Abstract
Knowing the age of individuals in a population is crucial to devise appropriate conservation strategies, especially on endangered long-lived species such as sea turtles. Sea turtles do not have external morphologic characters that indicate their age; therefore, age has been estimated by indirect methods such as biometric measurements or skeletochronological analysis. Previous skeletochronological studies have determined that sea turtle age can be estimated with skeletal growth marks (GMs) produced by annual seasonality. This study focused on the skeletochronological analysis of known-age loggerheads reared under seminatural conditions, showing that sea turtles kept under natural photoperiod and seawater temperature seasonality and fed periodically, did not present visible GMs. However, the animals suffering health problems affecting their growth did exhibit GMs. This suggests that skeletochronological studies in sea turtles must be taken with caution at least at early life stages and for the northeast Atlantic loggerhead population. Additionally, this study showed that seawater temperature seasonality strongly influences sea turtles’ growth rates.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Knowing the age of the individuals in a population is essential to identify life cycle stages and to conduct demographic studies, particularly on endangered and threatened long-lived species (Zug et al. 2002). Identification of complete lifecycles allows researchers to study the structure of a population, which is required to establish conservation strategies and management tools for threatened populations (Avens et al. 2017; Guarino et al. 2020; Chasco et al. 2020).
In general, young sea turtles present a rapid early growth that helps reduce the risk of predation (Salmon and Scholl 2014), which slows as they get older. A close look reveals that sea turtles exhibit highly variable growth rates during oceanic stages due to an “opportunistic feeding behavior”, which depends on genetics, sex, fitness or environmental factors during their life in the open ocean (Heppell et al. 2003; Bjorndal et al. 2003). Because young loggerhead sea turtles develop in oceanic habitats that are considered extremely stochastic environments, they exhibit compensatory growth–also known as “catch-up” growth–or the accelerated growth of an organism exposed to good conditions after a period of reduced growth due to suboptimal environmental conditions (Bjorndal et al. 2003).
The absence of external morphologic characters that indicate the age of sea turtles (Avens and Snover 2013), together with their growth variability along with the “lost years” period, when post-hatchling turtles develop away from the coastline, make it difficult to estimate the age of sea turtles. Thus, researchers look for indirect methods of estimating the age of sea turtles, such as using the carapace length, examining growth rates or using skeletochronological approaches (Chaloupka and Musick 1997; Snover and Hohn 2004; Avens and Snover 2013; Guarino et al. 2020). However, some studies have suggested that high variability in growth rates does not allow the establishment of a clear relationship between age and carapace length (Braun-McNeill et al. 2008; Piovano et al. 2011; Avens and Snover 2013). Thus, the general consensus in the sea turtle scientific community is that skeletochronology is currently the best and most accurate way to establish a sea turtle’s age (Avens and Snover 2013; Goshe et al. 2016; Avens et al. 2017; Guarino et al. 2020).
Skeletochronology is based on intermittent bone growth where growth cycles are reflected in bone deposition generating growth lines and growth rings in long bones (Zug et al. 1986; Avens and Snover 2013). Dark thin lines, called “lines of arrested growth (LAGs)”, correspond to the deceleration or arrest of skeletal growth, while light broad sections reflect active bone formation during high growth rates periods (Zug et al. 1986; Avens and Snover 2013). Both lines together identify a growth cycle and are called “skeletal growth marks (GMs)” (Snover and Hohn 2004). Traditionally, one GM has been interpreted as one year of life considering that endogenous physiological rhythms are synchronized with environmental parameters, such as temperature and foraging rates (Snover and Hohn 2004), and above all in reptiles that, being ectotherms, their metabolism is largely regulated by temperature (Taylor et al. 2021). Consequently, GMs can be used to estimate the age of the individual (Avens and Snover 2013; Goshe et al. 2016; Guarino et al. 2020). In wild loggerhead sea turtles (Caretta caretta), the annual growth periodicity has been verified using oxytetracycline in juveniles (from 40 to 80 cm straight carapace length) in Chesapeake Bay (USA) (Klinger and Musick 1992), and in an adult female (Coles et al. 2001), and in two known-age loggerhead adults by Snover and Hohn (2004). Skeletochronology provides estimated ages (Zug et al. 1997) because the number of GMs represents an estimation (Klinger and Musick 1992). It is important to consider that not every turtle will have visible GMs; for example, the absence of GMs has been observed in animals living in some tropical areas, where this phenomenon is attributed to a lack of seasonality (Bjorndal et al. 1998), although in other tropical areas GMs have been observed (Snover et al. 2011; Petitet et al. 2015; Goshe et al. 2016). Also, there are some difficulties when reading a bone, such as the compression of LAGs in the outermost zone of the bone (Snover and Hohn 2004), the presence of accessory LAGs, the presence of discontinuous or irregular LAGs (Zug et al. 1986), and the loss of early GMs by endosteal resorption and the expansion of the medullary cavity, getting more imprecise as turtles get older (Klinger and Musick 1992; Snover and Hohn 2004; Avens et al. 2020). Endosteal resorption is particularly extreme in long-lived species (Snover and Hohn 2004), therefore, GMs annual development must be tested for each species and population (Castanet et al. 1992).
