Abstract
Seagrasses are threatened globally by multiple anthropogenic disturbances, and management of these threats requires detailed information on where losses are occurring and why. Seagrass distribution is determined by processes operating at multiple scales, yet most assessments of change to seagrass extent are done at a single spatial scale. This study applied a multi-scale approach to quantify changes in the extent of the endangered seagrass Posidonia australis over the last 10–18 years using high-resolution mapping from 15 estuaries in New South Wales, Australia. Changes in P. australis extent and relationships with anthropogenic disturbances were examined at two spatial scales: across entire estuaries and at sub-estuary “local” scales within 50 × 50 m grids. Although increases in P. australis area were observed in ten estuaries, losses at local scales were prevalent in all estuaries. No disturbances correlated with seagrass change at the estuary scale; however, the greatest losses occurred in Botany Bay which is a highly modified estuary with a heavily urbanised catchment. At local scales, losses of P. australis were strongly associated with large areas of artificial structures and distance to the sea, and the greatest increases were observed in areas with marine reserves and no oyster aquaculture. These findings highlight the importance of quantifying changes in seagrass extent at multiple scales, as estuary-scale trends can mask localised losses if they are offset by increases in other parts of the estuary. Identifying hotspots of declines and the disturbances causing them are essential for applying focussed management actions to conserve seagrasses.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seagrass meadows have considerable ecological and socio-economic importance, yet they are suffering global declines owing to multiple anthropogenic stressors, including the ongoing development and urbanisation of coastal areas (Orth et al. 2006; Waycott et al. 2009; Dunic et al. 2021). Seagrasses provide many ecosystem functions and services, such as being important habitat for a range of flora and fauna (including commercial and recreational species), stabilising sediments, attenuating wave energy, maintaining water quality, nutrient cycling and storing carbon (Orth 1977; Lepoint et al. 2002; de Boer 2007; Gillanders 2006; Macreadie et al. 2014). However, seagrasses are susceptible to a range of anthropogenic disturbances which are linked to the loss and fragmentation of meadows (Orth et al. 2006; Waycott et al. 2009; Dunic et al. 2021; Swadling et al. 2022). Given the predicted increases in human population and development along coastlines worldwide, it is important to quantify any losses of seagrasses and better understand where and why such losses are occurring. This information is critical to help develop management strategies that promote ecologically sustainable development (Kilminster et al. 2015; Adams et al. 2016; Unsworth et al. 2019; McMahon et al. 2022).
Spatial scale is an essential consideration when identifying the mechanisms underpinning variability in species distributions, as it influences the patterns we observe, and abiotic or biotic variables also vary depending on the size of sampling unit (grain) and extent (Wiens 1989; Levin 1992; O'Brien et al. 2018). Distributions of seagrasses, like those of other species, are influenced by environmental and anthropogenic processes operating at multiple spatial scales (Walker et al. 2006; Kendrick et al. 2008; O'Brien et al. 2018; McMahon et al. 2022). For instance, land-use changes from agricultural practises or urbanisation can cause losses at the meadow and landscape scales through increased nutrient loading and sedimentation, contaminants and poor water quality (Cambridge et al. 1986; Short and Burdick 1996; Bester 2000; Hauxwell and Valiela 2004; Orth et al. 2006; Saunders et al. 2017; Lambert et al. 2021). In comparison, physical damage associated with individual boating infrastructure or fisheries (e.g. aquaculture or trawling) generally occurs over smaller spatial scales (10s–100s m) (Fyfe and Davis 2007; Demers et al. 2013; Glasby and West 2018; Howarth et al. 2022). However, the construction and operation of infrastructure can occur across large areas and at high densities, so their combined impacts may cause substantial loss and fragmentation of seagrass meadows (Glasby and West 2018; Unsworth et al. 2019; Swadling et al. 2022). Failing to investigate species–environment relationships at the appropriate scales can lead to incomplete or misleading information on the factors impacting their distribution, which may in turn compromise management and conservation actions (Andrew and Mapstone 1987; Underwood 1993).
The long-lived seagrass genus Posidonia has a limited capacity to recover from anthropogenic disturbances as its species are slow growing, and recolonisation by seedlings can be infrequent (Kilminster et al. 2015; O'Brien et al. 2018). In New South Wales (NSW) Australia, P. australis Hook. f occurs in 17 estuaries and has suffered losses in its extent at several locations, particularly in heavily urbanised estuaries (Larkum 1976; Larkum and West 1990; West 2011; Evans et al. 2018; West and Glasby 2021). Estimates of P. australis decline differ depending on sampling design. For example, rates of decline in P. australis meadows in Port Jackson (Sydney Harbour) have been estimated to be 10% yr−1 from investigations at specific sites known to be disturbed (Evans et al. 2018), whereas estimates are much lower (1.2% yr−1) when comparing meadow change across the entire estuary (West and Glasby 2021). Given that changes in seagrass meadows and disturbances vary spatially, targeted local-scale studies are unlikely to provide a representative estimate of meadow change across estuaries, although they are useful for quantifying specific impacts. In contrast, studies of meadow change at the scale of estuaries (or at broader scales such as regionally or globally) can provide a more accurate estimate of the overall trajectory of seagrass extent, yet the coarse resolution means that hotspots of decline and the drivers of loss may be overlooked. A multi-scale approach that quantifies changes in seagrass extent at both the estuary and sub-estuary scales will provide a more comprehensive understanding of seagrass trajectories and help prioritise management actions for protecting species within and amongst estuaries. The need for spatially explicit information on changes in P. australis extent is further underscored by the species’ endangered status in six NSW estuaries under state legislation (NSW Fisheries Management Act, 1994), plus an additional three estuaries under national legislation (Environment Protection and Biodiversity Conservation Act, 1999) due to anthropogenic threats operating at multiple scales.
In this study, we quantified changes in the extent of P. australis within 15 estuaries and examined whether these were related to multiple anthropogenic disturbances at 2 spatial scales: (1) across entire populations in estuaries, and (2) at sub-estuary “local” scales (i.e. in 50 × 50 m grids). We used the high-resolution habitat mapping from West and Glasby (2021) to quantify changes in P. australis extent over 10–18 years in all but two of the estuaries where the species occur in NSW, Australia. We built on this previous research using an augmented dataset (updated maps for six estuaries) and by correlating changes in P. australis area at local scales with key disturbances. The disturbances selected were based on previous findings (West 2011; West and Glasby 2021; Swadling et al. 2022) and availability of data at the appropriate scales, and included human population density, catchment land usage, terrestrial nutrient inputs, artificial structures, moorings and oyster aquaculture leases. We hypothesised that estuaries with highly populated, urbanised catchments and high nutrient loading would suffer greater losses in P. australis area, whereas meadow area in less disturbed estuaries would be relatively stable. At the sub-estuary scale, it was hypothesised that losses have occurred where there are artificial structures (jetties, marinas, pontoons and rock groynes), aquaculture leases and moorings, as these disturbances are known to affect seagrasses (Fyfe and Davis 2007; West 2011; Glasby and West 2018; Howarth et al. 2022; Swadling et al. 2022). Many of the surveyed estuaries contained marine reserves (locally known as sanctuary zones within either marine parks or aquatic reserves), where human impacts to seagrasses are minimised via regulations preventing activities that may harm or remove fauna or flora. We, therefore, included the presence of marine reserves in the analyses, predicting that the loss of P. australis meadows would be less in estuaries and at local sites containing reserves when compared to non-protected areas.
