becker and Ludwigs 2004). During the first days after hatching, chicks are not yet able to thermoregulate and depend on their parents to maintain their body temperature (Cabot and Nisbet 2013). During this period, females spend more time brooding the chicks, while males do most of the food provisioning (Wiggins and Morris 1987).
We studied the foraging behaviour of common terns from a long-term study population located at the Banter See in Wilhelmshaven, at the German North Sea coast (Fig. 1; 53° 30′ 40′′ N, 08° 06′ 20′′ E). This colony consists of six artificial concrete islands each measuring 10.7 × 4.6 m and surrounded by 60 cm high walls, that protect against flooding and prevent chicks from leaving the colony before fledging. Since 1992, all locally hatched chicks have been ringed and those that survive until fledgling are molecularly sexed (following Becker and Wink 2003) and subcutaneously marked with a transponder (TROVAN ID-100 BC, Trovan, Germany), which allows non-invasive life-long individual identification using an automatic antenna system. This antenna system reads the transponder code when birds are within a radius of 11 cm. For this study, mobile antennas were placed around 10 nests to identify incubating individuals and ensure their experienced breeder status before capturing 6 males and 4 females to deploy, and later recover, a GPS device.
Location of the study area (red rectangle) at the German North Sea coast (left), the location of the breeding colony (yellow diamond), the tide reference locations (dark blue circles with the respective names), and the Banter See area (in light blue) with the bathymetry raster (grey gradient) as a background (right)
GPS deployment and retrieval
Birds were trapped on the 8th of June 2019, 9–14 days after clutch initiation, using the antenna system and an electronically released drop trap. The captured birds, one at each nest, were weighed and tagged with a GPS device (nanoFix® GEO, Pathtrack Ltd, Otley, UK) set to collect one position every 5 min, a resolution chosen based on battery life to achieve the highest resolution possible within a reasonable deployment period. The GPS device was attached to the four central tail feathers, below the uropygial gland, using Tesa® 4651 tape. This tail attachment method is quicker than the back attachment method, and more secure as tail feathers are stronger than body feathers. The total mass of the GPS device and tape was 3.13–3.40 g, corresponding to 2.33–2.76% of the body mass of the tracked birds at deployment, thus below the recommended threshold of 3% (Phillips et al. 2003; Vandenabeele et al. 2012). All individuals resumed incubation after being handled (i.e., no clutch was abandoned) and were recaptured and weighed on the 13th of June 2019, although only nine GPS devices were recovered, as one male lost its device. Of the 9 recovered GPS devices, one had malfunctioned, such that the data showed large gaps and could not be used for analysis.
No discernible sex difference was found in the timing between GPS deployment and the first foraging trip after being tagged. To evaluate potential effects of GPS deployment on reproductive performance, we compared fledging success (i.e., the number of fledged chicks divided by the number of hatched chicks) and mass of fledglings of the tracked birds (nine nests) to that of 10 control birds from nests initiated at the same time using the function t.test from the ‘stats’ package (R Core Team 2021).
Foraging behaviour
We used the ‘Residence in Space and Time’ method (Torres et al. 2017) to infer the behaviour of the birds at each of their GPS positions. This method assigns a state of resting, foraging, or travelling based on the distance travelled and time spent within a specific radius. A bird’s behaviour was (algorithmically) assigned as (1) resting if the bird spent a long period of time and travelled little, (2) foraging if the bird spent a long period of time and travelled much, or (3) travelling if the bird spent a short period of time and travelled much within that specific radius. The specific radius was calculated for each individual using the diagnostic tool provided in the RST package by testing several potential radii: 0.01 km, 0.05 km, and from 0.10 to 5 km in 0.1 km intervals. As foraging within the Banter See is rare (pers. obs.), while resting and preening, as well as social behaviour such as joining panic flights or interacting with kleptoparasites, are more common, we re-assigned all foraging positions (623 out of 10,960, i.e., 5.7%) within this area to resting.
Temporal and spatial variables
To test for effects of the daylight cycle on the foraging behaviour of common terns, we calculated the number of hours since civil sunrise (‘time since sunrise’; when the sun was 6° below the horizon) for each GPS position. This variable was negative when a position occurred prior to civil sunrise and positive when it occurred after it. Civil sunrise times were extracted from https://www.timeanddate.com/sun/germany/wilhelmshaven and converted to Greenwich Mean Time (GMT). We also estimated for each GPS position the number of hours since the nearest low tide based on the tidal calendar of the closest tide reference locations (Fig. 1; Bundesamt für Seeschiffahrt und Hydrographie 2019).
To test for potential spatial drivers of the foraging behaviour of common terns, we determined the distance from the colony (in km), the minimum distance from the coast (in km), the bathymetry (in m), and the main subtidal sediment component for each GPS position. The distance from the colony was calculated using the function deg.dist of the package ‘fossil’ (Vavrek 2011) and the minimum distance from the coast was calculated based on the Europe coastline shapefile obtained from https://www.eea.europa.eu/data-and-maps/data/eea-coastline-for-analysis-2/gis-data/eea-coastline-polyline. We updated this shapefile in QGIS 3.10.2 (QGIS.org 2020) using the Vertex Editor tool to include the land area of the EUROGATE Container Terminal Wilhelmshaven. We also defined the water area of the Banter See, which in the original shapefile was defined as land. We obtained the minimum distance of each GPS position to the adapted coastline using the function dist2Line of the package ‘geosphere’ in R (Hijmans et al. 2019). The raster of bathymetry (resolution of 10 m) and the polygons of the main subtidal sediment components present in the study area (resolution of 100 m) were downloaded from https://mdi-de.baw.de/easygsh/EasyEN_DownloadG.html#2016 and https://mdi-de.baw.de/easygsh/Easy_DownloadS.html#home. We downloaded these two rasters from the most recent year available (2016), and do not expect large changes to have occurred between 2016 and 2019. As the GPS devices used have a latitude and longitude position accuracy of 20 m (information given by Pathtrack Ltd), we reduced the resolution of the bathymetry raster to 20 m using the function aggregate of the package ‘raster’ (Hijmans 2021). Subsequently, we used the function extract of the same package to obtain the bathymetry value and the function over of the package ‘sp’ (Pebesma and Bivand 2005; Bivand et al. 2013) to obtain the main sediment component (silt, fine sand, and medium and coarse sand; Fig. S1) for each GPS position.
Statistical analyses
We conducted two analyses to address two distinct questions: (i) when common terns are resting or searching for food, and (ii) which spatial environmental variables influence their foraging behaviour. Combining these questions would not have been feasible, because this species rests on land (in the colony), such that we lack information on bathymetry and sediment type for those resting positions.
