Abstract
In this study, we explored the feasibility of using the beaks of the African cuttlefish Sepia bertheloti for age estimation and growth analysis. The rostrum sagittal section (RSS) of the lower beak was the most suitable region in the species. It was applied in samples caught off Morocco and Guinea-Bissau between June 2018 and January 2020. A maximum life expectancy of around 14 months was observed (specifically 419 days for cuttlefishes from Morocco and 433 from Guinea-Bissau). The males presented greater longevity, as the maximum age of the females was between 9 and 11 months. Sepia bertheloti showed a negative allometric growth; however, the exponential model better describes each population growth. By sexes, the males of both locations followed an asymptotic growth model while the females exhibited a non-asymptotic growth. The growth rates were different between locations, with the highest values in Guinea-Bissau. The males, in turn, grew faster for both study locations. In Guinea-Bissau, these growth differences were influenced by the hatching season since individuals born between autumn and winter were the fastest-growing. Samples from Morocco did not show growth differences between the hatching season and other seasons. These results indicate that the RSS of lower beaks are suitable for estimating the age, growth pattern, and population structure of Sepia bertheloti.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The African cuttlefish Sepia bertheloti (Orbigny, 1839) is distributed in the Eastern Atlantic from the Canary Islands and Western Sahara to Angola, predominantly occupying sandy bottoms from 20 to 160 m (Jereb and Roper 2005; Guerra et al. 2014). It can reach sizes of a 180 mm mantle length for males and 130 mm for females (Guerra et al. 2014). This species is caught by bottom trawlers at depths between 70 and 140 m (Roper et al. 1984), with greater frequency in the fisheries operating off the West African coast (FAO Fishing Area 34). It is one of the most productive regions of the world (Martos and Peralta 1995; Doumbouya et al. 2017) due to the upwelling systems off of the Western Sahara, Senegal, and Guinea-Bissau (Berrit and Rebert 1977).
There are no separate capture statistics for S. bertheloti, as it is often traded together with S. officinalis in Moroccan/Saharan waters, with S. hierredda in Guinean waters or treated as commercial by-catch. Sepia bertheloti accounts for 11–35% of reported by-catch and is marketed fresh and/or deep-frozen for export (Jereb and Roper 2005). The target cephalopod species for the Western Sahara/Morocco area are Octopus vulgaris, S. officinalis, and Loligo vulgaris (Hernández García and Castro 1994), while for the Guinea-Bissau fishing grounds, the target species are O. vulgaris and S. hierredda (FAO 2021). Furthermore, probably due to the low density and commercial value, the biological and fishery information about S. bertheloti is very limited in comparison to the fishing industry’s cuttlefishes. Most of this information has been acquired by analyzing the stomach contents of its natural predators (Delgado de Molina et al. 1993; Monzón-Argüello et al. 2018), by conducting exploratory fishing campaigns to assess the state of the resources (Balguerías et al. 1993; Rocha et al. 2017; García-Isarch et al. 2009; Perales-Raya et al. 2010a), or from the analysis of metal concentrations (Ama-Abasi and Akpan 2008). However, there is no information about age and growth of S. bertheloti, which are both key factors in estimating life history parameters and understanding its population dynamics in order to conduct an appropriate biomass estimation available for fishing (Arreguín-Sanchez et al. 1996).
The use of indirect methods off growth estimation based on length–frequency analysis is not suitable for age estimation in cephalopods since they are semelparous species with short life cycles and high variations among individuals, making their estimation imprecise (Jackson 2004). Direct methods using hard structures, such as statoliths, beaks, lenses, or gladius, based on the study of growth increments have proven to be the most useful method for estimating the absolute age and the growth of cephalopods. Growth increment analysis in jaws has been shown to be an appropriate technique for age determination, validated in other benthic cephalopods, such as O. vulgaris (e.g., Hernández-López et al. 2001; Canali et al. 2011; Perales-Raya et al. 2014b; Armelloni et al. 2020), O. maya (e.g., Rodríguez-Domínguez et al. 2013; Bárcenas et al. 2014), or Sepia officinalis (Guerra-Marrero et al. 2023). The recent revision of Xavier et al. (2022) provides detailed information on the attempted study of other cephalopod species using beak increment analysis, as well as those with confirmed daily deposition and validated ontogenetic stages.
The processing of cephalopod’s hard structures is usually a time-consuming process, but it enables age estimates with a high rate of precision and is useful for identifying seasonal cohorts, an important component of cephalopod stock assessment. Nevertheless, the great variety of the growth rates (exhibited by individuals of the same age with different lengths) makes age-based models impractical for real-time stock assessment (Arkhipkin et al. 2021). Arkhipkin et al. (2021) propose a range of methodologies for assessing cephalopod populations, while warning that there is a high data requirement with a constant catch per unit effort report (CPUE) required. The abundance estimates of S. bertheloti in the area encompass other cuttlefish species, such as S. officinalis for Northwest Africa and S. hierredda for Central-West Africa (Jereb and Roper 2005); therefore, age analysis using hard structures is a suitable approach for future stock assessments.
Based on previous studies of other cuttlefish species, it is known that sepias have high growth rates (Perales-Raya et al. 1994; Perales-Raya 2001; Bettencourt and Guerra 2001; Guerra 2006) although size/weight differences have been observed between the populations of these species along the large Canary Current marine ecosystem (Hernández-García and Castro 1994; Hernández-López 2000; Almonacid-Rioseco 2006; Jurado-Ruzafa et al. 2014).
This study contributes to (i) age and life span estimations of S. bertheloti in wild populations using the beak microstructure, (ii) the calculation of the hatching periods, (iii) determining the best model to describe the population growth pattern, and (iv) assessing the growth rates by seasons, with the final goal of obtaining these data for the future stock assessment of the African cuttlefish.