Materials and methods
Study area
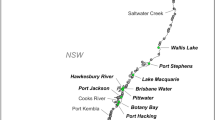
Meadows of Posidonia australis were mapped in 15 estuaries across ~ 750 km of coastline in NSW, Australia (32.27°–37.11° S; Fig. 1). These sites spanned the entire distribution of the species in NSW estuaries, ranging from Wallis Lake in the north to Twofold Bay in the south (Fig. 1). Surveyed estuaries experienced different levels of disturbances, with the highest rates of urbanisation and infrastructure for estuaries within the Greater Sydney Region (i.e. Brisbane Water, Pittwater, Port Jackson, Botany Bay, Port Hacking; Fig. 1). The total extent of P. australis across the 15 study estuaries was ~ 2994 ha, representing 99.6% of the total known area of the species in NSW. Only two estuaries (Cowan Creek and Batemans Bay) containing P. australis were excluded from the analyses due to recent mapping being unavailable, and combined these only represent a small area (< 11 ha) of the species. Cowan Creek, located in the Hawkesbury River, was the only population classified as endangered that was not included in our study (Fig. 1).
Location of estuaries containing Posidonia australis in NSW, Australia. Estuaries in bold were included in the study and those underlined have populations listed as endangered under state or national legislation. Pie charts illustrate the proportion of 50 × 50 m grids experiencing negative (red), positive (blue) or no change (grey) in P. australis area within estuaries between mapping times. Maps on the right show change in area for 50 × 50 m grids across a Port Stephens, and b Pittwater
Quantifying seagrass change
Change in P. australis area was quantified using detailed habitat mapping from two time periods, 10–18 years apart (mean ~ 15 years difference) depending on the estuary (Table 1). Two different techniques were used to map seagrass, as outlined in West and Glasby (2021). Maps produced between 2000 and 2008 (T1; Table 1) were created using a digital GIS method where individual seagrass patches were manually captured using onscreen digitising at a scale of 1:1500. This was done using ortho-rectified scanned or digital aerial imagery with the smallest identifiable features ≥ 4 m2. A refined mapping method was used for maps created after 2017 (T2; Table 2), which involved using object-based image analysis (OBIA) techniques in Trimble eCognition™ v10.2. The OBIA method segments high-resolution ortho-rectified aerial imagery into polygon objects of similar colour, texture and shape. The derived image objects are then manually classified into estuarine habitats, with seagrass meadows classified to a species level. These latter maps were more detailed and captured seagrass patches ≥ 1.5 m2. Initial presumptive seagrass polygons produced by both techniques were extensively validated in the field using a range of visual methods including towed underwater videos, drones (for latest mapping), side-scan sonar and differential GPS. In all maps, P. australis meadows were defined as any polygons containing the species, regardless of density (which was not estimated). Although we could not determine the extent to which any differences in seagrass area were associated with the change in the mapping method, previous comparisons have shown that more detailed mapping typically results in smaller area estimates for mapped polygons (West and Glasby 2021). Therefore, if there is any methodological bias it would likely be towards detecting reductions in area over time. Furthermore, it was assumed that the relative difference between times and methods was similar for each estuary and so unlikely to influence comparisons of change amongst estuaries. Older maps are available, but they are not as accurate as contemporary mapping and may overestimate seagrass extent (West and Glasby 2021). We, therefore, used only recent maps created over the last 10–18 years and these contemporary changes are the most practical to respond to from a managerial perspective. The latest seagrass maps from this project are available at the NSW Fisheries Spatial Data Portal (NSW DPI 2021a). The entire dataset (latest and historic mapping) plus changes in P. australis area over time at both estuary and local scales can be viewed on the NSW Estuarine Habitat Dashboard (NSW DPI 2021b).
Changes in P. australis area were quantified at two spatial scales, across entire estuaries and at the sub-estuary scale (hereafter “local scale”). At the estuary scale, the area of P. australis for each mapping time was calculated by summing the area of all polygons in respective estuaries (Table 1). Differences in P. australis area between times at the local scale were determined within 50 × 50 m grids (hereafter 50 m grids), created using the fishnet tool in ArcGIS v10.8.1 across the species distribution in NSW.
Abiotic variables
All abiotic variables used data representative of disturbances in the present day (T2), as historical data (for T1) were not available. Data on the catchment and sub-catchments for each estuary, land use and the quantity of nitrogen entering estuaries from terrestrial run-off were sourced from Roper et al. (2011) and the NSW SEED data portal (NSW DPIE 2019). Land usage was classified into the proportion of catchments with either natural or disturbed areas, with natural areas containing natural or near natural vegetation and disturbed areas being agricultural or urban areas. Terrestrial nitrogen input data were modelled values (expressed as kg/ha) for each sub-catchment in estuaries. Values for nitrogen inputs were summed for all sub-catchments bordering estuaries within tidal limits. Human population density in estuarine catchments was calculated using data from the 2021 Australian Census (Australian Bureau of Statistics 2021). Human population was included in the analyses as it can act as a surrogate for a variety of different anthropogenic stressors and has been used previously to identify impacts of humans in marine systems (Todd et al. 2019; Rees et al. 2021; Swadling et al. 2022). Human population data were calculated per hectare of catchment for each estuary.
We considered three different types of marine infrastructure in our analyses, specifically artificial structures, oyster leases and the number of boat moorings. The area of artificial structures was mapped from aerial imagery using OBIA techniques and included jetties, pontoons, marinas and rock groynes, and the total area was calculated per 50 m grid. Structures < 2 m in either length or breadth could not be reliably and efficiently mapped, meaning that structures used in oyster aquaculture were not mapped. Spatial data on currently active leases were, therefore, derived from maps of allocated leases (NSW DPI 2021a). It is also important to note that the arrangement and density of structures within a lease area can change over time. Because of these two limitations, we have only effectively considered gross changes in P. australis area at the scale of entire aquaculture leases, despite changes in seagrass extent being captured at much smaller scales.
Originally, at the local scale, the area of oyster leases within 50 m grids was calculated. However, these data were highly skewed (large numbers of zeroes or total coverage (2500 m2) of a grid) so the variable was transformed to the presence or absence of leases within grids. The total number of boat moorings was summed within each 50 m grid. At the estuary scale, the area of artificial structures and oyster leases and the number of boat moorings were summed from all grids in a respective estuary. This meant that only disturbances in, or directly adjacent to (i.e. within 50 m), seagrass meadows were included for estuary-scale analyses. Marine reserves in this study were defined as sanctuary zones in either aquatic reserves or marine parks, both of which have protection measures to prevent harm to marine vegetation. Marine reserves were present in grids containing P. australis in five estuaries (Port Stephens, Botany Bay, Port Hacking, Jervis Bay and Wagonga Inlet), and their presence or absence was recorded for each 50 m grid. It was notable that the marine reserve in Port Hacking (Shiprock Aquatic Reserve) is focussed over a rocky reef but contains a section of an extensive P. australis meadow, so it was retained in the analysis.
The distance of local sites from the sea (i.e. estuary mouth) was included as a predictor variable because it can influence water quality traits that affect seagrass health such as turbidity, temperature and salinity (Ferguson et al. 2018). This is important to account for when quantifying the distribution of P. australis, as the species is largely restricted to marine-dominated, well-flushed locations (West et al. 1989; Roy et al. 2001). Distance to the sea was calculated at the centre point of the 50 m grids using the ‘cost distance tool’ in the spatial analyst extension in ArcGIS. This tool was used as it measured the distance between sites and the ocean without crossing land. The geomorphology of estuaries was included because it can also be a driver of water quality, hydrodynamics and sedimentology (Roy et al. 2001; Ferguson et al. 2018).
Statistical analyses
The influence of anthropogenic disturbances and location covariates (distance to the sea and estuarine geomorphology) on the change in P. australis area at the estuary and local scales was investigated using generalised additive models (GAMs). GAMs were selected for the analyses as they account for non-linear and non-monotonic relationships between the response and explanatory variables (Guisan et al. 2002). At the estuary scale, the response variable was the annual rate of change in P. australis area (% increase or decrease), calculated as the difference between times 1 and 2 divided by the number of years between mapping times (Table 1). At the local scale, the response variable was the difference in P. australis area (m2) between time periods, which was divided by the number of years between mapping times to standardise for the varying temporal scales in sampling. Given that marine reserves and oyster aquaculture leases were not present in all estuaries, preliminary analyses were done to compare relationships between these variables and the change in P. australis area using the full dataset (containing all estuaries) and a restricted dataset (only estuaries with these variables). These analyses revealed little differences in the results and did not affect the interpretation of the relationship (Fig. S1), so the full dataset containing all 15 estuaries was used in the formal analyses.
