Abstract
The intensification of coastal development poses potential threats for coastal seabirds, and understanding their habitat use is a key factor to guide conservation and management. In sub-arctic areas, black guillemots (Cepphus grylle) use coastal habitats year-round, which makes them vulnerable to the increasing human activities in these areas. In mainland Norway, one of the species’ strongholds, black guillemots are red-listed after substantial population declines. However, their fine-scale foraging behaviour has received little attention to date. We collected and analysed GPS tracking data from adult black guillemots at three sites located over a latitudinal gradient of 250 km in central/northern Norway. Maximum foraging ranges of 33 km at Sklinna (65°12′N) for incubating birds, and 18 km at both Vega (65°34′N) and Sklinna for chick-rearing birds, are among the longest reported for this species. At all three sites, foraging probability was highest in shallow waters (< 50 m depth) close to the colony and declined with increasing water depth and distance from colony. However, birds from Vega also foraged over deeper waters. Kelp presence was of high importance at Sklinna, but apparently less important at Røst (67°26’N) and Vega. We also found distinct differences in foraging activity across the day and with tidal height among the sites. Inter-site differences in habitat use and foraging activity may be explained by differences in the availability of habitats and suitable prey. Our study highlights the importance of shallow marine areas for black guillemots and shows that habitat use can vary substantially between sites.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Seabirds are increasingly used as indicator species for ecosystem health and food availability (Piatt et al. 2008). This is because many of the species are highly sensitive to environmental changes, which also make them among the most threatened taxonomic groups of birds (Croxall et al. 2012). Seabirds are facing a multitude of anthropogenic stressors including introduced predators, climate change, and bycatch (Dias et al. 2019). In addition to changes in the food web due to ocean warming, spread of invasive species, pollution and human exploitation of fish stocks (Sydeman et al. 2017), human activities in the coastal zone are increasing worldwide (Crain et al. 2008; Brown et al. 2018). This coastal development includes aquaculture (Quick et al. 2004), harvesting of kelp forests (Christensen-Dalsgaard et al. 2020) and wind power installations at sea (Benjamins et al. 2020; Peschko et al. 2020a; 2020b), as well as increasing ship and boat traffic (Fliessbach et al. 2019; Dehnhard et al. 2020b), all of which may cause disturbance and lead to displacement of seabirds.
The foraging habitats used by seabirds during the breeding season are particularly important and relevant to take into account for sustainable marine spatial planning and protection plans, since reduced prey availability around the breeding site can have severe consequences for breeding success (Wanless et al. 2005; Ponchon et al. 2014; Divoky et al. 2015). This is amplified by the fact that the spatial range in which seabirds may find food resources is limited particularly during the breeding season, when parental birds engage in central-place foraging to optimize time at their nest sites attending their eggs and chicks (Bell 1990). Especially in those species that carry single prey items back to the nest to feed their chicks (‘single-prey loaders’, e.g. guillemots and terns), prey availability in close proximity to the breeding site during the chick-rearing period is of high importance, such that chicks receive a sufficiently high number of suitably sized and energy-rich prey items throughout each day (Monaghan et al. 1994; McLeay et al. 2009; Gaglio et al. 2018).
Black guillemots (Cepphus grylle) breed in the temperate and arctic zones of the Atlantic and Pacific Ocean and associated seas. The species is a single-prey loader that raises up to two chicks (Cairns 1987b; Divoky et al. 2021). In the Arctic, the species is often associated with sea ice (Divoky et al. 2016). In sub-arctic and temperate seas, however, black guillemots stay within the coastal zone (here including the continental shelf) and usually within 100 km of the nearest land all year-round and typically undertake rather short migrations (< 500 km) during the non-breeding season (Ewins 1988; Ewins and Kirk 1988; Bakken et al. 2003; Baak et al. 2021). They are therefore particularly sensitive to regional or local changes in coastal habitats. Black guillemots are among the seabird species most vulnerable to bycatch in coastal gillnet fisheries (Fangel et al. 2015; Bærum et al. 2019; Christensen-Dalsgaard et al. 2019), and the introduced American mink (Neogale vison) affects breeding success and adult survival of black guillemots in many colonies in Norway (Nordström et al. 2003; Fjeld et al. 2020). Increasing boat traffic and the associated disturbance may also be a problem for many coastal species (e.g. Dehnhard et al. 2020b) including black guillemots (Ronconi and St. Clair 2002). In addition, climate change can lead to changes in their food base and is expected to negatively affect population trends at the southern boundary of the species’ distribution range in the Baltic Sea (Buchadas and Hof 2017). Climate change is also considered a severe threat to kelp forest ecosystems (Smale 2020; Smith et al. 2023), which represent important foraging areas for black guillemots in Scotland (Owen 2015; Johnston et al. 2021). This also means that kelp harvesting poses a potential threat for black guillemots. Large-scale experiments have shown that kelp harvesting affects fish assemblages and is thought to decrease ecosystem resilience (Norderhaug et al. 2020). Assessments of impacts of kelp harvesting on seabirds are scarce, and so far limited to European shags (Gulosus aristotelis) (Christensen-Dalsgaard et al. 2020) and great cormorants (Phalacrocorax carbo) (Lorentsen et al. 2010b), while the industry in Norway is expanding northwards (Fiskeridirektoratet 2022).