Materials and methods
The sample
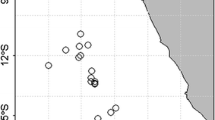
A total of 1124 individuals of the African cuttlefish Sepia bertheloti were collected from June 2018 to January 2020 in two locations of Northwest Africa (449 individuals from Morocco and 625 from Guinea-Bissau) from commercial trawlers operating in each study location (Fig. 1). The subsequent data sampling is summarized in Table 1.
The cuttlefish were immediately frozen at −20ºC after fishing and remained frozen until they were processed in the laboratory. Dorsal mantle length (DML) and body weight (BW) were measured to the nearest 1 mm and 0.01 g respectively. Sex was noted and maturity stages were identified according to the macroscopically maturity scale proposed by ICES (2010) for Sepia officinalis (namely, 0 is undetermined, 1 is Immature, 2a as Developing, 2b is Maturing, 3a is Mature/Spawning and 3b is Spent). Beak extraction and measurements were taken according to Perales-Raya et al. (2010b); Hernández-García (2003), respectively. The individuals caught in both locations were categorized according to the capture season (spring, summer, autumn, and winter).
Length–weight relationship
The length–weight relationship (LWR) was calculated using the equation BW = aDMLb (power function), where a and b are the regression parameters estimated by linear regression of the data logarithmically transformed and adjusted by the least squares method. Student’s t test was used to verify the ‘b’ values to determine whether they have isometric (b = 3) or allometric (negative allometric b < 3, and positive allometric b > 3) growth.
Beak analysis
After dissection, the beaks were extracted, cleaned, and stored in distilled water at a temperature of 4ºC, according to the procedure described by Perales-Raya et al. (2014b). A beak subsample of 78 specimens from Morocco and 128 from Guinea-Bissau were analyzed. A randomized categorizing process of the specimens was performed by size range within 5 mm of DML for both sexes.
After an analysis of the different regions of the upper and lower jaws was performed, the rostrum sagittal sections (RSS) of the lower beak were selected according to Perales-Raya et al. (2010b; 2014a). Once the beaks were processed, they were analyzed using a Nikon Microscope Multizoom AZ100 with a UV epi-illumination attachment (providing vertical reflected light) and different magnifications (100–400x) to observe the growth increments (Fig. 2).
The observed increments were counted twice by the same trained reader. The coefficient of variation (CV) was used to estimate the precision of the readings and the reproducibility of the method. The CV was calculated as the ratio of the standard deviation over the mean:
where R1 and R2 were the numbers of increments from the first and the second reading, respectively. R was the average of the number of increments of the two readings. According to Campana (2001), to avoid any bias, the mean of CV was averaged for each study location, and a CV < 7.6% was taken as valid, with higher values of CV being rejected.
The relationship between the rostral length of lower beak (LRL) and the number of increments (NI) was calculated to observe the growth of the reading area according to the estimated age. The analysis of covariance (ANCOVA) was carried out to determine possible significant differences in LRL–DML and LRL–NI relationships according to sex and study locations. These relationships and all the different analysis were performed using the second reading (R2) according to Perales-Raya et al. (2010b), who postulate that R2 is more reliable since the reader has greater experience and practice.
Growth models and growth rate estimation
Length-at-age data of Sepia bertheloti were fitted into seven growth models (lineal, power, exponential, von Bertalanffy, Gompertz, logistic, and Schnute (a ≠ 0 and b ≠ 0) models). According to Bolser et al. (2018), model parameters for von Bertalanffy, Gompertz, logistic, and Schnute were estimated using a non-linear least squares regression and the Levenberg–Marquardt algorithm, and confidence limits were placed around parameter estimates in R studio (using the R packages Ogle 2017; Elzhov et al. 2015; Baty et al. 2015):
-
von Bertalanffy growth model (Von Bertalanffy 1938): \({ }L\left( t \right) = L_{\infty } \left[ {1 - e^{{ - K\left( {t - t_{0} } \right)}} } \right]\)
-
Gompertz growth model (Gompertz 1825): \({ }L\left( t \right) = L_{\infty } e^{{\left( { - \left( \frac{1}{K} \right)e^{{ - K\left( {t - t_{0} } \right)}} } \right)}}\)
-
Logistic growth model (Ricker 1975): \({ }L\left( t \right) = L_{\infty } \left[ {1 + e^{{ - K\left( {t - t_{0} } \right)}} } \right]^{ - 1}\)
-
Schnute growth model (Schnute 1981): \({ }L\left( t \right) = \left[ {L_{1}^{b} + \left( {L_{2}^{b} - L_{1}^{b} } \right)\frac{{1 - e^{{ - a\left( {t - T_{1} } \right)}} }}{{1 - e^{{ - a\left( {T_{2} - T_{1} } \right)}} }}} \right]^{{{\raise0.7ex\hbox{$1$} \!\mathord{\left/ {\vphantom {1 b}}\right.\kern-0pt} \!\lower0.7ex\hbox{$b$}}}}\)
where L(t) is length (in mm DML) at age t, L∞ is the maximum average length (in mm DML), K is the growth rate coefficient (in year −1), and t0 is the theoretical age at which length is zero (in years). For the Schnute growth model, T1 is the first specified age, T2 is the second specified age, L1 is length at age T1; L2 is length at age T2, a is the constant relative rate of relative growth (in year −1), and b is the incremental relative rate of relative growth (dimensionless). Since the Schnute model does not calculate the parameter L∞ directly, the following equation must be used (Schnute 1981):
The parameters L1, L2, T1, and T2 are the same as used in the Schnute equation previously, while the parameters a and b are the resulting parameters of the growth model.
The best model for each sex and area was determined using the Akaike’s information criterion (AIC) (Akaike 1974), transformed to Akaike weight (AICw) (Burnham and Anderson 2002) and the Bayesian Information Criterion (BIC) using the “AICcmodavg” package in R (Mazerolle and Mazerolle 2017). Akaike weights provide relative likelihood of each model from the tested set of models.