To assess whether the tide and daylight cycles influence when terns are resting or looking for food (travelling or foraging), we constructed a set of candidate Generalised Additive Mixed Models (GAMMs) with a binary response variable in which 0 corresponded to resting positions and 1 to active (foraging or travelling) positions. To avoid temporal autocorrelation issues, we randomly removed 5% of the positions of each individual. As smoothing factors, we included the times since sunrise and the nearest low tide, both defined as cyclic variables. We also included sex, both as a main effect and in interaction with each of the smoothing factors. In all models, individual identity was added as a random effect. We fitted our models with a binomial family using the function gamm4 from the package ‘gamm4’ (Wood and Scheipl 2020) and checked the pairwise concurvity between each pair of explanatory variables using the function concurvity from the package ‘mgcv’ (Wood 2011). The concurvity varies from 0, indicating no problem, to 1, indicating a total lack of identifiability between the two tested variables. As the highest value of concurvity was 0.18, we assumed concurvity to be unimportant and included all variables in our candidate models. To assess the importance of variables and select which variables would be included in the final set of candidate models, we compared models with and without these terms through Likelihood Ratio Tests (LRTs) using the function anova in the package ‘stats’ (R Core Team 2021). We excluded from the final set of candidate models those variables for which the LRT was not significant, unless the LRT indicated that an interaction was significant, in which case both factors of the interaction were included in the final set of candidate models. The most parsimonious model (i.e., the best model explaining the data using the fewest parameters) was selected based on the lowest Akaike’s Information Criterion (AIC) value of the set of candidate models using a maximum-likelihood approach (ML), and parameter estimates (± SE) of the fixed factor(s) and effective degrees of freedom (edf) of the smooth factors were obtained after re-constructing the most parsimonious model using restricted maximum likelihood (REML).
To assess whether spatial environmental variables affect foraging behaviour, we constructed a second set of candidate GAMMs, this time with a binary response variable in which 0 corresponded to travelling positions and 1 to foraging positions. To avoid temporal autocorrelation issues, we again randomly removed 5% of the positions of each individual. As common terns perform several foraging trips per day (Becker et al. 1993), we included trip ID nested within individual identity as a random effect to avoid pseudo-replication. To obtain the trip ID, we considered that a trip began when a GPS position was at least 150 m from the colony and lasted for at least 15 min, to avoid including panic flights. By doing so, we may have lost nine short foraging trips (4.1% of the total number of trips), as terns can perform their first foraging attempt in less than 5 min from leaving the colony, although this is usually a failed attempt and birds continue to forage for longer time (Schwemmer et al. 2009). Furthermore, we excluded 19 on-land positions, as some variables (e.g., bathymetry) were only available for at-sea areas. As smoothing factors, we included the distance from the colony and the minimum distance from the coast. As the tide cycle influences the water column height, which unfortunately was not available for our study area, we included the interaction between bathymetry and time since the nearest low tide as a proxy for the water column height. As fixed effects, we included the main sediment component and sex, the latter both as a main effect and in interaction with distance from the colony and the minimum distance from the coast. As the highest pairwise concurvity between each pair of explanatory variables was below 0.50, we again assumed concurvity to be negligible and included all variables in our candidate model set. We also fitted a Generalised Linear Mixed Model (GLMM) with glmer function of the package ‘lme4’ (Bates et al. 2015) with a binomial family, the same random effect structure and with the same variables and interactions as the full GAMM model to test for multi-collinearity among variables using the function vif of the package 'car’ (Fox and Weisberg 2019). This function calculates the variance inflation factor for each predictor variable in the model and indicates how much the variance of the estimated regression coefficient is inflated due to multi-collinearity. A variance inflation factor value of 1 indicates no multi-collinearity, while a value greater than 5 or 10 is cause for concern (Hair 2010). In our case, all values were < 2.8, such that all variables could be retained in the set of candidate GAMMs. Model selection was performed as described above.
In addition to analysing our data at the location level, we also calculated the following main characteristics for each trip ID: the maximum distance travelled from the colony (in km) and the total duration of the trip (in hours), and we constructed a set of candidate GAMMs assuming a Gaussian family for both. As smoothing cyclic factors, we included the times since sunrise and the nearest low tide of the first GPS position of each trip. These models included individual as a random effect, and sex as a main fixed effect, as well as in interaction with each of the smoothing factors. The highest pairwise concurvity (estimate measure) value was 0.22, such that all variables were included in the candidate model set. Furthermore, we analysed variation in the maximum speed per trip (in km h−1) using a Linear Mixed Model (LMM) using the function lmer from the package ‘lme4’ (Bates et al. 2015) and defining sex as a fixed factor, and individual identity as a random effect. We used the function powerSim from the package ‘simr’ (Green and Macleod 2016) to calculate the statistical power of a model by repeating the following three steps for 1000 simulations: (1) generate new values for the response variable using the fitted model provided; (2) re-estimate the model using the simulated response variable; and (3) apply a statistical test to the simulated fit (Green and Macleod 2016). The statistical test used was the likelihood ratio test and the output obtained a percentage representing the number of tests in step three that detected a significant effect (p < 0.05) of the fixed effect evaluated relative to the total number of tests performed.
Finally, we summarised our data on the daily level, excluding the days of deployment and recovery, as birds differed in the number of hours tracked on these days. We analysed the total number of trips per day using the function glmer from the package ‘lme4’ (Bates et al. 2015) and a GLMM with a Poisson distribution, including sex as a fixed factor and individual identity as a random effect. Variation in the total distance travelled per day was analysed by fitting a LMM and defining sex as fixed factor, and individual identity as a random effect. We performed the LMM and tested the statistical power of these models as described above.
To determine the main foraging area used, we estimated each individual’s kernel density of at-sea foraging positions outside the Banter See. To do so, we first calculated the smoothing factor (h) using the function findScale from the package ‘track2KBA’ (Beal et al. 2020). Subsequently, we used the functions kernelUD and getverticeshr from the package ‘adehabitatHR’ (Calenge 2006) to determine the 50% and 95% Utilization Distribution (UD) contours kernel densities for each individual. We also used the function repAssess with 1000 iterations from the package ‘track2KBA’ (Beal et al. 2021) to calculate how representative our tracked individuals would be of the population, following the procedures described by Lascelles et al. (2016) and Beal et al. (2021). The level of representativeness ranges from 0 to 100%, with 100% achieved when the tracked sample fully represents the distribution of the entire population at the selected UD. Subsequently, we applied a conservative area projection (Lambert Equal area) to the 50% UD contour of each individual to estimate the kernel overlap between and within the sexes using the function kerneloverlaphr from the package ‘adehabitatHR’ (Calenge 2006). This yields the Bhattacharyya’s affinity index, which ranges from 0 (no overlap) to 0.50 (total overlap). To facilitate comparison across the different UD contours, we converted this index into a percentage.
To understand whether the sexes differed in their use of protected and non-protected areas, we calculated the frequency of foraging positions of each sex within the different protection zones of the Lower Saxony Wadden Sea National Park located in the study area (Zonierung Nationalpark Nds. Wattenmeer 2001) and tested for differences using the function chisq.test from the ‘stats’ package (R Core Team 2021).