A full-subset method was used to fit GAMs of all possible combinations (Fisher et al. 2018). Models were fit with a maximum of five predictors and variables with Pearson’s correlation greater than 0.4 were not permitted in the same model. The smoothing parameter was restricted to a simple spline to only allow monotopic relationships (k = 3). Models were fit with a Gaussian distribution and for the local-scale analyses, the estuary ID and an interaction term of latitude and longitude were included as random effects. Model selection was done using the Akaike’s information criterion for small sample sizes (AICc), with the best model having the lowest AICc units (Burnham and Anderson 2002). Models differing by less than two AICc units show weak evidence of support, and when multiple candidate models occurred (i.e. within 2 AICc), the most parsimonious model with the fewest predictors was selected (Burnham and Anderson 2002). Data manipulation, statistical analyses and plots were generated using the statistical computing programme ‘R’ (R Core Team 2022) and the packages ‘dplyr’ (Wickham et al. 2022), ‘FSSGAM’ (Fisher et al. 2018), ‘mgcv’ (Wood 2011) and ‘ggplot2’ (Wickham 2016).
Results
Across all 15 surveyed estuaries in NSW, there was an increase of 698.45 ha in P. australis area during the period (average ~ 15 years) between mapping times. Estuary wide increases (i.e. positive annual change) in area occurred in ten estuaries and overall declines were observed in five estuaries (Fig. 2). Port Hacking only had a minor increase (0.02%) and likely represents no change. The largest percent gains in P. australis area were found in St Georges Basin and Twofold Bay, which increased by 11.8% and 7.2% per year, respectively (Fig. 2). Notably, Twofold Bay had the smallest area of P. australis in NSW and despite the large percentage gained per year, the overall rate of increase was small (0.04 ha yr−1; Table 1). The greatest percent losses detected were in the highly urbanised Botany Bay (−2.6% yr−1), followed by Pambula Lake (−1.7% yr−1), Port Jackson (−1.2% yr−1), Bermagui River (−1.2% yr−1) and Lake Macquarie (−0.5% yr−1; Fig. 2). In terms of the overall area of P. australis lost per year, rates were generally similar amongst estuaries (> −1 ha yr−1) except for Botany Bay where declines were an order of magnitude greater (−8.12 ha yr−1; Table 1).
There was little evidence to suggest that change in P. australis area at the estuary scale was related to anthropogenic disturbances or estuarine geomorphology, with the null GAM model identified as the best model (Table 2). There were two models within ± 2 AICc of the null which contained either population density or the proportion of disturbed catchment. Interestingly, both variables had negative associations with the annual rate of change in seagrass area, with losses of P. australis predicted in estuaries with a population density > 27 people per hectare or with > 50% of disturbed catchment (Fig. S2).
Despite many estuaries experiencing increases in P. australis area, localised losses were identified in all surveyed estuaries (Fig. 1). Declines in seagrass meadows within 50 m grids ranged between 13% of grids in St Georges Basin and 61.3% of grids in Botany Bay. Out of the estuaries experiencing net gains, Port Hacking and Pittwater had large percentages of grids (44–46%) with declines. Four variables were found to best predict the change of P. australis at the local scale, and this model explained 12% of the variation (Table 2). The number of moorings was not present in the best model. Change in P. australis area per year declined with artificial structure area, and seagrass losses were predicted to occur where there was ≥ 310 m2 of artificial structures (i.e. pontoons, jetties or rock groynes) per 50 m grid (2500 m2), which equates to ≥ 12.4% of a grid occupied by artificial structures (Fig. 3a). A non-linear relationship was found between the distance to the sea and P. australis meadow change, with seagrass area most likely to be lost > 12.6 km from the sea (Fig. 3b). Upon further inspection, however, it appeared that this relationship varied with estuarine morphology (Fig. S3). Specifically, declines in P. australis far from the sea only occurred in drowned river valley estuaries, and these were the only estuarine morphology to have distances > 15 km. For instance, a gradient can be observed in the Port Stephens and Pittwater estuaries which are both drowned river valleys, where gains are more common at their mouths and losses are frequent in the upper sections of the estuaries (Fig. 1a, b). In comparison, the opposite relationship was observed for the other estuarine geomorphologies (coastal embayments and barrier estuaries) where declines occurred at the estuary mouths and increases were predicted far from the sea (Fig. S3).
Relationships between explanatory variables in the best model, a artificial structure area (m2), b distance to the sea (km), c oyster aquaculture presence, and d marine reserve presence and the total change in P. australis area per year (m2 y−1) at the local scale (within 50 × 50 m grids). Fitted GAMM prediction curves (solid line) are included, and ribbons and error bars represent ± SE
When analysed across all 15 estuaries, grids containing oyster aquaculture leases were associated with a 47.5% reduction in the rate of change in P. australis area (Fig. 3c). It was notable that whilst the overall change in area was still positive when averaged over all estuaries, constraining the analyses to only the nine estuaries containing oyster aquaculture resulted in losses of P. australis being apparent in some grids with leases, although, on average, small gains were still observed in the presence of aquaculture when measured at the scale of entire lease areas (mean ± SE = 1.75 ± 3.15 m2 y−1; Fig. S1a). Marine reserves were associated with increased gains in P. australis area (mean ± SE = 19.1 ± 2.9 m2 y−1) when compared to non-reserve areas (mean ± SE = 3.85 ± 2.7 m2 y−1; Fig. 3d).
Discussion
Although there are global trends of seagrass meadow loss, there are encouraging signs in some regions that declines are stabilising or have been reversed (Waycott et al. 2009; Dunic et al. 2021; Krause-Jensen et al. 2021). Changes in seagrass meadows are often estimated using studies or meta-analyses over regional, national or global scales, which provide insights into the overall trajectory of seagrass meadows (Waycott et al. 2009; Grech and Coles 2010; Dunic et al. 2021; Turschwell et al. 2021). However, it is also important to identify where seagrass losses are occurring within systems and what the specific causes are, with such assessments needed at scales relevant to potential disturbances (Duarte et al. 2006; O'Brien et al. 2018; West and Glasby 2021). Here, we used a comprehensive dataset to assess changes in the extent of the endangered seagrass Posidonia australis at 2 spatial scales across 15 estuaries spanning ~ 750 km of coastline. The area of P. australis was found to increase in 66% or 10 of the 15 estuaries surveyed over the 10–18 year period, which supports the findings of West and Glasby (2021). Whilst this result is promising and may indicate that management actions are working, investigation at smaller spatial scales (i.e. in 50 × 50 m grids) revealed localised losses are prevalent in all estuaries. Such findings demonstrate that estimates of estuary-scale change can mask smaller-scale losses if they are offset by gains in other areas. No disturbance variables were found to correlate with the change in P. australis area at the estuary scale (supporting West and Glasby 2021). In comparison, local losses were associated with the area of artificial structures and greatest increases were observed in marine reserves, or in areas without oyster aquaculture leases. Overall, these findings highlight the importance of applying multi-scale techniques for determining the trajectory of meadows across a system and identifying hotspots of decline. The latter is essential for applying focussed management actions and to optimise the allocation of efforts to reduce negative human impacts on seagrasses.