Estimated growth rates for length-at-age relationships were calculated for each 90-day age class according to the following equations (Forsythe and Van Heukelem 1987; Gonzalez et al. 1996):
a. Instantaneous relative growth rate, G (% DML d.−1)
b. Absolute growth rate, AGR (mm d.−1)
Hatching date estimation
To study the influence of seasonality on growth, the hatching date of each cuttlefish was back-calculated according to estimated age and the date of capture. According to hatch dates, four seasonal hatching groups were defined: Spring (March–May), Summer (June–August), Autumn (September–November), and Winter (December–February). Kruskal–Wallis test and post hoc Dunn test were used to describe possible differences between hatching seasons.
The normal distribution of the data was checked using the Shapiro–Wilk test in each analysis. When the data showed a normal distribution, a two-group independent t test was used to compare differences in age according to the location and differences in age by sex. ANCOVA was also used to analyze differences between locations and sexes. When a normal distribution was not achieved, a non-parametric Kruskal–Wallis test and a Dunn post hoc test were used. All statistical analyses were carried out using R v-4.1.1 (R Core Team 2022).
Results
Size–structure
Cuttlefish lengths and weights from Morocco and Guinea-Bissau are shown in Table 2. Mature males from Morocco ranged from 50 to 130 mm DML while mature females ranged from 60 to 110 mm (Fig. 3). Guinea-Bissau’s mature males fell within a range off 60–176 mm DML, while mature females ranged from 68 to 140 mm (Fig. 4). Immature and developing/maturing individuals were not found in Morocco (Fig. 3), while all reproductive stages were found in Guinea-Bissau (Fig. 4).
The regression parameters of the DML–BW relationship (power equation) for males and females from Morocco and Guinea-Bissau are shown in Table 3.
The ANCOVA test showed significant differences in DML–BW relationships between sex and locations (p < 0.0001). Males of both locations were larger and heavier than the females, and the specimens from Morocco were smaller and lighter than those from Guinea-Bissau.
In terms of growth, the LWRs exhibited negative allometry (See Table 3) for both sexes and locations. This growth model implies a faster growth in DML than in BW.
Age analysis
Of the 206 beaks analyzed, 183 allowed a reliable reading. Twenty-three beaks (11.17%) were discarded due to malformations (see image in supplementary material) or severe damage during grinding that made a reliable reading impossible. Of the 183 beaks, 69 belonged to cuttlefish from Morocco (Table 4) with 114 belonging to specimens taken from Guinea-Bissau (Table 5).
From the Morocco sample, the youngest specimen was a female (111 days old, 60 mm DML), and the oldest specimen was male (419 days, 140 mm DML). No significant difference in age was found between sexes (t test: p = 0.09034), with the median age being 186 ± 41 days in females and 220 ± 70 days in males. The oldest specimen was caught in summer 2018, while the youngest was caught during winter 2020 (see Table 4). Mature females were found between 111 and 234 days while the mature males were found between 11 and 370 days (see Fig. 5).
Cuttlefishes from Guinea-Bissau showed an estimated age between 94 and 433 days, with a mean age of 219 ± 74 days. The youngest specimen was a 94-day-old female (72 mm DML), and the oldest specimen was a 433-day-old male (160 mm DML). The youngest male was 122 days old (85 mm DML), and the oldest female was 316 days old (152 mm DML). Statistically significant difference in age was found between the sexes (t test: p = 0.0082), with a mean age of 198 ± 56 days in females and 235 ± 78 days in males. The oldest specimen was caught in summer 2019, while the youngest individual was caught during the fall of the same year (see Table 5). Mature females were found between 126 and 289 days while the mature males were found between 118 and 400 days (see Fig. 6).
No significant difference in age was found between locations (t test: p = 0.3285) and sexes (t test: p = 0.784 for females and p = 0.354 for males). The mean reading precision value (CV) off the beaks readings was 2.72 ± 3.95% for specimens from Guinea-Bissau and 2.61 ± 3.54% for specimens from Morocco. Correlations between NI, LRL, and DML for both locations are shown in Table 6. In Morocco, the relationship between LRL and DML for both sexes was best fitted by the exponential model (Table 6). Moreover, the exponential model was the best model to describe the relationship between LRL and NI (Table 7). ANCOVA showed that there were statistically significant differences between the sexes with respect to the DML–LRL relationship (p = 0.022), but no significant differences were found in the LRL–NI relationship (p = 0.587). Also, in the LRL–NI relationship, greater variability was observed in males than in females, which resulted in lower R2 values. In the case of Guinea-Bissau for both sexes, the relationship between LRL and DML was best described by a linear model (Table 7). In the case of females, the best LRL–NI relationship fits the linear model, while this relationship in males was best described by the power model (Table 7). ANCOVA showed that there was a statistically significant difference in the DML–LRL relationship of both sexes (p = 0.001) but not in the LRL–NI (p = 0.155) relationship. A greater variability of the DML–LRL and LRL–NI relationships was observed in males than in females, which resulted in lower R2 values observed in samples from Morocco.
Growth models and growth rates
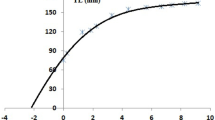
The estimated statistical parameters for the different growth models are shown in Table 8. According to AIC parameters, the Schnute and the exponential models best described the growth pattern of males and females, respectively, in the Morocco population. The exponential model was the best to describe the growth of the entire population (combining males and females). In the case of Guinea-Bissau’s Sepia bertheloti population, the von Bertalanffy model best described the growth pattern of males and the exponential model for females, while for the entire population (males and females combined), the exponential model showed the best fit. Figure 7 shows the best growth curves for males and females of each study location.
The asymptotic length (L∞), according to the Von Bertalanffy model for males from Guinea-Bissau, was 173 mm DML, and according to the Schnute model, the males from Morocco showed a L∞ of 140 mm DML.