Most skeletochronological analyses have been done with juvenile (Klinger and Musick 1992; Avens et al. 2013, 2015; Ramirez et al. 2017; Guarino et al. 2020) or adult wild sea turtles (Coles et al. 2001; Avens et al. 2015; Guarino et al. 2004, 2020). A few of these studies have been conducted with captive or with individuals of known age (Snover and Hohn 2004), but almost none with yearlings (Guarino et al. 2004, 2020), presumably due to the difficulty of finding these small animals in the wild. In addition, there is no skeletochronological study with yearlings of known age. Therefore, the goals of this study were to: (i) Evaluate the influence of annual seasonality (based on seawater temperature) on loggerhead yearlings’ growth rates; (ii) Determine if GMs were present in the early years of loggerhead turtles; and (iii) Test if growth rate seasonality was reflected in GM in known-age loggerhead yearlings.
Materials and methods
Animal origin and husbandry conditions
Loggerhead turtles used for this study had been reared under seminatural conditions since hatching in Gran Canaria Island (Canary Islands, Spain) between 2006 to 2010, under the corresponding CITES permits. All turtles included in this study died from different infections (i.e., dermatitis, rhinitis, pneumonia or stomatitis) as well as by undetermined causes (Oros et al. 2020). The turtles were reared in outdoor tanks under natural photoperiod and with continuous-flow untreated seawater pumped directly from the sea to keep rearing conditions as natural as possible. Consequently, the water had the same conditions as in the sea, ranging between 18 °C and 25 °C, where two seasons were defined: (i) warm season when temperatures were above 21 °C, and (ii) cold season when temperatures were below 21 °C. Water temperature was measured every two hours with HOBO TidbiT-v2 and Pro-v2 data loggers, and daily and monthly average temperatures were calculated.
Hatchlings were raised in 3000 L oval tanks, with 30–40 hatchlings per tank during the first year of life and decreasing to 10 to 15 hatchlings per tank on the second year. The turtles were fed the equivalent of 3% and 5% of their body weight (cold and warm months respectively) four times a week (Higgings 2003; Usategui-Martín et al. 2021). During the third year, animals were kept in 5000 L rectangular tanks, 5–10 yearlings per tank, and fed the equivalent 2.5% and 4% of their body weight (cold and warm months, respectively), three times per week. Sea turtles were fed a mix of fresh fishes, squids, and mussels (Higgings 2003; Usategui-Martín et al. 2021). Tanks were emptied and cleaned twice a week (Monday and Friday), eliminating turtle feces and algae with a scrub brush, then rinsed with fresh water and filled again with seawater.
The health of the animals was monitored daily by direct observation and weekly by weighing. When some turtles became ill (i.e., lost weight), were injured (i.e., wounds, bites, infected areas), or feeding problems arose (no reaction to food, or 3 feedings with less than 3 bites of food), sick animals were kept in separate tanks with warm water (24 °C) and fed ad libitum until total recovery. Care of sick animals was conducted by veterinarian staff of the “Gran Canaria Wildlife Recovery Center” of the Cabildo de Gran Canaria (Canary Islands, Spain).