Since common terns may vary their foraging areas throughout the tide cycle (Becker et al. 1993), we also calculated the 50% UD contour of the foraging positions of each tracked individual for each tide level. We defined the tide levels asymmetrically based on the temporal variation of the water height and current flow speed throughout the tide cycle (Morales 2022). High tide was defined as the period of 1 h before and 1 h after the time of high tide, during which water height reaches its maximum, but current speed is low. Prey may not easily be captured during this tide level as they can be at greater depths and thus less available for the terns. Conversely, low tide was defined as the period of 1 h before and 1 h after the time of low tide, when water height is at its minimum and current speed is low. During this tide level, we expect prey to be more easily accessible and occur at higher concentrations in areas where water is still accumulated. The remaining time between high and low tides and low and high tides were defined as ebb and flood tides, respectively. These periods are marked by significant fluctuations in water height and strong current flows, which may impact the ability of terns to locate and catch their prey due to the water's turbidity. Subsequently, we calculated, as explained above, the kernel overlaps between and within sexes of 50% UD kernel contours across the different tide levels as well as between sexes for each tide level.
All statistical analyses were carried out using R 3.6.2 (R Core Team 2021) with the level of significance set to p < 0.05.
Results
We found no difference in fledging success (meancontrol nests = 0.40 vs. meanGPS nests = 0.52; t = − 0.71, d.f. = 14.38, p = 0.487) or fledging mass (meancontrol nests = 113.88 g vs. mean GPS nests = 118.24 g; t = − 1.44, d.f. = 10.04, p = 0.181) between the GPS and control nests. Moreover, all tagged birds returned to the colony in the year after deployment.
After model selection (Tables S2A and S3), the most parsimonious GAMM concerning temporal factors influencing when common terns were resting or looking for food (travelling or foraging) included sex and interactions between sex and the times since sunrise and the nearest low tide. This model showed that males spend more time resting than females, that both sexes differentially vary their activity throughout the tide cycle (Fig. 2a and Table 1), and that males and females both rest during the night and present peaks of activity right after sunrise and 2–3 h before sunset but differ in their activity level mid-day (Fig. 2b and Table 1).
Output of the Generalised Additive Mixed Model showing how activity of female and male common terns, tracked with GPS during the incubation period, varied with a time since the nearest low tide (in hours) and b time since the civil sunrise (in hours). The y-axis represents the variation in the proportion of resting versus active behaviour, with 0 indicating only resting positions and 1 indicating only active positions (i.e., foraging or travelling). The solid lines represent the mean values, while the shaded areas represent the 95% confidence intervals
Regarding spatial factors explaining variation in travelling and foraging behaviour, the most parsimonious GAMM (see Tables S2B and S4 for model selection) included interactions between bathymetry and the time since the nearest low tide and between sex and minimum distance to the coast, as well as main effects of colony distance and main sediment (Table 2). This model showed that foraging behaviour was more intense in shallow waters (< 5 m of depth, Fig. S2), but also took place in deeper waters (10–12 m of depth) around low tide (Fig. 3a). Furthermore, males foraged less than females and showed a slightly, but significantly, higher proportion of foraging behaviour in waters < 2 km from the coast than females (Fig. 3b). Foraging occurred more often at > 10 km from the colony (Fig. 3c) and was less frequent in areas with medium and coarse sand (Fig. 3D, Table 2).
Output of the Generalised Additive Mixed Model performed on variation in travelling and foraging behaviour of common terns, tracked with GPS during the incubation period. Panel a illustrates the interaction between bathymetry (m) and time since the nearest low tide (h). The colour gradient indicates the variability in behaviour, ranging from 0 (indicated by purple pixels) where only travelling positions (represented by light grey dots) were observed, to 1 (indicated by yellow pixels) where only foraging positions (represented by black dots) were observed. Panel b illustrates how the proportion of travelling and foraging positions for males and females varied with the minimum distance from the coast (km). The y-axis values vary from 0 representing only travelling positions to 1 representing only foraging positions. The solid lines represent the mean values, and the shaded areas the 95% confidence intervals. Panel c represents how the proportion of travelling and foraging positions vary with the distance to the colony (km). The solid line represents the mean values, the dashed lines the 95% confidence intervals. Panel d shows the odds ratios of the fixed factors sex and main subtidal sediment. The odd ratios represent a measure of the effect size of these factors (Vaske et al. 2002), with an odds ratio value of 1 indicating no effect and values < 1 or > 1 representing a negative or positive correlation with the response variable, respectively. The asterisks represent the interval of the p values (*p value between 0.05 and 0.01 and ***p value < 0.001)
At the trip level, our selected best models (Tables 3A and S2C–E) showed that common terns foraged farther from the colony and their trips were longer in distance and duration during ebb than flood tide (Fig. 4 and Table 3). Time since sunrise was not important in any model at the trip level (Table S2C–E). Furthermore, males travelled farther from the colony than females (Table 3B) and at a higher maximum speed than females (LMM: t value = 2.53, d.f. = 5.94, p value = 0.045, power = 75%). At the daily level, we did not find differences between the sexes in the number of trips (GLMM: z value = − 1.16, p value = 0.247), nor the total distance travelled (LMM: t value = 1.66, d.f. = 6.00, p value = 0.147), but the power of these tests was low (21% and 42%, respectively).
Results of the Generalised Additive Mixed Models showing how the a maximum distance from the colony, b total distance travelled per day, and c total trip duration of common terns tracked with GPS during the incubation period varies with the tidal cycle. The solid lines represent the mean values, while the dashed lines represent the 95% confidence intervals
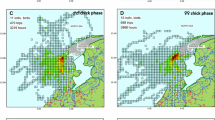
Despite the relatively small number of individuals tracked, the 50% and 95% UD kernel contours of the foraging distribution showed representativeness values of 87.8% and 96.7%, respectively. The 50% UD kernel contours of the foraging distribution of males and females overlapped by 33.8%, while this value was 52.6% and 25.5% among females and males, respectively (Table S6). Males and females foraged in slightly different areas, with males foraging more north and farther from the colony. Males also foraged more in areas with lower protection than females (χ2 = 35.798, d.f. = 2, p < 0.001; Fig. 5 and Table S5).
a Foraging positions (circles) and 50% UD kernel contours of each individual male (left) and female (right) common tern tracked with GPS during the incubation period in relation to the location of different protection zones of the Lower Saxony Wadden Sea National Park. The yellow diamond represents the colony and the light blue area represents the Banter See. b The percentage of male and female foraging positions within the differentially protected areas
Males and females shared a more similar distribution during low than during high tide (37.8% vs. 15.8% of 50% UD kernel overlap, Figures S3–S6). Males explored different areas during low and high tides (0% of overlap) (Figs. S3 and S5 and Table S6). In contrast, their distribution reached a maximum of 35.0% overlap during high and ebb tides (Fig. S3 and S4 and Table S6). Females showed a more consistent distribution throughout the tide cycle (Figs. S3–S6 and Table S6), with a minimum kernel overlap of 2.8% between high and flood tides and a maximum of 30.8% between low and ebb tides (Table S6).