The largest number of specimens was aged from 191 to 280 days (Table 8), for Morocco (51.47%) and Guinea-Bissau (41.22%), with the youngest age classes (< 100 days old) being the least present (Morocco: 0.00% and Guinea-Bissau: 0.88%). In the case of males, the lowest instantaneous relative growth rate (G) values were for the age classes of 281–370 and > 371 days old for Morocco and Guinea-Bissau, respectively. In the case of females, the lowest G values were for the 191–280 days old age classes for Guinea-Bissau. The fastest growth patterns were found in the lower age classes of 191–280 for males from Morocco and Guinea-Bissau, while for females, it was in the range of 101–190 days old for Guinea-Bissau. Females from Morocco have not been compared since just obtaining a value of G could misrepresent their results. Even so, the age range available for Morocco females (191–280 days old) exhibited a lower G than individuals of the same age from Guinea-Bissau. This also occurs for males as, those from Guinea-Bissau show a higher G for all age classes, except for individuals > 371 days old, where cuttlefishes from Morocco show a value of 0.137% DML d−1 and those from Guinea-Bissau a value of 0.131% DML d−1. In Table 9, all G and AGR data are summarized.
Significant differences in G and AGR growth rates between sexes (t test, p < 0.0001) and locations (t test, p < 0.0001) were found. The individuals from Guinea-Bissau showed a higher G value than the individuals from Morocco, showing faster growth at the same age (See Table 9). On the other hand, within each location, males had a higher growth rate than females. This is demonstrated by the fact that males showed larger sizes than females in both locations for the same age. The differences in G and AGR between locations are observed: individuals from Guinea-Bissau showed larger sizes than individuals from Morocco at the same age ranges.
Hatching season
The back-calculation method indicated that the cuttlefish hatched between June 2017 and September 2019 for Morocco, and between May 2018 and October 2019 for Guinea-Bissau. It was observed that cuttlefish hatched throughout the year in Morocco (Fig. 8A) and Guinea-Bissau (Fig. 8B); however, 45.59% of the total sample hatched in winter for Morocco, while Guinea Bissau’s the hatchings had two marked peaks—one in summer (30.70%) and another in winter (35.08%). Kruskal–Wallis test (W = 4.0462, p = 0.2565) did not result in significant differences in length ranges according to season of hatching for individuals from Morocco. In contrast, individuals from Guinea-Bissau showed significant differences in length ranges (Kruskal–Wallis test; W = 36.439, p < 0.0001). The post hoc Dunn test (p < 0.001) showed that these differences were due to the fact that individuals born during warm seasons (spring and summer) were smaller than those born in the autumn/winter. Individuals hatched in winter exhibited the largest sizes.
Growth rates for each hatching season group were analyzed for females and males separately (see supplementary material). In Morocco, the highest values of G for males and females were found for specimens born in summer, and for Guinea-Bissau, the highest values of G were found in autumn for females and in spring for the males. In both locations and sexes, a decreasing trend in the growth rhythm was observed with age.
Discussion
In this study, the length ranges obtained from commercial fisheries did not enable us to separate the catches into different ontogenetic groups (juveniles and adults) due to the lack of consensus regarding definition the juvenile phase. Sweeney et al. (1992) assigned the category of “juveniles” to the stage of development between hatching and the subadult stage (defining the category of “subadult stage” as that stage in which the morphological characteristics of cephalopod are sufficiently developed to determine the species and ending when sexual maturity is reached). Bellanger et al. (2005) defined the juvenile category for Sepia officinalis as individuals up to 3 months old.
Significant differences in mean length between the sexes have already been described for other cuttlefish, such as Sepia latimanus, S. koilados, S. rhoda, and S. subplana (Bettencourt and Guerra 2001; Dan et al. 2012), white males being larger than females. However, this is not a characteristic that can be extrapolated to the whole Sepiidae family since most species have a different growth pattern where females are larger than males (i.e., S. orbignyana and S. elegans among others; Jereb and Roper 2005).
Differences in growth conditioned by environmental factors (i.e., latitude, temperature, food, etc.) have been described in cephalopods (e.g., Arkhipkin et al. 1998; Semmens et al. 2004; Guerra 2006; Batista et al. 2021). In the case of S. bertheloti, it was observed that specimens from Guinea-Bissau (Central Africa) exhibited larger sizes than individuals captured in Morocco (North Africa) at the same age. The oceanographic differences of both zones, due to the influence of high productivity from the Western Sahara upwelling (Arístegui et al. 2009) and the different thermal ranges between the locations due to the seasonality of upwellings and winds (Arístegui et al. 2009; Pelegrí et al. 2017), might cause differences in the length frequency distribution. In relation to growth, males and females showed negative allometric patterns, growing faster in dorsal mantle length than in total weight, which is comparable to other species of cuttlefish such as S. officinalis (Vasconcelos et al. 2018).
In this study, the analysis of beak microstructure for Sepia bertheloti used rostrum sagittal sections to determine age. This is in contrast to other benthic cephalopods, such as Octopus vulgaris (e.g., Perales-Raya et al. 2010b; 2014a, b; Canali et al. 2011; Cuccu et al. 2012), O. insularis (Batista et al. 2021), or O. maya (Rodríguez-Domínguez et al. 2013) where the lateral wall surfaces (LWS) of upper beaks were successfully used for age estimation. In the case of S. bertheloti, and after exploring the RSS and LWS of several upper and lower jaws, the RSS of the lower beak showed the clearest pattern of growth increments. On the contrary, no clear sequence of growth increments were observed for age estimation of S. bertheloti in the LWS of the upper beaks.
Although differences were observed among the length distributions of specimens within the same age classes of both studied locations, the maximum age recorded for Sepia bertheloti (14 months) did not differ between locations. Nevertheless, according to our data, males showed a higher life span than females in both locations. The maximum estimated age for males was 14 months, while females showed a maximum age of 9–10 months. As samples do not cover the entire year in the northern location, some of the largest individuals may not have been caught; therefore, the maximum age could be underestimated. In this context, using age data from statoliths, Perales-Raya (2001) obtained a maximum age of 223 days (over 7 months) for both sexes for S. hierredda from Western Sahara, with males reaching larger sizes than observed in this study. Bettencourt and Guerra (2001) showed that the maximum age of S. officinalis under culture conditions was 420 days (14 months), although the number of increments in the statoliths could have been underestimated due to the large number of narrow increments close to the nucleus, which were very dark and difficult to detect and discern. While working with samples from the English Channel, Challier et al. (2005) reported an approximate life expectancy of 2 years for S. officinalis. Similarly, Nabhitabhata and Nilaphat (1999) described the life expectancy of S. pharaonis as one year or over two years depending on the season of birth. This characteristic of life expectancy was also described by Hernandez-López (2000); life span variations in O. vulgaris are dependent on the hatching season. Our results indicate life span of around one year for S. bertheloti in both fishing locations, although it should be noted that this age range corresponds to specimens captured by commercial fishing fleets. Additionally, a deeper study of both populations would be advisable in order to observe possible differences in life expectancy and sexual maturity as reported for other species (Moreno et al. 2002).