Black guillemots are coastal foragers with typically shallow—and often benthic—diving patterns in the range of about 15–20 m, although they can dive down to a depth of at least 43 m (Masden et al. 2013; Shoji et al. 2015; Divoky et al. 2021). Diving activity of black guillemots in Northern Ireland occurred only during daylight hours (Shoji et al. 2015). Studies from Iceland, Scotland and Northern Ireland showed that the foraging range of chick-rearing individuals is typically less than 10 km, although trips up to 24 km have been observed (Petersen 1981; Sawyer 1999; Owen 2015; Shoji et al. 2015; Johnston et al. 2018, 2021). In Hudson Bay, Canada, foraging distances up to 15 km were observed during the breeding season (both incubation and chick-rearing), including observations of non-breeders and failed breeders (Cairns 1987a). In Scotland, the species forages particularly in kelp forests and often in environments with strong tidal currents (Masden et al. 2013; Owen 2015; Waggitt et al. 2017; Johnston et al. 2021), while the distribution of sea ice determines the habitat use in the high Arctic (Cairns 1987a; Divoky et al. 2021).
Matching their coastal diving foraging behaviour, black guillemot diet has been characterised by a number of benthic and kelp-forest associated fish species, varying with latitude, including sculpins (Cottidae), butterfish (Pholis gunnellus), gadoids (Gadiformes), sandeels (Ammodytidae) and squat lobsters (sub-order Anamura) in more temperate climates, and Arctic cod (Boreogadus saida) in the high Arctic (Slater and Slater 1972; Ewins 1990; Lønne and Gabrielsen 1992; Barrett and Anker-Nilssen 1997; Byers et al. 2010; Barrett et al. 2016; this study—see Supplement 1). Most diet data originate from observations of chick-feeding adults at the colonies, and black guillemots typically show a high chick-feeding rate in the early morning and during evening hours (Ewins 1990; Shoji et al. 2015).
The overarching goal of this study was to gain a better knowledge about the foraging behaviour of adult black guillemots in Norway during the breeding season. At least 10% of the global population of black guillemots breed in mainland Norway, with approximately 35,000 breeding pairs in 2005 (Barrett et al. 2006). However, the population is declining in many areas and black guillemots are now listed as “near threatened” in mainland Norway, with an estimated overall decline in the range of 15–30% over the last 30 years (Stokke et al. 2021). GPS tracking or seabird-at-sea observation data that could inform about the habitat use of black guillemots have essentially been lacking for the whole of mainland Norway. A better understanding of the foraging behaviour is much needed for conservation purposes and marine spatial planning, concerning especially the threats from bycatch and the growing kelp-harvesting industry in Norway.
Our study specifically aimed to document the fine-scale spatial habitat use by black guillemots and the environmental characteristics associated with these areas, and assess potential differences in foraging activity and habitat preferences between three different populations spread around the Arctic circle in northern and central Norway. We tested for variation in spatial habitat use, trip duration and foraging distance between the sexes and among sites, as well as for variation in environmental habitat preferences among the three populations. In addition, we examined how black guillemot foraging activity changed across the tidal cycle, as this may influence energetic costs of benthic diving, but also prey distribution (Wirjoatmodjo and Pitcher 1984; Hall et al. 1996; Enstipp et al. 2006) and the birds’ diurnal cycle (Ewins 1990; Shoji et al. 2015). With almost permanent daylight at our study sites during summer, and twilight prevailing throughout the darkest hours of the night, we predicted that black guillemots would extend foraging into night-time hours.