Although localised losses of P. australis were observed in all estuaries, ten estuaries experienced net gains in meadow area over the last 10–18 years. The increases in P. australis were somewhat surprising, as the species is classified as a persistent seagrass which forms enduring meadows with only small variation in its extent (Kilminster et al. 2015). The colonisation rate of P. australis is considered to be slow, and it can take decades to recover from disturbances. This notion comes largely from estimates of spreading rates for P. australis to be 20–80 cm yr−1 in areas where disturbances have occurred (Meehan and West 2000; Paling and McComb 2000; Cambridge et al. 2002; Bastyan and Cambridge 2008). However, it has been proposed that growth rates of seagrass rhizomes may not scale up to the growth dynamics at the patch or meadow scales (Duarte and Sand-Jensen 1995; Vidondo et al. 1997; Kendrick et al. 2005). Indeed, the results of the current study suggest that P. australis meadows in estuarine environments may have a greater capacity to expand than previously thought (e.g. St Georges Basin 16.48 ha yr−1 and Port Stephens 14.35 ha yr−1). The mechanisms (e.g. rhizome extension, seedling recruitment, fragment dispersal) underpinning the large expansion of P. australis meadows and the colonisation of bare sediments observed in this study remain unclear. Future studies are, therefore, warranted on understanding the factors influencing the growth dynamics of P. australis meadows in NSW estuaries.
In the current study, there were overall losses of P. australis in five estuaries, including the two most heavily urbanised estuaries (Botany Bay and Port Jackson). Although there was some evidence that losses may occur in estuaries with higher populations (> 27 people/ha) or disturbed (> 50% of non-natural land-use) catchments (Fig. S2), no disturbances were found to predict changes in P. australis extent at the estuary scale. This supports the findings of West and Glasby (2021), who reported no relationships between catchment disturbances and changes in the extent of multiple seagrass species in NSW estuaries. We suggest that the drivers of P. australis loss at estuary scales may be estuary specific or due to factors not considered in this study. For example, large movements of sediment have occurred in areas of Bermagui River and Botany Bay, which may have smothered seagrass meadows (Duarte et al. 1997). It is notable, however, that P. australis is more tolerant to burial than other seagrass species with 50% mortality reported when buried at a depth of 19.5 cm (Cabaço et al. 2008). Furthermore, some losses in Botany Bay were observed at the deeper edge of P. australis meadows and not near over-water structures or moorings, and could indicate reduced water clarity, which has previously been rated poor in Botany Bay and its main tributary (NSW OEH 2010). In comparison, declines of P. australis in Port Jackson and Lake Macquarie occurred in areas with high densities of moorings or artificial structures, and in Pambula Lake, losses were evident near aquaculture leases. Such observations highlight that the disturbances driving change in P. australis extent at the estuary scale are likely context dependent, so it may be difficult to make generalisations from studies examining change in seagrasses at broad spatial scales.
The loss of seagrass due to marine infrastructure has been commonly observed worldwide (Skinner et al. 2014; Griffiths et al. 2020; Sagerman et al. 2020; Ferretto et al. 2022; Howarth et al. 2022), and the life-history traits of P. australis may limit the recovery potential relative to other seagrasses (Kilminster et al. 2015). Infrastructure can cause seagrass removal through mechanical damage during construction, scouring due to changed water flow, or vessels accessing the structures may scour seagrasses (Loflin 1995; Fyfe and Davis 2007; West 2011; Bulmer et al. 2012; Howarth et al. 2022). In addition, shading by overwater structures (e.g. jetties, pontoons and aquaculture) has been shown to reduce (or inhibit) photosynthesis, and cause localised seagrass loss or lower shoot densities (Bulthuis 1983; Fitzpatrick and Kirkman 1995; Collier et al. 2009; Eriander et al. 2017; Ferretto et al. 2022). Compared to other Posidonia species, P. australis seems more susceptible to light reductions with 90% shading halving growth rates after a month (Fitzpatrick and Kirkman 1995) or shoot densities reducing 75% after 3 months under oyster aquaculture trays (Ferretto et al. 2022). Furthermore, the recovery of Posidonia species can be slow or non-existent after 6 months of shading (Collier et al. 2009). Coastal defence structures such as rock groynes can alter the hydrology and sedimentation in an area which also cause losses of seagrass (Duarte 2002; Airoldi and Beck 2007). Our findings demonstrate that artificial structures are negatively impacting P. australis meadows in NSW estuaries. Losses associated to artificial structures were detected when structures covered > 12% of a grid (i.e. 310 m2). Given that most artificial structures had an area of ~ 20–70 m2, we postulate that larger losses of P. australis occurred in grids with multiple structures (e.g. jetties). In Pittwater for instance, losses were more prevalent along sections of foreshore with large numbers of boating infrastructure such as jetties, whereas the greatest gains were observed at the mouth where there were low disturbances and no boating infrastructure (Fig. 1a). It remains unclear how many structures (e.g. jetties, pontoons or rock groynes) are required in an area to generate substantial losses and whether any thresholds for structure density exist, but this is an avenue for future research.
There was some evidence that losses of P. australis were more prevalent in the upper reaches of estuaries, yet this pattern was only apparent in drowned river valley estuaries (Fig. S3). The opposite trend was observed in barrier estuaries and coastal embayments. These patterns may relate to the different hydrological and sediment characteristics of these three estuary types. Drowned river valley estuaries were much longer than the other morphologies and have a large tidal range which results in well-flushed estuarine mouths and more turbid conditions in the upper reaches. It is, therefore, not surprising that declines in P. australis occurred upstream as these areas would be at the edges of its distributional limits within these estuaries, because the species is generally restricted to well-flushed and marine conditions (West et al. 1989; Ferguson et al. 2018). In comparison, losses at the entrances of barrier estuaries could be attributed to the presence of narrow tidal deltas that commonly form here. Shifting sands in these deltas may smother seagrasses or erode meadows (West et al. 1989; Roy et al. 2001; Cabaço et al. 2008; West and Glasby 2021). This is likely for the many barrier estuaries (e.g. Bermagui River) with their entrances trained by break walls, which alter water flow and cause the entrance shoals to be more mobile (Ferguson et al. 2018). Finally, coastal embayments are subjected to strong wind and wave conditions (West et al. 1989) at their mouths, which may be exacerbated by dredging in Botany Bay (Larkum and West 1990), and this could result in the removal of P. australis via physical damage or sediments smothering meadows.
Marine reserves are being employed globally to conserve biodiversity (Spalding et al. 2008; Halpern et al. 2010; Knott et al. 2021), and seagrasses are regularly incorporated into reserve design due to their widespread degradation and ecological importance (Orth et al. 2006; Coles et al. 2015; Howard et al. 2017; Strachan et al. 2022). Whilst previous studies often report that seagrass may continue to decline despite being protected within marine reserves (Marbà et al. 2002; Fraschetti et al. 2013; Quiros et al. 2017; Tin et al. 2020), our results were consistent with P. australis meadows benefiting from protection afforded by reserves. The positive effects of protection are likely due to key disturbances in our study system being minimised in reserves. For example, seagrass loss in reserves has been linked to the anchoring of vessels or trampling (Milazzo et al. 2004; La Manna et al. 2015; Travaille et al. 2015). However, vessel anchoring in areas with seagrass in many NSW reserves is not permitted, and a few reserves have designated anchorages or public moorings. Furthermore, the development of artificial structures in marine reserves is restricted. Seagrass declines within reserves elsewhere have also been reported where adjacent disturbances flow into protected areas (e.g. land-use practises in watersheds; Quiros et al. 2017), but we did not identify any impacts of such disturbances at the estuary scale. Some ecological functions such as herbivory may be enhanced within marine reserves and have indirect positive impacts to seagrasses. Higher abundances of herbivores within reserves have been shown to increase grazing rates (Prado et al. 2008; Planes et al. 2011; Ferguson et al. 2016), and seagrass grazing can stimulate shoot production (Valentine et al. 1997; Gulick et al. 2021) or reduce epiphyte loading to allow greater light availability (Orth and van Montfrans 1984; Howard and Short 1986). Nevertheless, our findings suggest that marine reserves may present conservation benefits to P. australis populations, most likely by minimising localised negative impacts through increased regulatory mechanisms.