The use of asymptotic models for the growth analysis in cephalopods is something that has been under discussion for years. Several studies, e.g., Jackson et al. (2000), describe the inefficiency of the von Bertalanffy model for cephalopods. In contrast, authors such as Uozumi and Shiba (1993) or Brodziak and Macy (1996) recommend the use of the Gompertz and Schnute asymptotic models (Petric et al. 2021). Arkhipkin and Roa-Ureta (2005); Arkhipkin et al. (2021) recommend using the Schnute model to describe growth since the use of von Bertalanffy parameters for assessment models is inappropriate for cephalopods’ semelparous classification. In this study, seven growth models were tested, including four with asymptotic growth (Logistic, von Bertalanffy, Gompertz, and Schnute models). The absence of individuals of small and very large lengths means that our growth models were adapted to the fished portion of the population. Forsythe and Van Heukelem (1987) indicated that cephalopods grow differently in each life stage, so the presence of extreme length ranges determines which model fits best. According to the length distributions in our sample, the exponential model was the one that best described the growth of the S. bertheloti population of Morocco and Guinea-Bissau, which has also been described as adequate for other cephalopods species during the early stages of their life cycles (Forsythe and Van Heukelem 1987). However, the exponential model was not the best model to consider to each sex separately. The females, with a shorter maximum age, showed an exponential growth pattern, but the asymptotic models (Schnute’s for males from Morocco and Von Bertalanffy’s for males from Guinea-Guinea) was a better fit for males. This variation in growth between males and females may be due to life expectancy factors, since males have a longer life span than females and are probably able to survive a longer after reproduction. Furthermore, an asymptotic model in the final phase of life would be expected as the growth rate slows.
The instantaneous growth rates generally showed high values in the early stages of life and are expected to decrease with age. Many authors have already observed this growth pattern in cephalopods (Richard 1971; Dominguez et al. 2006; Petric et al. 2021 among others). Cuttlefish from Guinea-Bissau showed higher growth rates than those from Morocco in the same age bracket, although differences in date of capture might affect this. By sexes, males from both locations exhibited a longer life span than females. These differences between sexes and geographic locations have also been observed in other cephalopods such as Illex coindetti (Arkhipkin 1996) where females grow faster than males and specimens from Central Africa (Sierra Leone) grow faster than those from the Western Sahara. These observations are in keeping with the hypothesis that individuals from colder waters (i.e., Morocco) have a longer life span, a slower growth rate and later reproduction than cuttlefish from warmer waters (i.e., Guinea-Bissau), as suggested by several authors (e.g., Hernández-García and Castro 1998; Pelegrí et al. 2017).
Regarding growth differences by hatching season, specimens from Guinea-Bissau born during the warmer seasons (spring and summer) had smaller lengths at a given age than individuals born during the autumn and/or winter periods. Conversely, in the cuttlefish S. hierredda from the Western Sahara, the specimens born in spring showed larger sizes at a given age than those born in autumn (Perales-Raya 2001), though it should be noted that the author also found inter annual differences. The back-calculation analysis showed that S. bertheloti from Morocco and Guinea-Bissau hatch continuously throughout the year. A peak of hatching was observed in the winter off of Morocco, but two marked peaks were shown in Guinea-Bissau, one in summer and one in winter. These results should be considered with caution when managing these populations that are difficult to access as the sampled period does not cover the whole year, since a more complete study (> 18 months of sampling) would be necessary to confirm the hatching peaks. However, a continuous spawning has been observed for many benthic cephalopods, such as O. vulgaris, S. officinalis, and S. hierredda, in West Africa: however, like the Guinea-Bissau sample, they usually had two marked reproductive periods (Hatanaka 1979; Jurado-Ruzafa et al. 2014). In both locations, it was observed that the highest G values coincided with the greatest intensity of the upwellings (Ingham 1970; Arístegui et al. 2009), when the increased availability of nutrients at hatching makes cuttlefishes grow faster.
In conclusion, the RSSs of beaks are suitable structures for age estimation in cuttlefish. The results obtained from this study suggest that the life span of S. bertheloti is around 9–10 months for females and 14 months for males, with differences between the growth rates likely due to genetic, environmental and geographical factors, in keeping with observations off other cephalopod species (Guerra 2006). The growth of S. bertheloti showed a negative allometry following an exponential model in both study locations. By sex, this model was the best fit for all the females of the study. However, in the case of males, the Schnute model was the best adapted to Moroccan males with the von Bertalanffy model being best suited to the Guinea-Bissau sample. Growth rates were also different between locations and sexes. In both locations, males showed a faster relative instantaneous growth pattern than females. The population of Guinea-Bissau presented a higher overall growth rate than Morocco. In Guinea-Bissau, a sample’s hatching season marked a difference in growth patterns with individuals born in spring and summer being smaller than those born in autumn–winter. In contrast, no size differences were observed in relation to the hatching season in Morocco. Future efforts should be focused on accessing a wider range of lengths, seasons, and maturity stages among S. bertheloti to enhance understanding of their life cycles in the region. This information may enable the analysis of potential differences related to the water temperature and the influence of upwellings. This knowledge is indeed essential for the sustainable management of cuttlefishes in the Central Eastern Atlantic.