Minimum straight carapace length (SCLmin; mm) was obtained weekly or biweekly (before and after 12-15 months of life respectively) using a metal caliper to the nearest 0.01-mm. SCLmin divided by age (SCLmin/Age) was used to normalize data by size. Mean monthly growth rates (GR) were calculated for each cohort (years: 2006, 2007, 2008 and 2009) during the first two years of life to determine how their growth changed between seasons (growth seasonality), as well as the individual mean growth rate (indGR) of each yearling whose humerus was analyzed. For this, the formula GR (mm/month) = (LT2-LT1)/(T2-T1)), where L is the SCLmin; T1 is the week of initial measurement; and T2 is the week of final measurement (Stokes et al. 2006).
Skeletochronology
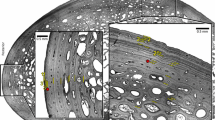
In this study, the right humerus (Zug et al. 1986) from 32 loggerhead turtles was used, ages between 12 to 44.4 months old. The right flippers were surgically removed from the body, removing flesh and skin remnants with scissors and forceps to get a clean humerus. From each humerus, six different measurements were collected using a caliper to the nearest 0.1 mm: (1) Maximum length (ML), distance from proximal-most tip of ulnar process to distal articular surface; (2) Longitudinal length (LL), distance from proximal surface of head to distal articular surface, parallel to longitudinal axis; (3) Medial width (MW), transverse distance from pre- to post axial surface at point of minimum width; (4) Width at deltopectoral crest (DpCW), transverse distance of shaft from pre- to postaxial surface at deltopectoral crest; (5) Distal width (DW), transverse distance from pre- to postaxial surface at juncture of articular condyles with shaft; and (6) Proximal width (PW), distance from preaxial surface of head to postaxial surface of ulnar process, perpendicular to longitudinal axis (Zug et al. 1986) (Fig. 1).
A five-millimeter section in length just below the deltopectoral scar (Zug et al. 1986; Avens and Snover 2013) was removed from each humerus and decalcified by immersion in Histofix® decalcifier 3 DC from 5 to 780 min. The immersion time depended on the diameter of the bone: the wider the bone, the longer the immersion time. After this, histological sections were sliced with a Leica CM 1950 cryostat and bone sections were individually placed inside Round Cryomold® Standard (Ø 23-mm), submerged in Tissue-Tek® and then froze the tissue in 24H. Frozen Tissue-Tek® disks were fixed to the specimen disks (Ø30-mm) and set up in the cryostat’s specimen head (adjusted to – 15 ºC), where five histological 25 µm thickness sections were made. Cryostat chamber temperature was set up at − 20 °C during the sectioning process.
The five 25-µm slices from each bone section were allocated in Costar® netwell inserts (Ø24-mm, membrane size 500-µm), filled with ddH2O to clean them for 3 min. Slices were stained with Harris hematoxylin and Eosin (Zug et al. 1986) using the free-floating technique (60 s Hematoxylin–water cleaning–30 s Eosin–water cleaning). Finally, all samples from the same bone section were deposited on a microscope slide, observed under an Olympus BX51 microscope and photographed with an Olympus SC30 digital camera. Samples were classified in two groups: samples without LAG and samples with LAG, both complete and partial. Final images included compositions of the best photos of the same sample.
Statistical analysis
All statistical analyses were conducted using R V.3.1.2 (R development Core Team 2014). Different correlation tests were conducted to examine the relationship between straight carapace length and the several humerus measurements (Humerus DW/SCLmin, Humerus DpCW/SCLmin and Humerus LL/SCLmin), between Age and SCLmin, as well as between rearing temperature and growth rates.
To determine if there were significant differences in growth rates between seasons, a general linear model (LM) was applied, with seasons as explanatory variable and cohort’s growth rates the response variable. LAG presence was analyzed in relation to Age, SCL, SCLmin/Age and indGR using LM, with presence or absence of LAGs as the explanatory variables. The significance level (α) of all statistical analyses was 0.05.
Results
Correlation tests indicated that SCLmin was strongly correlated with humerus MW (P < 0.05; ρ = 0.99, CI95% = 0.97–0.99); with humerus DW (P < 0.05; ρ = 0.95, CI95% = 0.90–0.99); also with humerus LL (P < 0.05; ρ = 0.98, CI 95% = 0.95–0.99) and with humerus DpCW (P < 0.05, ρ = 0.90, CI95% = 0.80–0.95). However, the correlation between SCLmin and age was low because although significant (P < 0.05), the correlation index was moderate (ρ = 0.52), and its 95% confidence interval (CI95%) was very wide and low (0.20–0.75).