Traditional block and chain boat moorings are the most common type of mooring in NSW, and they are known to physically scour seagrass meadows and generate scarring (Walker et al. 1989; West 2011; Demers et al. 2013; Glasby and West 2018). The size of mooring scars in P. australis is reported to increase at an average rate of 6.3 m2 per month, meaning it takes about 30 months to attain an average size of 185 m2 in water 2–6 m deep (Glasby and West 2018). We propose the lack of any association between mooring density and changes in the extent of P. australis over the last 10–18 years likely reflects the stable impact of moorings, rather than a lack of mooring effects. This is supported by many of the moorings included in this study being present in the same area for at least a decade (authors’ pers. obs. from aerial imagery).
The development of estuaries will undoubtedly increase as the human population continues to grow (Bulleri and Chapman 2010; Dafforn et al. 2015), making it important for management to minimise direct and indirect negative impacts to seagrasses or introduce seagrass-friendly infrastructure designs. Our findings suggest that one way to reduce the loss of P. australis in NSW estuaries is to prevent more artificial structures being constructed in or directly adjacent to seagrass meadows. Although current legislation (NSW Fisheries Management Act 1994) in NSW prevents certain types of marine infrastructure being built in P. australis meadows, there are increasing pressures to allow more of these structures and other legislative mechanisms can allow this to occur. Endangered populations of P. australis should be the highest priority for protection, particularly those in the Greater Sydney Region and Lake Macquarie where declines are still occurring. Developing and implementing seagrass-friendly designs for marine infrastructure is another option for research and management to help conserve seagrasses into the future. In the construction of jetties for instance, the use of mesh decking over timber or jetty designs which are high and narrow may provide greater light penetration and reduce negative impacts to seagrass (Burdick and Short 1999; Gladstone and Courtenay 2014).
In NSW, seagrass-friendly designs are being increasingly implemented in oyster aquaculture, with a shift from the use of traditional cultivation methods (trays and longline baskets) to floating bags and baskets. When floating bags are deployed in low densities and in water no shallower than 1 m, they can have no significant impacts on P. australis shoot density or growth over a 6-month period, whereas trays and longline baskets negatively affect photosynthesis and cause adverse impacts (Ferretto et al. 2022). The increasing implementation of floating structures may in part explain the overall gains in seagrass predicted where aquaculture was present (notwithstanding these gains being significantly less than in areas without aquaculture), as these new structures may allow P. australis to recolonise deeper areas (> 1 m) previously shaded by trays or longline baskets. It is notable that most oyster aquaculture leases in this study have been operational for almost 100 years, so many negative impacts to P. australis meadows had likely already occurred prior to mapping. An alternative explanation for the gains in seagrass around aquaculture may be the spatial scale of the assessment. Although changes in seagrass could be documented at small spatial scales (> 1.5 m2), it was not possible to map individual aquaculture infrastructure in the same way that other artificial structures were mapped. As such, we effectively assessed for changes in seagrass at the scale of entire oyster leases, which were almost always far larger areas than those covered by oyster infrastructure. Thus, any losses of P. australis under aquaculture structures would be obscured by gains of other parts of the lease where there were no structures. Future studies are required to examine changes in the cover of seagrasses around aquaculture structures at scales smaller than those used in this study.
In conclusion, this study has provided important insights into how the extent of the endangered seagrass P. australis is changing across NSW estuaries. Specifically, we revealed using a multi-scale assessment that although gains are evident in the species’ distribution at the estuary-scale, localised losses are still prevalent throughout estuaries. This approach also allowed us to identify anthropogenic disturbances responsible for local losses such as artificial structures and there were more gains in marine reserves and in the absence of oyster aquaculture leases. More broadly, our findings highlight the need for studies quantifying patterns in seagrass distribution to move away from single-scale approaches to provide a better understanding on patterns of seagrass change. Such information is essential to inform management strategies to combat the degradation of seagrass meadows and support ecologically sustainable development as the population increases along coastlines worldwide.
Data availability
The latest seagrass maps from this project are available at the NSW Fisheries Spatial Data Portal (https://www.dpi.nsw.gov.au/fishing/fisheries-research/spatial-data-portal). All other datasets generated and analysed in the study are available from the corresponding author on reasonable request.
References
Adams MP, Saunders MI, Maxwell PS, Tuazon D, Roelfsema CM, Callaghan DP, Leon J, Grinham AR, O’Brien KR (2016) Prioritizing localized management actions for seagrass conservation and restoration using a species distribution model. Aquat Conserv 26:639–659. https://doi.org/10.1002/aqc.2573
Airoldi L, Beck M (2007) Loss, status and trends for coastal marine habitats of Europe. Oceanogr Mar Biol 45:345–405
Andrew NL, Mapstone BD (1987) Sampling and the description of spatial pattern in marine ecology. Oceanogr Mar Biol 25:39–90
Australian Bureau of Statistics (2021) Census GeoPackages. https://datapacks.censusdata.abs.gov.au/geopackages/. Accessed 2 Nov 2021
Bastyan G, Cambridge ML (2008) Transplantation as a method for restoring the seagrass Posidonia australis. Estuar Coast Shelf Sci 79:289–299. https://doi.org/10.1016/j.ecss.2008.04.012
Bester K (2000) Effects of pesticides on seagrass beds. Helgol Mar Res 54:95–98. https://doi.org/10.1007/s101520050007
Bulleri F, Chapman MG (2010) The introduction of coastal infrastructure as a driver of change in marine environments. J Appl Ecol 47:26–35. https://doi.org/10.1111/j.1365-2664.2009.01751.x
Bulmer R, Kelly S, Jeffs AG (2012) Hanging basket oyster farming: assessing effects on seagrass using aerial photography. Aquacult Environ Interact 2:285–292. https://doi.org/10.3354/aei00046
Bulthuis DA (1983) Effects of in situ light reduction on density and growth of the seagrass Heterozostera tasmanica (Martens ex Aschers.) den Hartog in Western Port, Victoria, Australia. J Exp Mar Biol Ecol 67:91–103. https://doi.org/10.1016/0022-0981(83)90137-5
Burdick DM, Short FT (1999) The effects of boat docks on eelgrass beds in coastal waters of Massachusetts. Environ Manage 23:231–240
Burnham KP, Anderson DR (2002) A practical information-theoretic approach. Model selection and multimodel inference, 2nd edn. Springer, New York
Cabaço S, Santos R, Duarte CM (2008) The impact of sediment burial and erosion on seagrasses: a review. Estuar Coast Shelf Sci 79:354–366. https://doi.org/10.1016/j.ecss.2008.04.021