Data availability
The datasets generated during and/or analyzed during the current study are not publicly available due to are being processed for further analysis but are available from the corresponding author on reasonable request.
References
Akaike H (1974) A new look at the statistical model identification. IEEE Trans Auto Con 19(6):716–723
Almonacid-Rioseco EI (2006) Contribución a la ecología de Sepia officinalis (Cephalopoda: Sepiidae) en Gran Canaria, Doctoral dissertation
Ama-Abasi D, Akpan I (2008) Elemental concentration and size relationship in African cuttlefish Sepia bertheloti, from the coastal waters of Qua Iboe River, Nigeria. En: 22nd Annual Conference of the Fisheries Society of Nigeria (FISON), 12–16 Nov 2007, Kebbi, Nigeria, 245–249
Arístegui J, Barton E, Álvarez-Salgado X, Santos PA, Figueiras F, Kifani S, Hernández-León S, Mason E, Machú E, Demarcq H (2009) Sub-regional ecosystem variability in the Canary Current upwelling. Prog Ocea 83:33–48
Arkhipkin A (1996) Geographical variation in growth and maturation of the squid Illex coindetii (Oegopsida, Ommastrephidae) off the north-west African coast. J Mar Bioll Assoc UK 76:1091–1106
Arkhipkin AI, Roa-Ureta R (2005) Identification of ontogenetic growth models for squid. Mar Fresh Res 56(4):371–386
Arkhipkin AI, Laptikhovsky VV, Nigmatullin CM, Bespyatykh AV, Murzov SA (1998) Growth, reproduction and feeding of the tropical squid Ornithoteuthis antillarum (Cephalopoda, Ommastrephidae) from the central-east Atlantic. Sci Mar 62(3):273–288
Arkhipkin AI, Hendrickson LC, Payá I, Pierce GJ, Roa-Ureta RH, Robin JP, Winter A (2021) Stock assessment and management of cephalopods: advances and challenges for short-lived fishery resources. ICES J Mar Sci 78(2):714–730
Armelloni EN, Lago-Rouco MJ, Bartolome A, Felipe BC, Almansa E, Perales-Raya C (2020) Exploring the embryonic development of upper beak in Octopus vulgaris Cuvier, 1797: new findings and implications for age estimation. Fish Res 221:105375
Arreguín-Sánchez F, Solís M, Sánchez JA, Valero E, González ME (1996) Age and growth of the octopus (Octopus maya) from the continental shelf of Yucatan, Mexico. AquaDocs 1:485–498
Balguerías-Guerra E, Hernández-González CL, Fernández-Núñez M, Perales-Raya C (1993) Analysis of discards produced by the Spanish cephalopod-fishing fleet at the Sahara Bank. Bol Inst Esp Ocean 9(1):75–88
Bárcenas GV, Perales-Raya C, Bartolomé A, Almansa E, Rosas C (2014) Age validation in Octopus maya by counting increments in the beak rostrum sagittal sections of known age individuals. Fish Res 152:93–97
Batista BB, Matthews-Cascon H, Marinho RA, Kikuchi E, Haimovici M (2021) The growth and population dynamics of Octopus insularis targeted by a pot longline fishery in north-eastern Brazil. J Ma Biol Assoc UK 101(6):935–946
Baty F, Charles S, Flandrois J, Delignette-Muller ML (2015) The R package nlstools: a toolbox for nonlinear regression. J Stat Soft 66(5):1–21
Bellanger C, Halm MP, Dauphin F, Chichery R (2005) In vitro evidence and age-related changes for nicotinic but not muscarinic acetylcholine receptors in the central nervous system of Sepia officinalis. Neuro Lett 387(3):162–167
Berrit GR, Rebert JP (1977) Oceanographie physique et productivité primaire. In: Berri GR (ed) Le milieu marin de la Guinée Bissau et ses ressources vivantes. ORSTOM, Paris, pp 1–60
Bettencourt V, Guerra A (2001) Age studies based on daily growth increments in statoliths and growth lamellae in cuttlebone of cultured Sepia officinalis. Mar Biol 139(2):327–334
Bolser DG, Grüss A, Lopez MA, Reed EM, Mascareñas-Osorio I, Erisman BE (2018) The influence of sample distribution on growth model output for a highly-exploited marine fish, the Gulf Corvina (Cynoscion othonopterus). PeerJ 6:e5582
Brodziak JK, Macy WK (1996) Growth of long-finned squid, Loligo peali, in the northwest Atlantic. Fish Bull 94:212–236
Burnham KP, Anderson DR (2002) Avoiding pitfalls when using information-theoretic methods. J Wild Manage 66:912–918
Campana SE (2001) Accuracy, precision and quality control in age determination, including a review of the use and abuse of age validation methods. J Fish Boil 59(2):197–242
Canali E, Ponte G, Belcari P, Rocha F, Fiorito G (2011) Evaluating age in Octopus vulgaris: estimation, validation and seasonal differences. Mar Ecol Prog Ser 441:141–149
Challier L, Dunn MR, Robin JP (2005) Trends in age-at-recruitment and juvenile growth of cuttlefish, Sepia officinalis, from the English Channel. ICES J Mar Sci 62(8):1671–1682
Cuccu D, Mereu M, Cau Alessandro Pesci P, Cau A (2012) Reproductive development versus estimated age and size in a wild Mediterranean population of Octopus vulgaris (Cephalopoda: Octopodidae). J Mar Biol Assoc UK 93:843–849
Dan S, Hamasaki K, Yamashita T, Oka M, Kitada S (2012) Age-based life cycle traits of the broadclub cuttlefish Sepia latimanus confirmed through release-recapture experiments. Aqua Biol 17:181–195
Delgado de Molina-Acevedo A, Santana-Fernández JC, Ariz-Telleria J, Goni-Beltran de Garizurieta R (1993) Biological-fishing parameters of some species of the Sepiidae Keferstein, 1866, collected during the IBNSINA Survey (1980–1982). Bol Inst Esp Oceano 9(1):41–56