Methods
Fieldwork was conducted on Heimøya, Sklinna (65°12′N, 10°59′E) in 2013, 2018 and 2019, on Hernyken, Røst (67°25′N, 11°53′E) in 2019 and 2021, and on Muddvær, Vega (65°34′N, 11°41′E), in 2021. The population at Heimøya was stable with about 450 individual black guillemots during the study period, while the total number of birds in the entire Sklinna archipelago is likely somewhat higher. The population at Muddvær held about 100 individuals in 2021 and has a stable population trend since 2013, whereas Røst has more than 1500 pairs (Anker-Nilssen 2009), with 40–50 pairs at Hernyken and nearby islets, a population that has increased by 5% per year over the last decade (Anker-Nilssen unpubl. data).
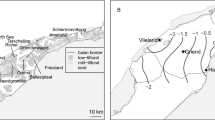
Both Sklinna and Røst are offshore archipelagos that are surrounded by deeper waters (Fig. 1). Sklinna measures about 4 by 4 km in size, with several islands and skerries (i.e. rocky islets). A few small skerries are located about 8 km south of Sklinna, while the nearest other land is 20 km to the south (outer islands of the larger Vikna archipelago, closer to the mainland). Twenty kilometres east of Sklinna is another extended shallow marine area with numerous skerries (Hortavær). Røst, by contrast, is about 20 by 10 km in size, with several hundred islands and skerries. From Hernyken, the nearest land outside Røst (Værøy) is found 37 km to the northeast (including 20 km of open sea). Vega is a large island separated from the mainland of Norway by 12 km of open water. Muddvær forms a small (3 by 2 km in size) archipelago, which is located 4 km south of Vega, and there are numerous skerries surrounding Muddvær.
Location of the three study sites (panel a, white stars) and GPS positions recorded at the different sites (white dots). Black guillemots were tracked during the chick-rearing period, but the datasets from Muddvær and Sklinna included individuals with unknown breeding status (and thus potential non-breeders). Black dots mark foraging locations as identified through EMbC (see Methods). Except for panel a, land areas are shown in grey, depths in shades of blue, with grey lines marking the 50 m isobaths. Green areas indicate kelp forests. Note that the scale differs between the plots
Black guillemots were tracked with 18 g ®Mataki GPS loggers (Debug Innovations, Cambridge, UK) in 2013 (Sklinna only) and 5–11 g ®PathTrack nanofix GPS loggers (Pathtrack Ltd, Otley, UK) in 2018, 2019 and 2021 (see Table 1). Both types of loggers remotely downloaded the recorded GPS data (at 5-min intervals for Mataki loggers, and either 30-min or 60-min intervals for PathTrack loggers) to one or two base stations placed in the colony. Mataki loggers were programmed to record data every 5 min, PathTrack loggers were programmed to record data at 4-min intervals at Sklinna and Muddvær, and 1- or 5-min intervals at Røst. Both logger types were attached with 1–2 g ®Tesa tape on the lower back of the birds and thus added mass represented between 1.6 and 5.5% of the birds’ body mass (Table 1). Birds were caught either by hand or with a crook on the nest, by mist net when approaching or leaving the colony, or with a leg-hold noose mat at a resting location at the edge of the colony. Birds were measured and weighed during handling, and for those that were not part of earlier studies, a small blood sample was taken from the foot web for molecular sexing. Handling time at deployment did not exceed 15 min. We attempted a second capture to recover loggers only in those cases where the nest site was known, but in most such cases, the logger had already fallen off.
Not all birds could be assigned to a nest, and the nest content was not known for all nests at Sklinna and Muddvær, while all birds at Røst were chick-rearing (see Table 1). We therefore cannot rule out that some of the birds that were tracked were non-breeders or had failed their breeding attempt. Loggers collected on average data for 2 days (range 1–7). We did not conduct systematic nest checks after deployment. Due to the short tracking durations per individual, we assumed that those birds that were incubating were doing so during the entire duration during which GPS loggers were attached and collected data. At Røst and Muddvær, chick-rearing individuals that were deployed with GPS loggers were observed to continue to feed their chicks. There were no indications that nests failed either due to handling of the birds or from other causes, while one of their parents was carrying a GPS logger. Since our study design was not balanced with respect to breeding stages (Table 1), we performed all statistical analyses both for the entire dataset (i.e. pooling all available data) and for chick-rearing individuals only (i.e. subsampling the data from Sklinna and Muddvær).