Cambridge M, Chiffings AW, Brittan C, Moore L, McComb A (1986) The loss of seagrass in Cockburn Sound, Western Australia. II. Possible causes of seagrass decline. Aquat Bot 24:269–285. https://doi.org/10.1016/0304-3770(86)90062-8
Cambridge M, Bastyan G, Walker D (2002) Recovery of Posidonia meadows in Oyster Harbour, southwestern Australia. Bull Mar Sci 71:1279–1289
Coles RG, Rasheed MA, McKenzie LJ, Grech A, York PH, Sheaves M, McKenna S, Bryant C (2015) The great barrier reef world heritage area seagrasses: managing this iconic Australian ecosystem resource for the future. Estuar Coas Shelf Sci 153:A1–A12. https://doi.org/10.1016/j.ecss.2014.07.020
Collier CJ, Lavery PS, Ralph PJ, Masini RJ (2009) Shade-induced response and recovery of the seagrass Posidonia sinuosa. J Exp Mar Biol Ecol 370:89–103. https://doi.org/10.1016/j.jembe.2008.12.003
Dafforn KA, Glasby TM, Airoldi L, Rivero NK, Mayer-Pinto M, Johnston EL (2015) Marine urbanization: an ecological framework for designing multifunctional artificial structures. Front Ecol Environ 13:82–90. https://doi.org/10.1890/140050
de Boer WF (2007) Seagrass–sediment interactions, positive feedbacks and critical thresholds for occurrence: a review. Hydrobiologia 591:5–24. https://doi.org/10.1007/s10750-007-0780-9
Demers MCA, Davis AR, Knott NA (2013) A comparison of the impact of ‘seagrass-friendly’ boat mooring systems on Posidonia australis. Mar Environ Res 83:54–62. https://doi.org/10.1016/j.marenvres.2012.10.010
Duarte CM (1991) Allometric scaling of seagrass form and productivity. Mar Ecol Prog Ser 77:289–300
Duarte CM (2002) The future of seagrass meadows. Environ Conserv 29:192–206. https://doi.org/10.1017/S0376892902000127
Duarte CM, Sand-Jensen K (1995) Seagrass colonization: patch formation and patch growth in Cymodocea nodosa. Mar Ecol Prog Ser 65:193–200
Duarte CM, Terrados J, Agawin NSR, Fortes MD, Bach S, Kenworthy WJ (1997) Response of a mixed Philippine seagrass meadow to experimental burial. Mar Ecol Prog Ser 147:285–294. https://doi.org/10.3354/meps147285
Duarte CM, Fourqurean JW, Krause-Jensen D, Olesen B (2006) Dynamics of seagrass stability and change. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, The Netherlands, pp 271–294
Dunic JC, Brown CJ, Connolly RM, Turschwell MP, Côté IM (2021) Long-term declines and recovery of meadow area across the world’s seagrass bioregions. Glob Change Biol 27:4096–4109. https://doi.org/10.1111/gcb.15684
Environment Protection and Biodiversity Conservation Act (1999) (s266B) Approved Conservation Advice (Including Listing Advice) for Posidonia Australis Seagrass Meadows of the Manning-Hawkesbury Ecoregion Ecological Community; Office of Legislative Drafting and Publishing, Canberra, Australia, 2015
Eriander L, Laas K, Bergström P, Gipperth L, Moksnes P (2017) The effects of small-scale coastal development on the eelgrass (Zostera marina L.) distribution along the Swedish west coast–Ecological impact and legal challenges. Ocean Coast Manage 148:182–194. https://doi.org/10.1016/j.ocecoaman.2017.08.005
Evans SM, Griffin KJ, Blick RAJ, Poore AGB, Vergés A (2018) Seagrass on the brink: decline of threatened seagrass Posidonia australis continues following protection. PLoS ONE 13:e0190370. https://doi.org/10.1371/journal.pone.0190370
Everett RA, Ruiz GM, Carlton JT (1995) Effect of oyster mariculture on submerged aquatic vegetation: an experimental test in a Pacific Northwest estuary. Mar Ecol Prog Ser 125:205–217. https://doi.org/10.3354/meps125205
Ferguson AM, Harvey ES, Knott NA (2016) Herbivore abundance, site fidelity and grazing rates on temperate reefs inside and outside marine reserves. J Exp Mar Bio Ecol 478:96–105. https://doi.org/10.1016/j.jembe.2016.02.008
Ferguson A, Scanes P, Potts J, Adams M, O’Brien K (2018) Seagrasses in the South-East Australian Region—distribution, metabolism, and morphology in response to hydrodynamic, substrate, and water quality stressors. In: Larkum AWD, Kendrick GA, Ralph PJ (eds) Seagrasses of Australia: structure ecology and conservation. Springer, Switzerland, pp 419–444
Ferretto G, Vergés A, Poore AGB, Gribben PE, Glasby TM (2022) Floating bags have the potential to minimise oyster farming impacts on Posidonia australis seagrass meadows. Aquaculture 560:738594. https://doi.org/10.1016/j.aquaculture.2022.738594
Fisheries Management Act (1994) NSW legislation. https://www.legislation.nsw.gov.au/view/html/inforce/current/act-1994-038. Accessed 05 May 2023
Fisher R, Wilson SK, Sin TM, Lee AC, Langlois TJ (2018) A simple function for full-subsets multiple regression in ecology with R. Ecol Evol 8:6104–6113. https://doi.org/10.1002/ece3.4134
Fitzpatrick J, Kirkman H (1995) Effects of prolonged shading stress on growth and survival of seagrass Posidonia australis in Jervis Bay, New South Wales. Mar Ecol Prog Ser 127:279–289. https://doi.org/10.3354/meps127279
Fraschetti S, Guarnieri G, Bevilacqua S, Terlizzi A, Boero F (2013) Protection enhances community and habitat stability: evidence from a mediterranean marine protected area. PLoS ONE 8:e81838. https://doi.org/10.1371/journal.pone.0081838
Fyfe SK, Davis AR (2007) Spatial scale and the detection of impacts on the seagrass Posidonia australis following pier construction in an embayment in southeastern Australia. Estuar Coast Shelf Sci 74:297–305. https://doi.org/10.1016/j.ecss.2007.04.022
Gillanders BM (2006) Seagrasses, fish, and fisheries. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, The Netherlands, pp 503–505
Gladstone W, Courtenay G (2014) Impacts of docks on seagrass and effects of management practices to ameliorate these impacts. Estuar Coast Shelf Sci 136:53–60. https://doi.org/10.1016/j.ecss.2013.10.023
Glasby TM, West G (2018) Dragging the chain: Quantifying continued losses of seagrasses from boat moorings. Aquat Conserv 28:383–394. https://doi.org/10.1002/aqc.2872
Grech A, Coles R (2010) An ecosystem-scale predictive model of coastal seagrass distribution. Aquat Conserv 20:437–444. https://doi.org/10.1002/aqc.1107
Griffiths LL, Connolly RM, Brown CJ (2020) Critical gaps in seagrass protection reveal the need to address multiple pressures and cumulative impacts. Ocean Coast Manage 183:104946. https://doi.org/10.1016/j.ocecoaman.2019.104946
Guisan A, Edwards TC, Hastie T (2002) Generalized linear and generalized additive models in studies of species distributions: setting the scene. Ecol Model 157:89–100. https://doi.org/10.1016/S0304-3800(02)00204-1
Gulick AG, Johnson RA, Pollock CG, Hillis-Starr Z, Bolten AB, Bjorndal KA (2021) Recovery of a cultivation grazer: a mechanism for compensatory growth of Thalassia testudinum in a Caribbean seagrass meadow grazed by green turtles. J Ecol 109:3031–3045. https://doi.org/10.1111/1365-2745.13718
Halpern BS, Lester SE, McLeod KL (2010) Placing marine protected areas onto the ecosystem-based management seascape. Proc Natl Acad Sci 107:18312–18317. https://doi.org/10.1073/pnas.090850310
Hauxwell J, Valiela I (2004) Effects of nutrient loading on shallow seagrass-dominated coastal systems: patterns and processes. In: Nielsen SL, Banta GT, Pedersen MF (eds) Estuarine nutrient cycling: the influence of primary producers. Kluwer Academic Publishers, The Netherlands, pp 59–82
Howard RK, Short FT (1986) Seagrass growth and survivorship under the influence of epiphyte grazers. Aquat Bot 24:287–302. https://doi.org/10.1016/0304-3770(86)90063-X