Dominguez PM, Bettencourt V, Guerra A (2006) Growth of Sepia officinalis in captivity and in nature. Vie Et Millleu Life Environ 56(2):109–120
Doumbouya A, Camara OT, Mamie J, Intchama JF, Jarra A, Ceesay S, Belhabib D (2017) Assessing the effectiveness of monitoring control and surveillance of illegal fishing: the case of West Africa. Front Mar Sci. https://doi.org/10.3389/fmars.2017.00050
Elzhov TV, Mullen KM, Spiess AN, Bolker BR, Mullen MK (2015) Interface to the levenberg-marquardt nonlinear least-squares algorithm found in MINPACK. R package version, 1–2
FAO (2021) FAO yearbook. Fishery and aquaculture statistics 2019
Forsythe JW, Van Heukelem WF (1987) Cephalopod growth. In: Boyle PR (ed) Cephalopod life cycles, vol II. Comparative reviews. Academic Press, London, pp 135–155
García-Isarch E, Burgos-Cantos C, Sobrino-Yraola I, Mendes de Almeida A, Barri I, Assau-Nahada V, Gomes-Correia-Funny R, Joaquim-Gomes M (2009) Informe de la campaña de evaluación de recursos demersales de la ZEE de Guinea Bissau a bordo del B/O Vizconde de Eza, “Guinea Bissau 0810”, Inst Esp Oceano. pp 2014
Gompertz B (1825) On the nature of the function expressive of the law of human mortality, and on a new mode of determining the value of life contingencies. In a letter to Francis Baily, Esq. FRS & c. Philo Trans Roy Soci Lon 115:513–583
González AF, Castro BG, Guerra A (1996) Age and growth of the short-finned squid Illex coindetii in Galician waters (NW Spain) based on statolith analysis. ICES J Mar Sci 53(5):802–810
Guerra A (2006) Ecology of Sepia officinalis. Vie et Milieu/Life & Environment, pp 97–107
Guerra A, González AF, Roeleveld M, Jereb P (2014) Cephalopods. In Carpenter KE, De Angelis N (eds) The living marine resources of the Eastern Central Atlantic. Volume 1: Introduction, crustaceans, chitons, and cephalopods, FAO Species Identification Guide for Fishery Purposes. Rome, FAO. pp 369–681
Guerra-Marrero A, Perales-Raya C, Lishchenko F, Espino-Ruano A, Jiménez-Alvarado D, Couce-Montero L, Castro JJ (2023) Age validation in early stages of Sepia officinalis from beak microstructure. Mar Biol 170(2):24
Hatanaka H (1979) Spawning season of the cuttlefish, Sepia officinalis officinalis, off the northwestern coast of Africa [Atlantic Ocean]. In: Meeting of the Ad Hoc Working Group on the Assessment of Cephalopod Stocks, Santa Cruz de Tenerife (Canary Islands), 18 Sep 1978
Hernández García V, Castro JJ (1994) A note on the DML distribution and catches of Sepia bertheloti and Sepia officinalis (Cephalopoda: Sepiidae) on the Saharan Bank. ICES J Mar Sci
Hernández López JL (2000) Biología, ecología y pesca del pulpo común Octopus vulgaris, Cuvier 1797 en aguas de Gran Canaria, Doctoral dissertation
Hernández-García V (2003) Growth and pigmentation process of the beaks of Todaropsis eblanae (Cephalopoda: Ommastrephidae). Berl Palal Abh Berlin 3:131–140
Hernández-López JL, Castro-Hernández JJ, Hernández-García V (2001) Age determined from the daily deposition of concentric rings on common octopus (Octopus vulgaris) beaks. Fish Bull 99(4):679–684
ICES (2010) Report of the workshop on sexual maturity staging of cephalopods (WKMSCEPH), 8–11 November 2010, Livorno, Italy. pp 97
Ingham MC (1970) Coastal upwelling in the northwestern Gulf of Guinea. Bull Mar Sci 20(1):1–34
Jackson GD (2004) Advances in defining the life histories of myopsid squid. Mar Fresh Res 55(4):357–365
Jackson GD, Alford RA, Choat JH (2000) Can length frequency analysis be used to determine squid growth?–An assessment of ELEFAN. ICES Jour Mar Sci 57(4):948–954
Jereb P, Roper CFE (2005) Cephalopods of the world. An annotated and illustrated catalogue of cephalopods species known to date, vol 1. Chambered nautiluses and sepioids (Nautilidae, Sepiidae, Sepiolidae, Sepiadariidae, Idiosepiidae and Sepirulidae). FAO Species Catalogue for Fishery Purposes
Jurado-Ruzafa A, Duque V, Carrasco MN (2014) Reproductive aspects of Octopus vulgaris, Cuvier 1797 (Cephalopoda: Octopodidae), caught in Mauritanian waters by the industrial Spanish fleet (NW Africa). Vieraea 42:149–164
Martos AR, Peralta LF (1995) Biology and fisheries of North-west African hakes (M. merluccius M. senegalensis and M. polli). Hake. Springer, Dordrecht, pp 89–124
Mazerolle MJ, Mazerolle MMJ (2017) Package ‘AICcmodavg’. R package, 281
Monzón-Argüello C, Cardona L, Calabuig P, Camacho M, Crespo-Picazo JL, García-Párraga D, Varo-Cruz N (2018) Supplemental feeding and other anthropogenic threats to green turtles (Chelonia mydas) in the Canary Islands. Sci Tot Env 621:1000–1011
Moreno A, Pereira J, Arvanitidis C, Robin JP, Koutsoubas D, Perales-Raya C, Cunha MM, Balguerias E, Denis V (2002) Biological variation of Loligo vulgaris (Cephalopoda: Loliginidae) in the eastern Atlantic and Mediterranean. Bull Mar Sci 71(1):515–534
Nabhitabhata J, Nilaphat P (1999) Life cycle of cultured pharaoh cuttlefish, Sepia pharaonis Ehrenberg, 1831. Phu Mar Biol Cen 19(1):25–40
Ogle D (2017) FSA: simple fisheries stock assessment methods v. R package version 0.8. 17
Pelegrí JL, Peña-Izquierdo J, Machín F, Meiners C, Presas-Navarro C (2017) Chapter 3 Oceanography of the cape verde basin and mauritanian slope waters. In: Ramos A, Ramil F, Sanz JL (eds) Deep-sea ecosystems off mauritania research of marine biodiversity and habitats in the Northwest African margin. Springer, Dordrecht, pp 119–153