Growth rates were moderately correlated with seawater temperature (P < 0.05, ρ = 0.65), where the LM showed significant differences in growth rates between cold and warm seasons (P < 0.05, t = 7.38) (Fig. 2). Here, the mean growth rate (± SE) during warm season was 5.75 ± 0.31-mm/month and 3.09 ± 0.18-mm/month during the cold season.
From the 32 sampled turtles for skeletochronology, only 31 provided useful samples, because one humerus was damaged during the decalcification process. All sampled turtles were subjected to strong seasonal variations along their life (in terms of seawater temperature) (Fig. 3A–D); however, 20 humeri out of 31 (64.5%), ranging in age from 12.24 to 44.52 months of life, did not show any type of LAG, neither complete nor incomplete (Fig. 3A1, 3B1, 3C1 and 3D1). The remaining 11 humeri (35.5%) presented some type of LAG; 8 of them had complete GM, 2 cuts only partial LAGs and the last one presented both, a complete and a partial one (Fig. 3 B1 and 4).
A. Monthly seawater temperature and mean monthly growth rate from 2006 cohort along their first two years of life. A1. Histological humerus section (× 2) from H5/06 turtle, from 2006 cohort (16.9 months old), without any GM nor LAG. B. Monthly seawater temperature and mean monthly growth rate from 2007 cohort along their first two years of life. B1. Histological humerus section (× 4) from 48/07 turtle, from 2007 cohort (13.5 months old), without GM but with partial LAG marked with black arrows and black lines. C. Monthly seawater temperature and mean monthly growth rate from 2008 cohort along their first two years of life. C1. Histological humerus section (× 4) from 22FV/08 turtle, from 2008 cohort (16.1 months old), with no GM nor LAG. D. Monthly seawater temperature and mean monthly growth rate from 2009 cohort along their first two years of life. D1. Histological humerus section (× 10) from 30/09 turtle, from 2009 cohort (44.6 months old), with no GM nor LAG. Upper right corner of the histological section is the outer part of the bone
Histological humerus sections (× 10) with GM marked with black arrows and black lines. A. Turtle C/08, 0.99 years old (11.9 months) and one LAG. B. Turtle E/08, 1.16 years old (13.9 months) and three LAGs. C. Turtle A9/08, 1.24 years old (14.9 months) and one LAG. D. Turtle J7/08, 1.15 years old (13.8 months) and one LAG. E. Turtle X10/08, 1.38 years old (16.6 months) and two LAGs. F. Turtle X15/08, 1.20 years old (14.4 months) and one LAG
Seven of the animals with complete GMs, ranging from 11.88 and 15.88 months old, presented only 1 LAG, and the other 2 individuals with GMs, with 16.56 and 13.92 months old, had 2 and 3 LAGs respectively. Finally, the 2 yearlings with only partial GMs, presented 12.6 and 14.64 months old.
LM analysis showed that the presence or absence of LAGs was not explained by the age of the animals (P > 0.05, F = 2.282). However, sea turtles with LAGs were significantly smaller when they died (P < 0.05, F = 7.827) compared to the ones without any type of LAG in their humerus, with a mean (± SE) SCLmin of 74.80 (± 1.93)-mm and 99.34 (± 2.51)-mm, respectively (Fig. 5). Also, the individual growth of yearlings with LAGs (indGR 1.025 ± 0.51-mm/month) was significantly lower (P < 0.05, F = − 3.830) than the ones with no LAGs in their humerus (indGR 4.052 ± 0.46-mm/month) (Fig. 6), the same result observed with the variable SCL/age.
Discussion
As expected and due to the natural growth of the animals, longitudinal growth, expressed by SCLmin, presented a strong correlation with skeletal growth, expressed by humerus measures. Indeed, humerus biometrics, both length and width, were strongly correlated with SCLmin, as evidenced by the high Pearson correlation coefficients (all above 0.9). In agreement with Braun-McNeill et al (2008), our study showed that there was low correlation between SCLmin and Age of the studied turtles, evidenced by a low correlation coefficient (ρ = 0.52), and a very wide and low confidence interval (0.20 – 0.75). This is consistent with the notion that age estimation based on carapace length is not reliable (Chaloupka and Musick 1997), at least for yearlings from the northeast loggerhead Atlantic population. Moreover, humerus size depends on the animal’s size and not on the animal’s age (Chaloupka and Musick 1997).