Discussion
The results obtained in this study aligned with our expectations, confirming that common terns only foraged during the day, predominantly in shallow water, and exhibited a higher frequency of foraging activity during low tide. However, we also found sex-specificity in the foraging areas used, with males foraging farther away from the colony and in areas of lower protection status than females, which may result in a differential exposure to at-sea threats.
Potential GPS effects on reproductive performance
We did not detect differences in measures of reproductive performance, fledging success, or fledging mass of the offspring produced, between tagged and control birds and all tagged birds survived to the following breeding season, suggesting no lasting effects of GPS deployment. Nevertheless, although all individuals resumed incubation after being handled, we cannot exclude potential short-term effects during the incubation period, on either the tagged birds or their partners (Seward et al. 2021).
Foraging strategies
At the species level, the resting and active behaviours of the tracked common terns were mainly influenced by the daylight cycle, with birds resting during the night and being most active soon after sunrise and before sunset. The absence of active behaviour during the night was expected based on findings of Frank and Becker (1992), but tested because some evidence of nightly foraging was found in the closely related Roseate tern (Sterna dougallii, Pratte et al. 2021). Foraging just after sunrise and prior to sunset could be a strategy to recover from, and prepare for, nocturnal fasting (Frank and Becker 1992) and/or a response to temporal variation in prey distribution, since prey might to be closer to the sea surface at dusk and dawn than during the day due to diel movement patterns (Burrows et al. 1994; Cardinale et al. 2003).
The choice between rest and activity was less influenced by the tide cycle than by the daylight cycle, which is not surprising given that none of the tracked terns foraged at night. When active, however, common terns foraged mainly in shallow (< 5 m depth) and coastal waters (< 2 km from coastline), usually in areas with sand or silt subtidal sediment, up to 20 km away from the colony and mostly at low tide and at the beginning of the flood tide, which is in line with the previous findings in this species (Becker et al. 1993; Schwemmer et al. 2009; Bracey et al. 2021; Martinović et al. 2023). Common terns have a restricted diving capacity (Cabot and Nisbet 2013), which limits their foraging activity to shallow waters where prey are close to the surface and thus better available. However, common terns can also forage in deeper tidal streams (Schwemmer et al. 2009), if the water column is reduced and local tide currents bring prey to the surface, as shown by the small peak of foraging behaviour of our tracked birds at waters of about 12 m depth during the low tide period. Furthermore, their small body reserves and the need to regularly relieve partners from their shared incubation explain why the tracked individuals, and terns in general, showed a small foraging range and performed several but short (in duration and distance) foraging trips per day (Becker et al. 1993; Schwemmer et al. 2009) in comparison to other seabird species of smaller body size, such as the European storm petrel Hydrobates pelagicus (Rotger et al. 2021). The greater number of foraging positions in fine sand and silt habitats was initially thought to be related to the habitat preferences of common tern prey. Two main prey species, the Atlantic herring (Clupea harengus) and sand eel (Ammodytes marinus), however, have been shown to prefer habitats with a low percentage of silt (Reid and Maravelias 2001; Holland et al. 2005; MarineSpace Ltd 2018). This apparent mismatch between the main sediment preferences of common terns and their prey might be attributed to several factors. A low spatial resolution (100 m) of the main sediment data used could have limited our understanding of the precise sediment characteristics of the main foraging areas used by common terns, and the complex habitats of the Wadden Sea were simplified into three main types of sediments, potentially overlooking important variation. Moreover, there might have been some spatial–temporal variation in the distribution of the main sediment type since 2016 (the year of the main sediment data collection) and 2019 (when our study was conducted).
Sexual segregation in foraging behaviour
We found common terns to show sex-specific aspects of foraging behaviour that can be examined within the context of different hypotheses of sexual segregation in animal foraging strategies.
The competitive exclusion hypothesis suggests that exclusion can be more intense during periods of low prey availability (Reyes‐González et al. 2021). In the case of the common tern, males have slightly larger wings, head, and bill measurements than females (Nisbet et al. 2007), which could provide them with some form of advantage in intra-specific competition between the sexes. If so, we could expect males to forage on the optimal habitat closer to the colony (to save energy), while females would forage farther, especially in periods of low prey availability, e.g., during high tide. During high tide, the overlap between the foraging distributions of males and females was indeed at its minimum value, which could support the competition exclusion hypothesis. However, males were the ones travelling farther from the colony in the remaining periods of the tide and showed more variability in their kernel distribution throughout the tide cycle, which seems to contradict this hypothesis. This is not surprising since the competitive exclusion hypothesis is more commonly supported in highly dimorphic species, but more detailed studies are needed to completely exclude this hypothesis in common terns, as the most optimal foraging habitat may not be the closest one to the colony and may change with the tide.
Alternatively, the slight sexual dimorphism in terns may allow males to capture larger/heavier prey and/or to have better flight capacity (Pennycuick 1987), leading to niche specialization. As such, our finding that males reached a higher maximum speed during foraging trips than females may support the niche specialization hypothesis. Nevertheless, it seems unlikely that males would be more adapted to fly longer distances or reach higher flight speeds due to the slightly biometric differences in the wings and/or tail lengths, since a geolocator study in the same study population did not find sex-specific speed during migration (Kürten et al. 2022). Alternatively, males may have reached a higher maximum speed because they foraged farther from the colony.
Regarding the possibility that males and females may forage for distinct resources (Gwiazda and Ledwoń 2015), studying the diet of common terns during the incubation period is difficult, as they usually swallow their prey at sea, precluding direct observation. During the chick-rearing period, however, a study conducted in the same study population across 2015–2020 found that although males and females brought prey of similar size and average nutritional values, males seemed to provide more Atlantic herring and fish larvae than females, which provided more shrimp, insects, and smelt (Osmerus eperlanus) (Cansse et al. in revision). If prey provided to the chicks reflect parental diet (Dänhardt et al. 2011), this would support sex-specific prey specialization. This also fits with the observation that herring seems more abundant at Minsener Oog, an area north of the Banter See closer to the foraging area mainly used by the tracked males, than in the Jade Bay area close to the Banter See colony, which was used by females throughout the tide cycle (Dänhardt and Becker 2011).
Our study was performed mid-incubation, when both sexes share duties, although females incubate slightly more than males, especially during the night (Wiggins and Morris 1987; Fasola and Saino 1995; Riechert and Becker 2017; Arnold et al. 2020). As these differences are not strong, and mainly occur during the non-foraging period, it seems unlikely that the reproductive role specialization hypothesis explains the sex-specific activity budget and foraging strategies we observed.