Perales-Raya C, Fernandez-Nuñez M, Balguerías E, Hernández-González CL (1994) Progress towards ageing cuttlefish Sepia hierredda from northwestern African coast using statoliths. Mar Eco Prog Ser 144(1–2):139–147
Perales-Raya C, Bartolomé A, García-Santamaría MT, Pascual-Alayón P, Almansa E (2010b) Age estimation obtained from analysis of octopus (Octopus vulgaris Cuvier, 1797) beaks: improvements and comparisons. Fish Res 106(2):171–176
Perales-Raya C, Jurado-Ruzafa A, Bartolomé A, Duque V, Carrasco MN, Fraile-Nuez E (2014a) Age of spent Octopus vulgaris and stress mark analysis using beaks of wild individuals. Hydro 725(1):105–114
Perales-Raya C, Almansa E, Bartolomé A, Felipe BC, Iglesias J, Sánchez FJ, Carrasco JF, Rodríguez C (2014b) Age validation in Octopus vulgaris beaks across the full ontogenetic range: beaks as recorders of life events in octopuses. J Shell Res 33(2):481–493
Perales-Raya C, Hernández-González CL, Gomes M, Jiménez S, Duque V, Falcón JM (2010a) Cephalopod abundance, distribution and assemblage structure in waters off Guinea-Bissau (North West Africa), SIEBMXVI, Póster, Alicante, 6–10 Septiembre
Perales-Raya C (2001) Determinación de la edad y estudio del crecimiento del choco (Sepia hierredda Rang, 1837), el calamar (Loligo vulgaris Lamarck 1798) y el pulpo (Octopus vulgaris Cuvier, 1797) de la costa noroccidental africana. Doctoral dissertation, Universidad de La Laguna, pp 192
Petrić M, Škeljo F, Šifner SK (2021) Age, growth and maturation of Illex coindetii (Cephalopoda: Ommastrephidae) in the eastern Adriatic Sea. Reg Stud Mar Sci 47:101935
R Core Team (2022) R: a language and environment for statistical computing. R foundation for statistical computing. https://www.R-project.org/
Richard A (1971) Contribution à l'étude expérimentale de la croissance et de la maturation sexuelle de Sepia officinalis L. (mollusque céphalopode). Doctoral dissertation, Université de Lille, France
Ricker WE (1975) Computation and interpretation of biological statistics of fish populations. Bull Fish Res Board Canada 191:1–382
Rocha F, Fernández-Gago R, Ramil F, Ramos A (2017) Cephalopods in Mauritanian Waters. Deep-Sea Ecosystems Off Mauritania. Springer Netherlands, Dordrecht, pp 393–417
Rodríguez-Domínguez A, Rosas C, Méndez-Loeza I, Markaida U (2013) Validation of growth increments in stylets, beaks and lenses as ageing tools in Octopus maya. Jour Exp Mar Biol Ecol 449:194–199
Roper C, Sweeney M, Nauen C (1984) Factors affecting reactions to Cephalopods of the world. An annotated and illustrated catalogue of species of interest to fisheries. FAO Species Cat 125(3):96–111
Schnute J (1981) A versatile growth model with statistically stable parameters. Can J Fish Aquat Sci 38(9):1128–1140
Semmens JM, Pecl GT, Villanueva R, Jouffre D, Sobrino I, Wood JB, Rigby PR (2004) Understanding octopus growth: patterns, variability and physiology. Mar Fresh Res 55:367–377
Sweeney MJ, Roper CF, Mangold KM, Clarke MR, Boletzky SV (1992) “ Larval” and juvenile cephalopods: a manual for their identification. Smithson Contrib Zoo. https://doi.org/10.5479/si.00810282.513
Uozumi Y, Shiba C (1993) Growth and age composition of Illex argentinus (Cephalopoda: Oegopsida) based on daily increment counts in statoliths. In: Okutani T, O’Dor RK, Kubodera T (eds) Rec adv ceph fish biol. Tokai Universtiy Press, Tokyo, pp 591–606
Vasconcelos P, Pereira F, Carvalho AN, Gaspar MB (2018) Weight-length relationships and relative growth of the cuttlefish (Sepia officinalis): causes and effects of hypoallometry. Thal Int Jour Mar Sci 34(2):323–331
Von Bertalanffy L (1938) A quantitative theory of organic growth (inquiries on growth laws II). Human Biol 10:181–213
Acknowledgements
AG-M was supported by a PhD-fellowship (PIFULPGC-2017- CIENCIAS-2) from the University of Las Palmas de Gran Canaria. CP-R would like to thank the support of the IEO project EPAFRIK-BIO2.
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. Guerra-Marrero was supported by a PhD-fellowship (PIFULPGC-2017-CIENCIAS-2) from the University of Las Palmas de Gran Canaria.
Author information
Authors and Affiliations
Contributions
All the authors contributed to the study conception and design. Material preparation, data collection, and analysis were performed by AGM, CPR, and JJC. AB greatly contributed to the material preparation. Data collection was also carried out by LCM, AER, and DJA. The first draft of the manuscript was written by AGM, and all the authors commented on previous versions of the manuscript. All the authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
No applicable.
Consent to participate
No applicable.
Consent to publish
Not applicable.
Additional information
Responsible Editor: R. Rosa .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Guerra-Marrero, A., Bartolomé, A., Couce-Montero, L. et al. Age, growth, and population structure of the African cuttlefish Sepia bertheloti based on beak microstructure. Mar Biol 170, 118 (2023). https://doi.org/10.1007/s00227-023-04272-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04272-7