Growth theory suggests that loggerhead sea turtles exhibit a great variability in growth rates depending on individual’s characteristics such as genetics, sex, or hatchlings’ fitness (Heppell et al. 2003), together with specific life history conditions found by each individual (i.e., water temperature, food resources, currents or diseases) (Bjorndal et al. 2003). In the present study, turtles analyzed exhibited special circumstances due to its rearing under seminatural conditions where they were not subjected to a stochastic environment, as wild animals are. Instead, the factors included in our study affected all animals equally; all of them were exposed to the same food regime (quantity and quality), the same temperature and photoperiod, the same water quality, the same space availability (volume and deep), and the same circumstances of no predation stress.
Results showed a clear effect of seawater temperature on the animals’ growth rate, even if this correlation was moderate. Other effects can be explained by the influence of factors such as the fitness or the genetics of each individual (Heppell et al. 2003). This is an important finding because even growth rate is linked to temperature in reptiles (Tylor et al. 2021). In sea turtles, that connection has been linked to different environmental or seasonal factors, but always with many co-factors interfering, not allowing the researchers to ascertain the specific effect of the temperature (Bjorndal et al. 2016; Balazs and Chaloupka 2004; Chaloupka et al. 2004). However, such correlation is clear in our study. In our case and considering that growth rates of sea turtles are affected by food intake and water temperature (Bjorndal et al. 2003), the correlation found in the yearlings was related only to seawater temperature and genetics, because all our animals had a continuous food supply. The effect of water temperature was strong enough to establish significant differences on growth rates between cold season (3.1-mm/month) and warm season (5.8-mm/month), showing a clear annual seasonality, where most years presented an alternation of 6 or 7 cold months with 6 or 7 warm months.
Nevertheless, and despite this seasonality in growth rates and the strong correlation between humerus dimension and carapace length, results suggested that bone growth was continuous (GMs absence) in 64.5% of turtles analyzed. Accordingly, we suggest that with lower temperatures, turtles prioritized bone tissue formation over longitudinal growth, which is reflected in a greater variability in carapace length. The growth rate decrease related to seawater temperature seasonality was not strong enough to produce LAGs on the bone, suggesting that LAG formation is controlled by a combination of factors, such as temperature plus food intake, because we showed in this study that the temperature on its own is not enough to disrupt bone formation. Thus, further research is needed to elucidate the effect of temperature on bone growth.
In general, skeletochronology has been used as a method to estimate turtle age under the premise that growing wild sea turtles present one GM per year due to natural marine seasonality (Zug et al. 1986), accepting that environmental seasonality is reflected in the humerus, where each cold season (slow/no growth) one LAG should be formed in the humerus (one per year), and one light broad band (rapid growth) is generated each warm season (one per year) (Zug et al. 1986, 1995; Klinger and Musick 1992, 1995; Bjorndal et al. 1998, 2003; Coles et al. 2001; Snover et al. 2007). However, it is unknown how yearlings may respond to changes in temperature. May be during that life stage growth is more dependent on environmental seasonality.
In this study, where all turtles were subjected to strong water temperature seasonality during their first years of life, 64.5% of them living from 12.24 to 44.52 months of age did not exhibit LAGs, whereas the other 35.5% showed some type of LAG, creating GMs or simply incomplete LAGs. Moreover, the presence of these LAGs seemed to have no relation with the turtle’s age. All turtles showing LAGs in their humerus exhibited growth deficiencies related to weakness or disease periods, resulting also in lower growth rates, suggesting that this could be the cause of LAGs generation. Weakness or illness (periods of metabolic depression) is considered stressful for an animal, similar to that produced by a lack of food, a situation likely quite common in the open ocean, known for its low productivity and food scarcity (Martin et al. 2002). On this extremely stochastic environment, food availability would depend largely on temperature seasonality (Valiela and Valiela 1995).