Identification of foraging locations
We identified the most likely foraging locations based on expectation–maximization binary clustering (EMbC) (Garriga et al. 2016b). EMbC uses velocity and turning angle to classify movement data into four different clusters aligned with likely behavioural states: low velocities and low turns (LL; “resting”), low velocities and high turns (LH; “intensive search”), high velocities and low turns (HL; “travelling”) and high velocities and high turns (HH; “extensive search”) (Garriga et al. 2016b). EMbC has been shown to be useful across a broad range of species (e.g. Cecere et al. 2020; De Pascalis et al. 2020; Dehnhard et al. 2020a; 2022) and comes with the advantage of requiring less supervision, less a-priori assumptions and less computational power than other approaches (Garriga et al. 2016b).
GPS data were analysed in the EMbC R-package (Garriga et al. 2016a), using the stack clustering function stbc, which accounts for potential among-individual behavioural differences by annotating behavioural states for each individual. GPS data were not interpolated, and thus, sampling intervals differed within and between individuals. EMbC has proven to be robust towards variation in sampling intervals (Garriga et al. 2016b). The same approach (i.e. stack-clustering of GPS data with different sampling intervals) worked well for European shags, where sampling intervals differed substantially more than in our current dataset (Dehnhard et al. 2022).
The stack clustering function was run on data that were pooled for all years and sites, in order to guarantee the same cut-offs among years and sites. The pre- and post-smoothing options were set to zero. Previous studies on European shags (Dehnhard et al. 2022) and Antarctic fulmarine petrels (Dehnhard et al. 2020a) found a good match between EMbC stages LL and LH with simultaneously collected diving and wet-dry data, respectively. Lacking simultaneously collected diving data in this current study, we here adopted the same approach and defined all GPS time stamps with EMbC states LL and LH as foraging locations in further analyses.
We excluded locations within 300 m of the centre of all colonies, since these locations are strongly associated with resting, preening and social display activities, besides some foraging that happens close to the colony. Distinguishing between foraging and non-foraging activities in this area is thus very challenging and would require the use of dive loggers in addition to GPS loggers. However, the proximity to the colony in itself means that this area is of high importance for the birds, while we lack information about the importance of areas further away. Similarly, any roosting places at or near foraging sites were removed, i.e. when GPS time stamps were located on land and not at sea. One apparent locational outlier was detected and removed.
When comparing foraging trip metrics between populations (Table 2), we excluded incomplete foraging trips. We defined foraging trips as movement paths ≥ 5 min ≥ 300 m away from the colony. Trips were considered incomplete when (1) locations at the colony were not available either immediately before or after the trip, (2) gaps of > 10 min existed between the last and/or first location in the trip and the next or previous location at the colony, and (3) gaps of > 30 min existed between locations during the trip. Due to the inability of GPS loggers to acquire locations when submerged (i.e. when the bird is diving), trips recorded by both logger types frequently included gaps between GPS locations. The 30-min cut-off to define incomplete trips was chosen as a conservative measure based on average trip duration (see Table 2) and the obtained GPS intervals. Since black guillemots breed in sheltered cavities under rocks, short visits to the nests to feed the chicks may be difficult to detect with the chosen settings of the GPS loggers and the above-named cut-offs. To make sure trips were identified correctly, we therefore also visually inspected distance from colony over time and could confirm that the above-listed thresholds also correctly identified such trips where parental birds only returned very briefly to their nests to feed the chicks.
Environmental variables
To identify marine habitat preferences, we selected three environmental variables that characterise the coastal habitat black guillemots typically exploit (Johnston et al. 2018): sea depth, seabed slope and the presence/absence of kelp. We downloaded bathymetry data from GEBCO (https://www.gebco.net/data_and_products/gridded_bathymetry_data/), which comes as a spatial grid with a resolution of 15 × 15 arc seconds. Slope (in degrees) was calculated from the bathymetry data as the maximum change from the location grid cell to its eight closest neighbours using the R-package terra (Hijmans 2021). Kelp data as modelled by the Norwegian Institute for Water Research (NIVA) based on depth, wave activity, salinity and several other factors at 10 m spatial resolution (Bekkby et al. 2009; 2020) were obtained from The Norwegian Environment Agency (https://geocortex01.miljodirektoratet.no/Html5Viewer/?viewer=naturbase). Sea bottom substrate data from the European Marine Observation and Data Network (EMODnet) (http://gis.ices.dk/geonetwork/srv/eng/catalog.search#/metadata/01bf1f24-fdcd-4ee7-af8b-e62cf72fe2f9) were considered, but since data coverage in the coastal areas was poor, we refrained from including this variable in our analyses.