Although the reproductive role of males and females is similar during the incubation period, their energetic or nutritional requirements may differ (energetic constraint hypothesis, Welcker et al. 2009; Elliott et al. 2010). We found that females rested less and foraged closer to the colony than males. These differences could reflect the need of females to recover from the cost associated with egg laying. Data on the plasma cholesterol values (as a proxy of body condition) of male and female common terns throughout the incubation period seem to support this idea (Bauch et al. 2010), given that males showed constant cholesterol values, suggestive of a constant body condition throughout the incubation period, whereas females showed lower values 3–5 days after clutch completion, but increases afterwards. Therefore, females may forage more than males to recover their body condition and forage closer to the colony to minimise the effort of travelling. That, however, raises the question of why males would forage farther from the colony and not in the same areas as the females. Perhaps males are somehow preparing themselves for the chick-rearing period by hunting for herring (see above) and by locating areas with fish larvae, given that they take on the main provisioning role in the first week of life of the chicks, while the female specialises on brooding (Cansse et al. in revision). This could also explain why males show more variability in the foraging areas they explored throughout the tide cycle.
Conservation implications
Independent of the drivers of the sexual segregation found, differences in foraging distribution between male and female common terns may have implications for their conservation. For instance, the spatial distribution of mercury, plastic particles, and trace element is not uniform within the Jade Bay (Jin et al. 2012; Beck et al. 2013; Dubaish and Liebezeit 2013). Consequently, male and female common terns may experience varying levels of exposure to these threats, the impact of which on their fitness and reproductive performance is not yet well understood. Indeed, male and female common terns in the study colony exhibit different levels of mercury (Bertram et al. in preparation) and a previous study on common terns breeding in North America showed exposure to mercury to depend on terns’ foraging distribution (Bracey et al. 2021). Moreover, some studies suggest that common terns may rely on fishery discards in years of food scarcity (Camphuysen et al. 1995; Oro and Ruiz 1997; Walter and Becker 1997; Abelló et al. 2003). Given that males foraged more frequently in less protected areas than females, there is a higher probability for them to interact with fisheries than females, as some beam trawls operate in this area (ICES 2020). Identifying the main foraging areas of terns of both sexes in years of different food availability will help to understand the potential impact of this threat.
On the other hand, our results confirmed that common terns forage predominantly in shallow and coastal waters < 20 km from the colony, which is consistent with the previous studies (Becker et al. 1993; Schwemmer et al. 2009; Bracey et al. 2021; Martinović et al. 2023). This information might be particularly valuable to identify potential foraging areas in colonies where conducting tracking studies may pose challenges and to focus conservation management efforts towards those areas if needed.
Conclusion
Our study demonstrated sex-specificity of various aspects of the foraging behaviour of common terns, highlighting the importance of assessing sexual segregation also in slightly dimorphic species. The main drivers of these patterns may relate to different energetic constraints between the sexes, but more studies are needed to evaluate whether sexual segregation occurs across the breeding cycle and throughout the year and how it is influenced by other intrinsic factors, such as age, breeding experience, or individual specialization.
Overall, we emphasize the importance of assessing sex-specificity of the foraging distribution of slightly dimorphic species, as neglecting it can lead to the misidentification of the main foraging areas used by these species and of the at-sea threats they are exposed to. This, in turn, can result in the implementation of inadequate conservation actions or misallocation of conservation efforts to less important areas.
Data availability
All GPS trip data is available on the Seabird Tracking Database http://www.seabirdtracking.org/ (dataset #2096) upon request.
References
Abelló P, Arcos JM, Gil de Sola L (2003) Geographical patterns of seabird attendance to a research trawler along the Iberian Mediterranean coast. Sci Mar 67:69–75. https://doi.org/10.3989/scimar.2003.67s269
Allen AM, Singh NJ (2016) Linking movement ecology with wildlife management and conservation. Front Ecol Evol 3:1–13. https://doi.org/10.3389/fevo.2015.00155
Arnold JM, Oswald SA, Nisbet ICT, Pyle P, Patten MA (2020) Common tern (Sterna hirundo). In: Birds of the world. https://doi.org/10.2173/bow.comter.01. Accessed 7 May 2022
Bates D, Mächler M, Bolker B, Walker S (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–51. https://doi.org/10.18637/jss.v067.i01
Bauch C, Kreutzer S, Becker PH (2010) Breeding experience affects condition: blood metabolite levels over the course of incubation in a seabird. J Comp Physiol B Biochem Syst Environ Physiol 180:835–845. https://doi.org/10.1007/s00360-010-0453-2
Beal M, Oppel S, Handley J, Pearmain L, Morera-Pujol V, Miller M, Taylor P, Lascelles BG, Dias MP (2020) BirdLifeInternational/track2kba: first release (Version 0.5.0). Zenodo. https://doi.org/10.5281/zenodo.3823902
Beal M, Oppel S, Handley J, Pearmain EJ, Morera-Pujol V, Carneiro APB, Davies TE, Phillips RA, Taylor PR, Miller MGR, Franco AMA, Catry I, Patrício AR, Regalla A, Staniland I, Boyd C, Catry P, Dias MP (2021) track2KBA: an R package for identifying important sites for biodiversity from tracking data. Methods Ecol Evol 2021:1–7. https://doi.org/10.1111/2041-210X.13713
Beck M, Böning P, Schückel U, Stiehl T, Schnetger B, Rullkötter J, Brumsack HJ (2013) Consistent assessment of trace metal contamination in surface sediments and suspended particulate matter: a case study from the Jade Bay in NW Germany. Mar Pollut Bull 70:100–111. https://doi.org/10.1016/j.marpolbul.2013.02.017
Becker PH, Ludwigs J-D (2004) Common Tern (Sterna hirundo). In: BWP update. Oxford University Press, Oxford, pp 97–137
Becker PH, Wink M (2003) Influences of sex, sex composition of brood and hatching order on mass growth in Common Terns Sterna hirundo. Behav Ecol Sociobiol 54:136–146. http://www.jstor.org/stable/25063245