Furthermore, we observed a significant difference between turtles with LAGs and without LAGs according to their SCLmin and growth. Smaller turtles (SCLmin < 80-mm) which presented lower growth rates were the turtles with LAG, and bigger turtles (SCLmin > 80-mm) with higher growth rates did not show LAGs. For example, (i) Turtle C/08, lived almost 12 months (362 days), hatched during a cold season and lived through 7 cold and 5 warm months. According to previous studies, bone structure should appear without GMs due to the decrease in bone growth during the cold season (Zug et al. 1986; Snover 2002), but the humerus of turtle C/08 showed 2 light broad bands and 1 LAG (Fig. 4A). Age based on humerus structure would be interpreted as 18 months or even 24 months, but this turtle did not live even 12 months; (ii) Turtle E/08, hatched during a cold season and lived 14 months, living through its first 7 cold months followed by 7 warm months. So, only one GM would be expected and interpreted as 12 months (Zug et al. 1986; Snover 2002). However, this turtle showed 3 LAGs (Fig. 4B) in its only 14 months of life; (iii) Turtle J7/08 also lived 14 months, under the same seawater conditions of the previous turtle, but unlike that turtle, J7/08 only presented 1 GM (Fig. 4D). Comparing SCL from both turtles, with similar age of 14 months old, E/08 (3 LAGs) was smaller than J7/08 (1 LAG).
Turtles A9/08 (1 LAG, Fig. 4C), X10/08 (2 LAGs, Fig. 4E) and X15/08 (1 LAG, Fig. 4F), neither fit the established models presented by Zug et al (1986) and Snover (2002). Turtles A9/08 and X15/08 should not present any LAG, because they lived along 7 cold months, 7 warm months, and 1 cold month, but both presented only 1 LAG. Turtle X10/08 lived along only 2 more cold months and presented 2 LAGs.
The main difference between the turtles from this study and wild turtles was that the studied turtles had a constant food supply and exhibited limited locomotion. Considering that together with the fact that most of the studied turtles did not show GMs and those with LAGs had feeding/health problems, we suggest that differences in food intake and genetics could be the primary reason of GM generation, and not the water temperature seasonality.
In conclusion, the results of this study suggest that the generation of GMs in an individual’s humerus does not depend on seawater temperature seasonality. Additionally, we also observed that deficiencies in food intake, suggestive of a state of sickness, could also play and important role in the generation of GMs. Thus, the estimation of age by skeletochronology in yearlings must be conducted with caution, at least for the Northeastern Atlantic loggerhead population. This in agreement with Castanet et al. (1992), who indicated that GM annual formation dynamics needs to be examined for each study or population, to better understand life cycles on sea turtles to establish adequate conservation strategies and management tools on threatened populations.
Data availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Avens L, Snover ML (2013) Age and age estimation in sea turtles. In: Wyneken J, Lohmann KJ, Musick JA (eds) The biology of sea turtles, vol 3. CRC Press, Florida, pp 97–134
Avens L, Goshe LR, Pajuelo M, Bjorndal KA, MacDonald BD, Lemons GE, Bolten AB, Seminoff JA (2013) Complementary skeletochronology and stable isotope analyses offer new insight into juvenile loggerhead sea turtle oceanic stage duration and growth dynamics. Mar Ecol Prog Ser 491:235–251
Avens L, Goshe LR, Coggins L, Snover ML, Pajuelo M, Bjorndal KA, Bolten AB (2015) Age and size at maturation-and adult-stage duration for loggerhead sea turtles in the western North Atlantic. Mar Biol 162(9):1749–1767
Avens L, Goshe LR, Coggins L, Shaver DJ, Higgins B, Landry AM Jr, Bayley R (2017) Variability in age and size at maturation, reproductive longevity, and long-term growth dynamics for Kemp’s ridley sea turtles in the Gulf of Mexico. PLoS ONE 12(3):e0173999