Howard J, McLeod E, Thomas S, Eastwood E, Fox M, Wenzel L, Pidgeon E (2017) The potential to integrate blue carbon into MPA design and management. Aquat Conserv 27:100–115. https://doi.org/10.1002/aqc.2809
Howarth L, Lewis-McCrea L, Kellogg L, Apostolaki E, Reid G (2022) Aquaculture and eelgrass Zostera marina interactions in temperate ecosystems. Aquacult Environ Interact 14:15–34. https://doi.org/10.3354/aei00426
Kendrick GA, Duarte CM, Marbà N (2005) Clonality in seagrasses, emergent properties and seagrass landscapes. Mar Ecol Prog Ser 290:291–296. https://doi.org/10.3354/meps290291
Kendrick GA, Holmes KW, Van Niel KP (2008) Multi-scale spatial patterns of three seagrass species with different growth dynamics. Ecography 31:191–200. https://doi.org/10.1111/j.0906-7590.2008.5252.x
Kilminster K, McMahon K, Waycott M, Kendrick GA, Scanes P, McKenzie L, O’Brien KR, Lyons M, Ferguson A, Maxwell P, Glasby TM, Udy JW (2015) Unravelling complexity in seagrass systems for management: Australia as a microcosm. Sci Tot Environ 534:97–109. https://doi.org/10.1016/j.scitotenv.2015.04.061
Knott NA, Williams J, Harasti D, Malcolm HA, Coleman MA, Kelaher BP, Rees MJ, Schultz A, Jordan A (2021) A coherent, representative, and bioregional marine reserve network shows consistent change in rocky reef fish assemblages. Ecosphere 12:e03447. https://doi.org/10.1002/ecs2.3447
Krause-Jensen D, Duarte CM, Sand-Jensen K, Carstensen J (2021) Century-long records reveal shifting challenges to seagrass recovery. Glob Change Biol 27:563–575. https://doi.org/10.1111/gcb.15440
La Manna G, Donno Y, Sarà G, Ceccherelli G (2015) The detrimental consequences for seagrass of ineffective marine park management related to boat anchoring. Mar Pollut Bull 90:160–166. https://doi.org/10.1016/j.marpolbul.2014.11.001
Lambert V, Bainbride ZT, Collier CJ, Lewis SE, Adams MP, Carter AB, Saunders MI, Brodie J, Turner RDR, Rasheed MA, O’Brien KR (2021) Connecting targets for catchment sediment loads to ecological outcomes for seagrass using multiple lines of evidence. Mar Pollut Bull 169:112494. https://doi.org/10.1016/j.marpolbul.2021.112494
Larkum AWD (1976) Ecology of Botany Bay. I. Growth of Posidonia australis (Brown) Hook. f. in Botany Bay and other bays of the Sydney basin. Mar Fresh Res 27:117–127. https://doi.org/10.1071/MF9760117
Larkum AWD, West RJ (1990) Long-term changes of seagrass meadows in Botany Bay, Australia. Aquat Bot 37:55–70. https://doi.org/10.1016/0304-3770(90)90064-R
Lepoint G, Millet S, Dauby P, Gobert S, Bouquegneau JM (2002) Annual nitrogen budget of the seagrass Posidonia oceanica as determined by in situ uptake experiments. Mar Ecol Prog Ser 237:87–96. https://doi.org/10.3354/meps237087
Levin SA (1992) The problem of pattern and scale in ecology: the Robert H. MacArthur award lecture. Ecology 73:1943–1967. https://doi.org/10.2307/1941447
Loflin RK (1995) The effects of docks on seagrass beds in the Charlotte Harbor estuary. Flor Sci 58:198–205
Macreadie P, Baird M, Trevathan-Tackett S, Larkum A, Ralph P (2014) Quantifying and modelling the carbon sequestration capacity of seagrass meadows—a critical assessment. Mar Pollut Bull 83:430–439. https://doi.org/10.1016/j.marpolbul.2013.07.038
Marbà N, Duarte CM, Holmer M, Martínez R, Basterretxea G, Orfila A, Jordi A, Tintoré J (2002) Effectiveness of protection of seagrass (Posidonia oceanica) populations in Cabrera National Park (Spain). Environ Conserv 29:509–518. https://doi.org/10.1017/S037689290200036X
McMahon K, Kilminster K, Canto R, Roelfsema C, Lyons M, Kendrick GA, Waycott M, Udy J (2022) The risk of multiple anthropogenic and climate change threats must be considered for continental scale conservation and management of seagrass habitat. Front Mar Sci 9:837259. https://doi.org/10.3389/fmars.2022.837259
Meehan AJ, West RJ (2000) Recovery times for a damaged Posidonia australis bed in south eastern Australia. Aquat Bot 67:161–167. https://doi.org/10.1016/S0304-3770(99)00097-2
Milazzo M, Badalamenti F, Ceccherelli G, Chemello R (2004) Boat anchoring on Posidonia oceanica beds in a marine protected area (Italy, western Mediterranean): effect of anchor types in different anchoring stages. J Exp Mar Biol Ecol 299:51–62. https://doi.org/10.1016/j.jembe.2003.09.003
NSW Department of Primary Industries (2021a) Fisheries spatial data portal. https://webmap.industry.nsw.gov.au/Html5Viewer/index.html?viewer=Fisheries_Data_Portal
NSW Department of Primary Industries (2021b) NSW estuarine habitat dashboard. https://nsw-dpi.shinyapps.io/NSW_Estuarine_Habitat/
NSW OEH (2010) State of the catchments report 2010. NSW Office of Environment and Heritage, Sydney
NSW Department of Planning, Industry and Environment (2019) NSW landuse 2017. https://datasets.seed.nsw.gov.au/dataset/nsw-landuse-2017
O’Brien KR, Waycott M, Maxwell P, Kendrick GA, Udy JW, Ferguson AJP, Kilminster K, Scanes P, McKenzie LJ, McMahon K, Adams MP, Samper-Villarreal J, Collier C, Lyons M, Mumby PJ, Radke L, Christianen MJA, Dennison WC (2018) Seagrass ecosystem trajectory depends on the relative timescales of resistance, recovery and disturbance. Mar Pollut Bull 134:166–176. https://doi.org/10.1016/j.marpolbul.2017.09.006
Orth R (1977) The importance of sediment stability in seagrass communities. In: Coull BC (ed) Ecology of marine benthos. University of South Carolina Press, Columbia, pp 281–300
Orth RJ, van Montfrans J (1984) Epiphyte-seagrass relationships with an emphasis on the role of micrograzing: a review. Aquat Bot 18:43–69. https://doi.org/10.1016/0304-3770(84)90080-9
Orth RJ, Carruthers TJB, Dennison WC, Duarte CM, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Olyarnik S, Short FT, Waycott M, Williams SL (2006) A global crisis for seagrass ecosystems. Bioscience 56:987–996. https://doi.org/10.1641/0006-3568(2006)56[987:AGCFSE]2.0.CO;2
Paling EI, McComb AJ (2000) Autumn biomass, below-ground productivity, rhizome growth at bed edge and nitrogen content in seagrasses from Western Australia. Aquat Bot 67:207–219. https://doi.org/10.1016/S0304-3770(00)00092-9
Planes S, Raventos N, Ferrari B, Alcoverro T (2011) Fish herbivory leads to shifts in seagrass Posidonia oceanica investments in sexual reproduction. Mar Ecol Prog Ser 431:205–213. https://doi.org/10.3354/meps09089
Prado P, Farina S, Tomas F, Romero J, Alcoverro T (2008) Marine protection and meadow size alter fish herbivory in seagrass ecosystems. Mar Ecol Prog Ser 371:11–21. https://doi.org/10.3354/meps07662
Quiros TAL, Croll D, Tershy B, Fortes MD, Raimondi P (2017) Land use is a better predictor of tropical seagrass condition than marine protection. Biol Conserv 209:454–463. https://doi.org/10.1016/j.biocon.2017.03.011
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/
Rees MJ, Knott NA, Hing ML, Hammond M, Williams J, Neilson J, Swadling DS, Jordan A (2021) Habitat and humans predict the distribution of juvenile and adult snapper (Sparidae: Chrysophrys auratus) along Australia’s most populated coastline. Estuar Coas Shelf Sci 257:107397. https://doi.org/10.1016/j.ecss.2021.107397
Roper T, Creese B, Scanes P, Stephens K, Williams R, Dela-Cruz J, Coade G, Coates B, Fraser M (2011) Assessing the condition of estuaries and coastal lake ecosystems in NSW, monitoring, evaluation and reporting program, technical report series. NSW Office of Environment and Heritage, Sydney
Roy PS, Williams RJ, Jones AR, Yassini I, Gibbs PJ, Coates B, West RJ, Scanes P, Hudson JP, Nichol S (2001) Structure and function of south-east Australian estuaries. Estuar Coast Shelf Sci 53:351–384. https://doi.org/10.1006/ecss.2001.0796