Becker PH, Frank D, Sudmann SR (1993) Temporal and spatial pattern of common tern (Sterna hirundo) foraging in the Wadden Sea. Oecologia 93:389–393
Bivand RS, Pebesma EJ, Gomez-Rubio V (2013) Applied spatial data analysis with R, 2nd edn. Springer, New York
Boecker M (1967) Vergleichende Untersuchungen zur Nahrungs- und Nistökologie der Fluβseechwalbe (Sterna hirundo L.) und der Küstenseeschwalbe (Sterna paradisaea Pont.). Bonner Zool Beiträge 18:15–126
Bracey AM, Etterson MA, Strand FC, Matteson SW, Niemi GJ, Cuthbert FJ, Hoffman JC (2021) Foraging ecology differentiates life stages and mercury exposure in common terns (Sterna hirundo). Integr Environ Assess Manag 17:398–410. https://doi.org/10.1002/ieam.4341
Briggs KT, Tyler WB, Lewis DB (1985) Comparison of ship and aerial surveys of birds at sea. J Wildl Manag 49:405–411
Bundesamt für Seeschiffahrt und Hydrographie (2019) Gezeitenkalender: Hoch- und Niedrigwasserzeiten für die Deutsche Bucht und deren Flussgebiete. Germany
Burke CM, Montevecchi WA, Regular PM (2015) Seasonal variation in parental care drives sex-specific foraging by a monomorphic seabird. PLoS ONE 10:e0141190. https://doi.org/10.1371/journal.pone.0141190
Burrows MT, Gibson RN, Robb L, Comely CA (1994) Temporal patterns of movement in juvenile flatfishes and their predators: underwater television observations. J Exp Mar Biol Ecol 177:251–268. https://doi.org/10.1016/0022-0981(94)90240-2
Cabot D, Nisbet ICT (2013) Terns, vol 123. Harper Collins Publisher, London
Calenge C (2006) The package “adehabitat” for the R software: a tool for the analysis of space and habitat use by animals. Ecol Modell 197:516–519. https://doi.org/10.1016/j.ecolmodel.2006.03.017
Camphuysen CJ, Calvo B, Durinck J, Ensor K, Follestad A, Furness RW, Garthe S, Leaper G, Skov H, Tasker ML, Winter CJN (1995) Consumption of discards by seabirds in the North Sea. Final report EC DG XIV research contract BIOECO/93/10. NIOZ Rapport 1995-5. Netherlands Institute for Sea Research, Texel
Camphuysen KCJ, Shamoun-Baranes J, Van Loon EE, Bouten W (2015) Sexually distinct foraging strategies in an omnivorous seabird. Mar Biol 162:1417–1428. https://doi.org/10.1007/s00227-015-2678-9
Cardinale M, Casini M, Arrhenius F, Håkansson N (2003) Diel spatial distribution and feeding activity of herring (Clupea harengus) and sprat (Sprattus sprattus) in the Baltic Sea. Aquat Liv Resour 16:283–292. https://doi.org/10.1016/S0990-7440(03)00007-X
Catry P, Phillips RA, Croxall JP (2005) Sexual segregation in birds: patterns, processes and implications for conservation. In: Ruckstuhl KE, Neuhaus P (eds) Sexual segregation in vertebrates: ecology of the two sexes. Cambridge University Press, Cambridge, pp 351–378
Cleasby IR, Wakefield ED, Bodey TW, Davies RD, Patrick SC, Newton J, Votier SC, Bearhop S, Hamer KC (2015) Sexual segregation in a wide-ranging marine predator is a consequence of habitat selection. Mar Ecol Prog Ser 518:1–12. https://doi.org/10.3354/meps11112
Dänhardt A, Becker PH (2011) Does small-scale vertical distribution of juvenile schooling fish affect prey availability to surface-feeding seabirds in the Wadden Sea? J Sea Res 65:247–255. https://doi.org/10.1016/j.seares.2010.11.002
Dänhardt A, Fresemann T, Becker PH (2011) To eat or to feed? Prey utilization of common terns Sterna hirundo in the Wadden Sea. J Ornithol 152:347–357. https://doi.org/10.1007/s10336-010-0590-0
Descamps S, Gaillard JM, Hamel S, Yoccoz NG (2016) When relative allocation depends on total resource acquisition: implication for the analysis of trade-offs. J Evol Biol 29:1860–1866. https://doi.org/10.1111/jeb.12901
Dubaish F, Liebezeit G (2013) Suspended microplastics and black carbon particles in the Jade system, southern North Sea. Water Air Soil Pollut 224:1352. https://doi.org/10.1007/s11270-012-1352-9
Dunn EK (1972) Studies on terns with particular reference to feeding ecology. Ph.D. thesis. Durham University
Elliott KH, Gaston AJ, Crump D (2010) Sex-specific behavior by a monomorphic seabird represents risk partitioning. Behav Ecol 21:1024–1032. https://doi.org/10.1093/beheco/arq076
Erikstad KE, Fauchald P, Tveraa T, Steen H (1998) On the cost of reproduction in long-lived birds: the influence of environmental variability. Ecology 79:1781–1788
Fasola M, Saino N (1995) Sex-biased parental-care allocation in three tern species (Laridae, Aves). Can J Zool 73:1461–1467. https://doi.org/10.1139/z95-172
Fasola M, Bogliani G, Saino N, Canova L (1989) Foraging, feeding and time-activity niches of eight species of breeding seabirds in the coastal wetlands of the Adriatic sea. Bolletino Di Zool 56:61–72. https://doi.org/10.1080/11250008909355623
Fox J, Weisberg SB (2019) An {R} companion to applied regression, 3rd edn. Sage, Thousand Oaks. https://socialsciences.mcmaster.ca/jfox/Books/Companion/. Accessed 22 May 2023
Frank D (1992) The influence of feeding conditions on food provisioning of chicks in Common terns Sterna hirundo nesting in the German Wadden Sea. Ardea 80:45–55
Frank D, Becker PH (1992) Body-mass and nest reliefs in common terns Sterna hirundo exposed to different feeding conditions. Ardea 80:57–69
González-Solís J, Croxall JP, Wood AG (2000) Sexual dimorphism and sexual segregation in foraging strategies of northern giant petrels Macronectes halli during the incubation period. Oikos 90:390–398. https://doi.org/10.1034/j.1600-0706.2000.900220.x
González-Solís J, Sokolov E, Becker PH (2001) Courtship feedings, copulations and paternity in common terns Sterna hirundo. Anim Behav 61:1125–1132. https://doi.org/10.1006/anbe.2001.1711
Green P, Macleod CJ (2016) SIMR: an R package for power analysis of generalized linear mixed models by simulation. Methods Ecol Evol 7:493–498. https://doi.org/10.1111/2041-210X.12504
Gwiazda R, Ledwoń M (2015) Sex-specific foraging behaviour of the whiskered tern (Chlidonias hybrida) during the breeding season. Ornis Fenn 92:15–22
Hair JF (2010) Multivariate data analysis: a global perspective, vol 7. Pearson Education, Upper Saddle River
Heithaus MR, Delius BK, Wirsing AJ, Dunphy-Daly MM (2009) Physical factors influencing the distribution of a top predator in a subtropical oligotrophic estuary. Limnol Oceanogr 54:472–482. https://doi.org/10.4319/lo.2009.54.2.0472
Hernández-Pliego J, Rodríguez C, Bustamante J (2017) A few long versus many short foraging trips: different foraging strategies of lesser kestrel sexes during breeding. Mov Ecol 5:1–16. https://doi.org/10.1186/s40462-017-0100-6
Hijmans RJ (2021) raster: geographic data analysis and modeling. R package version 3.4-10