Avens L, Ramirez MD, Hall AG, Snover ML, Haas HL, Godfrey MH, Goshe LR, Cook M, Heppell SS (2020) Regional differences in Kemp’s ridley sea turtle growth trajectories and expected age at maturation. Mar Ecol Prog Ser 654:143–161
Balazs GH, Chaloupka M (2004) Spatial and temporal variability in somatic growth of green sea turtles (Chelonia mydas) resident in the Hawaiian Archipelago. Mar Bio 145:1043–1059. https://doi.org/10.1007/s00227-004-1387-6
Bjorndal KA, Bolten AB, Bennett RA, Jacobson ER, Wronski TJ, Valeski JJ, Eliazar PJ (1998) Age and Growth in Sea Turtles: Limitations of Skeletochronology for Demographic Studies. Copeia 1998:23–30
Bjorndal KA, Bolten AB, Dellinger T, Delgado C, Martins HR (2003) Compensatory growth in oceanic loggerhead sea turtles: response to a stochastic environment. Ecology 84:1237–1249
Bjorndal KA, Chaloupka M, Saba VS, Diez CE, van Dam RP, Krueger BH et al (2016) Somatic growth dynamics of West Atlantic hawksbill sea turtles: a spatio-temporal perspective. Ecosphere. 7:01279. https://doi.org/10.1002/ecs2.1279
Braun-McNeill J, Epperly SP, Avens L, Snover ML, Taylor JC (2008) Growth rates of loggerhead sea turtles (Caretta caretta) from the western North Atlantic. Herpetol Conser Biol 3:273–281
Castanet J, Francillon-Viellot H, Meunier FJ, DeRicqles A (1992) Bone and individual aging. In: Hall BK (ed) Bone: A Treatise, vol 7. Crc Press, Florida, pp 245–283
Chaloupka MY, Musick JA (1997) Age, growth, and population dynamics. In: Lutz PL, Musick JA (eds) The Biology of Sea Turtles, vol I. CRC Press, Boca Raton, Florida, pp 233–276
Chaloupka MY, Limpus C, Miller J (2004) Green turtle somatic growth dynamics in a spatially disjunct Great Barrier Reef metapopulation. Coral Reefs 23:325–335. https://doi.org/10.1007/s00338-004-0387-9
Chasco BE, Thorson JT, Heppell SS, Avens L, McNeill JB, Bolten AB, Bjorndal KA, Ward EJ (2020) Integrated mixed-effect growth models for species with incomplete ageing histories: a case study for the loggerhead sea turtle Caretta caretta. Mar Ecol Prog Ser 636:221–234
Coles WC, Musick JA, Williamson LA (2001) Skeletochronology validation from an adult loggerhead (Caretta caretta). Copeia 2001:240–242
Goshe LR, Snover ML, Hohn AA, Balazs GH (2016) Validation of back-calculated body lengths and timing of growth mark deposition in Hawaiian green sea turtles. Ecol Evol 10:3208–3215. https://doi.org/10.1002/ece3.2108
Guarino FM, Di Maio A, Caputo V (2004) Age estimation by phalangeal skeletochronology of Caretta caretta from the Mediterranean Sea. Ital J Zool 71:175–179
Guarino FM, Di Nocera F, Pollaro F, Galiero G, Laccarino D, Iovino D, Mezzasalma M, Petraccioli A, Odierna G, Maio N (2020) Skeletochronology, age at maturity and cause of mortality of loggerhead sea turtles Caretta caretta stranded along the beaches of Campania (south-western Italy, western Mediterranean Sea). Herpetozoa 33:39–51
Heppell SS, Snover ML, Crowder LB (2003) Sea Turtle Population Ecology. In: Lutz PL, Musick JA, Wyneken J (eds) The Biology of Sea Turtles, vol 2. CRC Press, Florida, pp 275–306
Higgins BM (2003) Sea turtle husbandry. In: Lutz PL, Musick JA, Wyneken J (eds) The Biology of Sea Turtles, vol 2. CRC Press, Florida, pp 411–440
Klinger RC, Musick JA (1992) Annular growth layers in juvenile loggerhead turtles (Caretta Caretta). Bull Mar Sci 51:224–230
Klinger RC, Musick JA (1995) Age and growth of loggerhead turtles (Caretta caretta) from Chesapeake Bay. Copeia 22:204–209
Martin AP, Richards KJ, Bracco A, Provenzale A (2002) Patchy productivity in the open ocean. Global Biogeochem Cycles 16:9–1
Orós J, Suárez-Saavedra A, Liria-Loza A, Arencibia A (2020) Lesions observed postmortem in post-hatchling loggerhead sea turtles (Caretta caretta) from a head start programme. J Comp Pathol 174:73–80