Sagerman J, Hansen JP, Wikström SA (2020) Effects of boat traffic and mooring infrastructure on aquatic vegetation: a systematic review and meta-analysis. Ambio 49:517–530. https://doi.org/10.1007/s13280-019-01215-9
Saunders MI, Atkinson S, Klein CJ, Weber T, Possingham HP (2017) Increased sediment loads cause non-linear decreases in seagrass suitable habitat extent. PLoS ONE 12:e0187284. https://doi.org/10.1371/journal.pone.0187284
Short FT, Burdick DM (1996) Quantifying eelgrass habitat loss in relation to housing development and nitrogen loading in Waquoit Bay, Massachusetts. Estuaries 19:730–739. https://doi.org/10.2307/1352532
Skinner MA, Courtenay SC, McKindsey CW, Carver CE, Mallet AL (2014) Experimental determination of the effects of light limitation from suspended bag oyster (Crassostrea virginica) aquaculture on the structure and photosynthesis of eelgrass (Zostera marina). J Exp Mar Biol Ecol 459:169–180. https://doi.org/10.1016/j.jembe.2014.05.012
Spalding M, Fish L, Wood LJ (2008) Toward representative protection of the world’s coasts and oceans—progress, gaps, and opportunities. Conserv Lett 1:217–226. https://doi.org/10.1111/j.1755-263X.2008.00030.x
Strachan LL, Lilley RJ, Hennige SJ (2022) A regional and international framework for evaluating seagrass management and conservation. Mar Pol 146:105306. https://doi.org/10.1016/j.marpol.2022.105306
Swadling DS, West GJ, Gibson PT, Laird RJ, Glasby TM (2022) Don’t go breaking apart: anthropogenic disturbances predict meadow fragmentation of an endangered seagrass. Aquat Conserv 33:56–69. https://doi.org/10.1002/aqc.3905
Tin HC, Uyen NT, Hieu DV, Ni TN, Tu NH, Saizen I (2020) Decadal dynamics and challenges for seagrass beds management in Cu Lao Cham Marine Protected Area, Central Vietnam. Environ Dev Sustain 22:7639–7660. https://doi.org/10.1007/s10668-019-00540-z
Todd PA, Heery EC, Loke LH, Thurstan RH, Kotze DJ, Swan C (2019) Towards an urban marine ecology: characterizing the drivers, patterns and processes of marine ecosystems in coastal cities. Oikos 128:1215–1242. https://doi.org/10.1111/oik.05946
Travaille KL, Salinas-de-León P, Bell JJ (2015) Indication of visitor trampling impacts on intertidal seagrass beds in a New Zealand marine reserve. Ocean Coast Manage 114:145–150. https://doi.org/10.1016/j.ocecoaman.2015.06.002
Turschwell MP, Connolly RM, Dunic JC, Sievers M, Buelow CA, Pearson RM, Tulloch VJD, Côté IM, Unsworth RKF, Collier CJ, Brown CJ (2021) Anthropogenic pressures and life history predict trajectories of seagrass meadow extent at a global scale. Proc Natl Acad Sci 118:e2110802118. https://doi.org/10.1073/pnas.2110802118
Underwood AJ (1993) The mechanics of spatially replicated sampling programmes to detect environmental impacts in a variable world. Austral J Ecol 18:99–116. https://doi.org/10.1111/j.1442-9993.1993.tb00437.x
Unsworth RKF, McKenzie LJ, Collier CJ, Cullen-Unsworth LC, Duarte CM, Eklöf JS, Jarvis JC, Jones BL, Nordlund LM (2019) Global challenges for seagrass conservation. Ambio 48:801–815. https://doi.org/10.1007/s13280-018-1115-y
Valentine JF, Heck KL, Busby J, Webb D (1997) Experimental evidence that herbivory increases shoot density and productivity in a subtropical turtlegrass (Thalassia testudinum ) meadow. Oecologia 112:193–200. https://doi.org/10.1007/s004420050300
Vidondo B, Duarte CM, Middelboe AL, Stefansen K, Lützen T, Nielsen SL (1997) Dynamics of a landscape mosaic: size and age distributions, growth and demography of seagrass Cymodocea nodosa patches. Mar Ecol Prog Ser 158:131–138. https://doi.org/10.3354/meps158131
Walker DI, Lukatelich RJ, Bastyan G, McComb AJ (1989) Effect of boat moorings on seagrass beds near Perth, Western Australia. Aquat Bot 36:69–77. https://doi.org/10.1016/0304-3770(89)90092-2
Walker DI, Kendrick GA, McComb AJ (2006) Decline and recovery of seagrass ecosystems—the dynamics of change. In: Larkum AWD, Orth RJ, Duarte CM (eds) Seagrasses: biology, ecology and conservation. Springer, The Netherlands, pp 551–565
Waycott M, Duarte CM, Carruthers TJB, Orth RJ, Dennison WC, Olyarnik S, Calladine A, Fourqurean JW, Heck KL, Hughes AR, Kendrick GA, Kenworthy WJ, Short FT, Williams SL (2009) Accelerating loss of seagrasses across the globe threatens coastal ecosystems. Proc Natl Acad Sci 106:12377. https://doi.org/10.1073/pnas.0905620106
West R (2011) Impacts of recreational boating activities on the seagrass Posidonia in SE Australia. Wetlands Austr J 26:3–13. https://doi.org/10.31646/wa.295
West GJ, Glasby TM (2021) Interpreting long-term patterns of seagrasses abundance: how seagrass variability is dependent on genus and estuary type. Estuar Coast 45:1393–1408. https://doi.org/10.1007/s12237-021-01026-w
West R, Larkum AWD, King R (1989) Regional studies—seagrasses of south eastern Australia. In: Larkum AWD, McComb A, Shepherd S (eds) Biology of seagrasses. A treatise on the biology of seagrasses with special reference to the Australian region. Elsevier, The Netherlands, pp 230–260
Wickham H (2016) ggplot2: elegant graphics for data analysis. Springer-Verlag, New York
Wickham H, François R, Henry L, Müller K (2022). dplyr: a grammar of data manipulation. R package version 1.0.10. https://CRAN.R-project.org/package=dplyr.
Wiens JA (1989) Spatial scaling in ecology. Funct Ecol 3:385–397. https://doi.org/10.2307/2389612
Wood SN (2011) Fast stable restricted maximum likelihood and marginal likelihood estimation of semiparametric generalized linear models. J Roy Stat Soc (b) 73:3–36. https://doi.org/10.1111/j.1467-9868.2010.00749.x
Acknowledgements
The authors acknowledge the traditional owners and custodians of the coastal environments where this research was completed and pay our respect to Elders past, present and future. We would like to thank the numerous people who contributed to the creation of the maps, in particular Ron West, Rob Williams, Trudy Walford, Chris Gallen, Isabelle Thiebaud, Brooke McCartin, Joe Neilson, Danielle Brown and Graham Housefield. We thank Carla Ganassin, Rodney James, Lesley Diver, Matthew Rees, Rachel Przeslawski and Anthony Zammit for discussion and review of the draft manuscript.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This work was funded by the NSW Government under the Marine Estate Management Strategy.
Author information
Authors and Affiliations
Contributions
DSS: conceptualisation, data curation, formal analysis, investigation, methodology, writing—original draft, writing—review and editing. GJW: conceptualisation, data curation, investigation, methodology, writing—review and editing. PTG: data curation, investigation, writing—review and editing. RJL: data curation, investigation, writing—review and editing. TMG: conceptualisation, data curation, investigation, methodology, project administration, resources, supervision, writing—original draft, writing—review and editing.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare no conflict of interest.
Ethical approval
No ethics approval was required for this study.
Additional information
Responsible Editor: C. Wild.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Swadling, D.S., West, G.J., Gibson, P.T. et al. Multi-scale assessments reveal changes in the distribution of the endangered seagrass Posidonia australis and the role of disturbances. Mar Biol 170, 147 (2023). https://doi.org/10.1007/s00227-023-04279-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04279-0