Hijmans RJ, Williams E, Vennes C (2019) Spherical trigonometry: package ’geosphere’R package version 1.5-10, pp 1–45
Holland GJ, Greenstreet SPR, Gibb IM, Fraser HM, Robertson MR (2005) Identifying sandeel Ammodytes marinus sediment habitat preferences in the marine environment. Mar Ecol Prog Ser 303:269–282. https://doi.org/10.3354/meps303269
Huey RB, Bennett AF, John-Alder H, Nagy KA (1984) Locomotor capacity and foraging behaviour of Kalahari lacertid lizards. Anim Behav 32:41–50
ICES (2020) Greater North Sea ecoregion—fisheries overview, including mixed-fisheries considerations. ICES Advice 2020
Jeanniard-Du-dot T, Trites AW, Arnould JPY, Guinet C (2017) Reproductive success is energetically linked to foraging efficiency in Antarctic fur seals. PLoS ONE 12:1–19. https://doi.org/10.1371/journal.pone.0174001
Jin H, Liebezeit G, Ziehe D (2012) Distribution of total mercury in surface sediments of the Western Jade Bay, Lower Saxonian Wadden Sea, Southern North Sea. Bull Environ Contam Toxicol 88:597–604. https://doi.org/10.1007/s00128-012-0530-1
Kienle SS, Friedlaender AS, Crocker DE, Mehta RS, Costa DP (2022) Trade-offs between foraging reward and mortality risk drive sex-specific foraging strategies in sexually dimorphic northern elephant seals. R Soc Open Sci 9:210522. https://doi.org/10.1098/rsos.210522
Kürten N, Schmaljohann H, Bichet C, Haest B, Vedder O, González-Solís J, Bouwhuis S (2022) High individual repeatability of the migratory behaviour of a long-distance migratory seabird. Mov Ecol 10:5. https://doi.org/10.1186/s40462-022-00303-y
Lascelles BG, Taylor PR, Miller MGR, Dias MP, Oppel S, Torres L, Hedd A, Le Corre M, Phillips RA, Shaffer SA, Weimerskirch H, Small C (2016) Applying global criteria to tracking data to define important areas for marine conservation. Divers Distrib 22:422–431. https://doi.org/10.1111/ddi.12411
Lemon WC (1991) Fitness consequences of foraging behaviour in the zebra finch. Nature 352:153–155
Lieber L, Langrock R, Nimmo-Smith WAM (2021) A bird’s-eye view on turbulence: seabird foraging associations with evolving surface flow features. Proc R Soc B Biol Sci 288:20210592. https://doi.org/10.1098/rspb.2021.0592
Ludynia K, Dehnhard N, Poisbleau M, Demongin L, Masello JF, Voigt CC, Quillfeldt P (2013) Sexual segregation in rockhopper penguins during incubation. Anim Behav 85:255–267. https://doi.org/10.1016/j.anbehav.2012.11.001
MarineSpace Ltd (2018) Atlantic herring potential spawning habitat and sandeel habitat asssessment baseline 2018—Anglian Region. A report for Bristish Marine Aggregates Producers Association
Martinović M, Plantak M, Jurinović L, Kralj J (2023) Importance of shallow river topography for inland breeding common terns. J Ornithol 164:705–716. https://doi.org/10.1007/s10336-023-02060-0
Mauck RA, Pratte I, Hedd A, Pollet Il, Jones PL, Montevecchi WA, Ronconi RA, Gjerdrum C, Adrianowyscz S, McMahon C, Acker H, Taylor LU, McMahon J, Dearborn DC, Robertson GJ, McFarlane Tranquilla LA (2023) Female and male Leach’s Storm Petrels (Hydrobates leucorhous) pursue different foraging strategies during the incubation period. Ibis (Lond 1859) 165:161–178. https://doi.org/10.1111/ibi.13112
Moore D, Siegfried D, Wilson R, Rankin MA (1989) The influence of time of day on the foraging behavior of the honeybee, Apis mellifera. J Biol Rhythms 4:305–325. https://doi.org/10.1177/074873048900400301
Morales JA (2022) Tide processes. In: Coastal geology. Springer textbooks in earth sciences, geography and environment. Springer, Cham, Switzerland, p 477. https://doi.org/10.1007/978-3-030-96121-3_8
Morehouse NI, Nakazawa T, Booher CM, Jeyasingh PD, Hall MD (2010) Sex in a material world: why the study of sexual reproduction and sex-specific traits should become more nutritionally-explicit. Oikos 119:766–778. https://doi.org/10.1111/j.1600-0706.2009.18569.x
Nisbet ICT, Bridge ES, Szcys P, Heidinger BJ (2007) Sexual dimorphism, female-female pairs, and test for assortative mating in common terns. Waterbirds 30:169–179
Oro D, Ruiz X (1997) Exploitation of trawler discards by breeding seabirds in the north-western Mediterranean: differences between the Ebro Delta and the Balearic Islands areas. ICES J Mar Sci 54:695–707. https://doi.org/10.1006/jmsc.1997.0246
Owen-Smith N (2008) Effects of temporal variability in resources on foraging behaviour. In: Prins HHT, Van Langevelde F (eds) Resource ecology. Wageningen UR Frontis Series, vol 23. Springer, Dordrecht, pp 159–181
Paredes R, Jones IL, Boness D (2006) Parental roles of male and female thick-billed murres and razorbills at the Gannet Islands, Labrador. Behaviour 143:451–481. https://doi.org/10.1163/156853906776240641
Pebesma EJ, Bivand RS (2005) S classes and methods for spatial data: the sp package. R News 5:1–21
Pennycuick CJ (1987) Flight of seabirds. In: Croxall JP (ed) Seabirds: feeding biology and role in marine ecosystems. Cambridge University Press, Cambridge, pp 43–62
Perrow MR, Skeate ER, Gilroy JJ (2011) Visual tracking from a rigid-hulled inflatable boat to determine foraging movements of breeding terns. J F Ornithol 82:68–79. https://doi.org/10.1111/j.1557-9263.2010.00309.x
Peters W, Grubb JTC (1983) An experimental analysis of sex-specific foraging in the downy woodpecker, Picoides pubescens. Ecology 64:1437–1443
Phalan B, Phillips RA, Silk JRD, Afanasyev V, Fukuda A, Fox JW, Catry P, Higuchi H, Croxall JP, Georgia S (2007) Foraging behaviour of four albatross species by night and day. Mar Ecol Prog Ser 340:271–286
Phillips RA, Xavier JC, Croxall JP (2003) Effects of satellite transmitters on albatrosses and petrels. Auk 120:1082–1090
Phillips RA, Silk JRD, Phalan B, Catry P, Croxall JP (2004) Seasonal sexual segregation in the two Thalassarche albatross species: competitive exclusion, reproductive role specialization or foraging niche divergence? Proc R Soc B 271:1283–1291. https://doi.org/10.1098/rspb.2004.2718
Pratte I, Ronconi RA, Craik SR, McKnight J (2021) Spatial ecology of endangered roseate terns and foraging habitat suitability around a colony in the western North Atlantic. Endang Species Res 44:339–350. https://doi.org/10.3354/ESR01108
QGis.org (2020) QGis 3.10. Geographic information system. QGis Association
R Core Team (2021) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 22 May 2023
Reid DG, Maravelias CD (2001) Relationships between herring school distribution and seabed substrate derived from RoxAnn. ICES J Mar Sci 58:1161–1173. https://doi.org/10.1006/jmsc.2001.1111