Petitet R, Avens L, Castilhos JC, Kinas PG, Bugoni L (2015) Age and growth of olive ridley sea turtles Lepidochelys olivacea in the main Brazilian nesting ground. Mar Ecol Prog Ser 541:205–218. https://doi.org/10.3354/meps11532
Piovano S, Clusa M, Carreras C, Giacoma C, Pascual M, Cardona L (2011) Different growth rates between loggerhead sea turtles (Caretta caretta) of Mediterranean and Atlantic origin in the Mediterranean Sea. Mar Biol 158:2577–2587
R Development Core Team (2014) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. www.r-project.org
Ramirez MD, Avens L, Seminoff JA, Goshe LR, Heppell SS (2017) Growth dynamics of juvenile loggerhead sea turtles undergoing an ontogenetic habitat shift. Oecologia 183:1087–1099
Salmon M, Scholl J (2014) Allometric growth in juvenile marine turtles: possible role as an antipredator adaptation. Zoology 117:131–138
Snover ML (2002) Growth and ontogeny of sea turtles using skeletochronology: methods, validation and application to conservation. Dissertation, Duke University
Snover ML, Hohn AA (2004) Validation and interpretation of annual skeletal marks in loggerhead (Caretta caretta) and Kemp’s ridley (Lepidochelys kempii) sea turtles. Fish Bull 102:682–692
Snover ML, Avens L, Hohn AA (2007) Back-calculating length from skeletal growth marks in loggerhead sea turtles Caretta caretta. Endang Spec Res 3:95–104
Snover ML, Hohn AA, Goshe LR, Balazs GH (2011) Validation of annual skeletal marks in green sea turtles Chelonia mydas using tetracycline labeling. Aquat Biol 12:197–204. https://doi.org/10.3354/ab00337
Stokes L, Wyneken J, Crowder LB, Marsh J (2006) The influence of temporal and spatial origin on size and early growth rates in captive loggerhead sea turtles (Caretta caretta) in the United States. Herpetol Conserv Biol 1:71–80
Taylor EN, Diele-Viegas LM, Gangloff EJ, Hall JM, Halpern B, Massey MD, Rödder D, Rollinson N, Spears S, Sun B, anD Telemeco RS, (2021) The thermal ecology and physiology of reptiles and amphibians: A user’s guide. J Exp Zool 335:13–44. https://doi.org/10.1002/jez.2396
Usategui-Martín A, Liria-Loza A, Valverde RA, Pinós-Crosas J, Tuya F, Carbajal A, López-Bejar M, Montero D (2021) Assessment of captive rearing conditions on loggerhead hatchlings: Effect of handling frequency and stocking density. J Exper Zool 335:489–498. https://doi.org/10.1002/jez.2469498|
Valiela I, Valiela I (1995) Marine ecological processes, vol 686. Springer, New York
Zug GR, Wynn AH, Ruckdeschel C (1986) Age determination of loggerhead sea turtles, Caretta caretta, by incremental growth marks in the skeleton. Smithson Contrib Zool. 427:34
Zug GR, Balazs GH, Wetherall JA (1995) Growth in juvenile loggerhead sea turtles (Caretta caretta) in the north pacific pelagic habitat. Copeia 195:484–487
Zug GR, Kalb HJ, Luzar SJ (1997) Age and growth in wild Kemp’s ridley sea turtles Lepidochelys kempii from skeletochronological data. Biol Conser 80:261–268
Zug GR, Balazs GH, Wetherall JA, Parker DM, Murakawa SK (2002) Age and growth of Hawaiian sea turtles (Chelonia mydas): an analysis based on skeletochronology. Fish Bull 100:117–127
Funding
Open Access funding provided thanks to the CRUE-CSIC agreement with Springer Nature. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. Material preparation, data collection and analysis were performed by Alejandro Usategui-Martín, Yania Paz-Sánchez and Ana Liria-Loza. The first draft of the manuscript was written by Alejandro Usategui-Martín and all authors commented on previous versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This is an observational study, so an ethical approval is not required.
Additional information
Responsible Editor: P. Casale .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Usategui-Martín, A., Valverde, R.A., Ostiategui-Francia, P. et al. First skeletochronological analysis on loggerhead yearlings (Caretta caretta) in the Canary Islands. Mar Biol 170, 95 (2023). https://doi.org/10.1007/s00227-023-04239-8
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04239-8