Reyes-González JM, De Felipe F, Morera-Pujol V, Soriano-Redondo A, Navarro-Herrero L, Zango L, García-Barcelona S, Ramos R, González-Solís J (2021) Sexual segregation in the foraging behaviour of a slightly dimorphic seabird: influence of the environment and fishery activity. J Anim Ecol 1365–2656:13437. https://doi.org/10.1111/1365-2656.13437
Riechert J, Becker PH (2017) What makes a good parent? Sex-specific relationships between nest attendance, hormone levels, and breeding success in a long-lived seabird. Auk 134:644–658. https://doi.org/10.1642/AUK-17-13.1
Rock JC, Leonard ML, Boyne AW (2007) Foraging habitat and chick diets of roseate tern, Sterna dougallii, breeding on Country Island, Nova Scotia. Avian Conserv Ecol 2:4
Rotger A, Sola A, Tavecchia G, Sanz-Aguilar A (2021) Foraging far from home: GPS-tracking of Mediterranean storm-petrels Hydrobates pelagicus melitensis reveals long-distance foraging movements. Ardeola 68:3–16. https://doi.org/10.13157/arla.68.1.2021.ra1
Ruckstuhl KE (2007) Sexual segregation in vertebrates: proximate and ultimate causes. Integr Comp Biol 47:245–257. https://doi.org/10.1093/icb/icm030
Ruckstuhl KE, Neuhaus P (2005) Sexual segregation in vertebrates: ecology of the two sexes. Cambridge University Press, Cambridge
Sahri A, Herwata Putra MI, Kusuma Mustika PL, Kreb D, Murk AJ (2021) Cetacean habitat modelling to inform conservation management, marine spatial planning, and as a basis for anthropogenic threat mitigation in Indonesia. Ocean Coast Manag 205:105555. https://doi.org/10.1016/j.ocecoaman.2021.105555
Schwemmer P, Adler S, Guse N, Markones N, Garthe S (2009) Influence of water flow velocity, water depth and colony distance on distribution and foraging patterns of terns in the Wadden Sea. Fish Oceanogr 18:161–172. https://doi.org/10.1111/j.1365-2419.2009.00504.x
Selander RK (1966) Sexual dimorphism and differential niche utilization in birds. Condor 68:113–151. https://doi.org/10.1073/pnas.0703993104
Seward A, Taylor RC, Perrow MR, Berridge RJ, Bowgen KM, Dodd S, Johnstone I, Bolton M (2021) Effect of GPS tagging on behaviour and marine distribution of breeding Arctic terns Sterna paradisaea. Ibis (Lond 1859) 163:197–212. https://doi.org/10.1111/ibi.12849
Shaffer SA, CostaWeimerskirch DPH (2003) Foraging effort in relation to the constraints of reproduction in free-ranging albatrosses. Funct Ecol 17:66–74. https://doi.org/10.1046/j.1365-2435.2003.00705.x
Torres LG, Orben RA, Tolkova I, Thompson DR (2017) Classification of animal movement behavior through residence in space and time. PLoS ONE 12:e0168513. https://doi.org/10.1371/journal.pone.0168513
van Noordwijk AJ, de Jong G (1986) Acquisition and allocation of resources: their influence on variation in life history tactics. Am Nat 128:137–142
Vandenabeele SP, Shepard EL, Grogan A, Wilson RP (2012) When three per cent may not be three per cent; device-equipped seabirds experience variable flight constraints. Mar Biol 159:1–14. https://doi.org/10.1007/s00227-011-1784-6
Vaske JJ, Gliner JA, Morgan GA (2002) Communicating judgments about practical significance: effect size, confidence intervals and odds ratios. Hum Dimens Wildl 7:287–300. https://doi.org/10.1080/10871200214752
Vavrek M (2011) fossil: palaeoecological and palaeogeographical analysis tools. Palaeontol Ellectronica 14(1T):16p
Walter U, Becker PH (1997) Occurrence and consumption of seabirds scavenging on shrimp trawler discards in the Wadden Sea. ICES J Mar Sci 54:684–694. https://doi.org/10.1006/jmsc.1997.0239
Wanless S, Harris MP, Morris JA (1990) A comparison of feeding areas used by individual common murres (Uria aalge), razorbills (Alca torda) and an Atlantic puffin (Fratercula arctica) during the breeding season. Colon Waterbirds 13:16–24
Wearmouth VJ, Sims DW (2008) Sexual segregation in marine fish, reptiles, birds and mammals: behaviour patterns, mechanisms, and conservation implications. In: Sims DW (ed) Advances in marine biology. Academic Press, Amsterdam, pp 107–170
Weimerskirch H (2018) Linking demographic processes and foraging ecology in wandering albatross—conservation implications. J Anim Ecol 87:945–955. https://doi.org/10.1111/1365-2656.12817
Welcker J, Steen H, Harding AMA, Gabrielsen GW (2009) Sex-specific provisioning behaviour in a monomorphic seabird with a bimodal foraging strategy. Ibis (Lond 1859) 151:502–513. https://doi.org/10.1111/j.1474-919X.2009.00931.x
Wendeln H, Becker PH (1996) Body mass change in breeding common terns Sterna hirundo. Bird Study 43:85–95
Wiggins DA, Morris R (1987) Parental care of the common tern Sterna hirundo. Ibis (Lond. 1859) 129:533–540
Wood S, Scheipl F (2020) gamm4: generalized additive mixed models using “mgcv” and “lme4”. R package version 0.2-6
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J R Stat Soc Ser B (Stat Methodol) 73:3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x
Ydenberg RC, Welham CVJ, Schmid-Hempel R, Schmid-Hempel P, Beauchamp G (1994) Time and energy constraints and the relationships between currencies in foraging theory. Behav Ecol 5:28–34
Zonierung Nationalpark Nds. Wattenmeer (2001). https://geoportal.geodaten.niedersachsen.de/harvest/srv/api/records/B9CC416D-84C9-45E8-A138-0DAA78BB772B. Accessed 11 May 2023
Acknowledgements
This paper is dedicated to the memory of Maria Helena M. Gautier Neto. The authors would like to thank Götz Wagenknecht for valuable help in the field, Virginia Morera-Pujol for statistical advice and Jacob González-Solís for the GPS devices and scientific advice. We are also grateful for the valuable and constructive comments provided by three anonymous reviewers. The authors declare no conflict of interest.
Funding
Open Access funding enabled and organized by Projekt DEAL. This work was supported by the Ministerio de Ciencia, Innovación y Universidades (PID2020-117155GB-I00/AEI/10. 13039/ 501100011033). N.K. received financial support from the German Federal Environmental Foundation (DBU).
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. GPS deployment and recovery and nest monitoring were performed by NK, and data collection and analysis was performed by TM. The first draft of the manuscript was written by TM and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was carried out under licenses provided by the city of Wilhelmshaven and the Lower Saxony State Office for Consumer Protection and Food Safety, Germany.
Additional information
Responsible Editor: V. Paiva .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Militão, T., Kürten, N. & Bouwhuis, S. Sex-specific foraging behaviour in a long-lived seabird. Mar Biol 170, 132 (2023). https://doi.org/10.1007/s00227-023-04280-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04280-7