To assess whether foraging activity differed with tidal height, we downloaded hourly water level observations for the three sites from the website of the Norwegian Mapping Authority (https://www.kartverket.no/en/at-sea/se-havniva). We accessed daily data for time of sunrise and sunset at the breeding sites from www.timeanddate.no. These were not included in statistical analyses but used for visualisation.
Statistics
All statistical procedures were carried out in R (version R-4.2.2; Development Core Team 2022). Due to the low number of complete foraging trips in some of the years, we pooled all available data per site and refrained from investigating inter-annual effects. We investigated differences in foraging trip metrics between sites, breeding stages (where known) and sexes, using linear mixed models (LMMs) in the R-package lme4 (Bates et al. 2015). P-values were calculated with lmerTest (Kuznetsova et al. 2014). Models were run with either maximum distance from colony or trip duration as dependent variable, and bird ID as random factor. We ran two LMMs with site, breeding stage and sex (no interaction terms) as explanatory variables. Breeding stage could influence both maximum distance from colony and trip duration. Since chick-rearing was the only breeding stage during which birds from all three sites were tracked, we additionally ran two LMMs as described above using only data from this stage to check whether breeding stage had a strong influence on model results and thus inter-site differences.
To investigate marine habitat preferences, we used resource-selection functions (Boyce and McDonald 1999; Signer et al. 2019; Northrup et al. 2022), i.e. we compared covariates associated with the foraging locations of the birds with random locations within the spatially available habitat. We defined available habitat as the area within reach for birds around their colonies, thus creating a circular buffer around each colony. As in other studies comparing the habitat preferences of birds originating from different colonies (Christensen-Dalsgaard et al. 2017; Péron et al. 2018; Dehnhard et al. 2022), the radius was set as the maximum distance between a registered foraging location and the colony, separately per site (cf. Table 2). Since there was evidence for breeding stage to have an effect on maximum distance from colony, and particularly chick-rearing birds to travel shorter distances than incubating birds (see Results), we applied a different radius for those birds from Sklinna that were known to be chick-rearing. The radius was thus largest for incubating birds and those with unknown breeding status from Sklinna (33 km). Chick-rearing birds from Sklinna had a radius of 18 km, and thus the same as chick-rearing birds and those with unknown breeding status from Muddvær (both 18 km). With 11 km, birds from Røst (all chick-rearing) had the smallest radius. To create a representative sample of the available habitats within these areas, five point locations were created randomly for each foraging location, separately per individual, within the defined available area, using the R-package sf (Pebesma 2018). Land areas within the circular buffers were removed before generating random locations. All foraging locations and random locations were intersected with the environmental layers using the R-packages sf and terra.
We then ran generalised additive mixed models (GAMMs) with a binomial distribution (1 = foraging locations, 0 = available, i.e. random locations). Like generalised additive models, GAMMs allow the fitting of nonlinear responses to predictor variables, which is a major advantage as animals rarely respond linearly to their environment (Aarts et al. 2008; Dehnhard et al. 2020a). The GAMMs were run using the R-package mgcv (version 1.8–38; Wood 2017) with a logit link function. Similarly to Christensen-Dalsgaard et al. (2017), models were fitted using thin-plate regression smoothing (Wood 2017). We initially set the maximum number of knots for smooth terms to 5 in order to avoid overfitting and used the gam.check function to check whether models with less knots had a better fit. GAMMs were run separately for each site. For Muddvær and Sklinna, where the breeding stage was unknown for some individuals, we ran models twice: once with the full dataset and once only for chick-rearing birds. We followed a forward-stepwise approach to add environmental covariates and modelled the environmental habitat preferences separately for each site. The initial models therefore contained one environmental covariate, either as fixed factor (kelp) or as smooth term (depth, slope, distance from colony) as well as Bird ID as random effect. After identifying the best-performing environmental variable, we assessed the inclusion of a second and third environmental variable. To avoid collinearity, we only included environmental covariates in the same model which had a mutual Spearman’s rank correlation coefficient of ≤ 0.5 (for the Muddvær dataset depth and slope were correlated, and for the Røst dataset depth and distance from colony were correlated). Model selection was based on the Akaike information criterion (AIC), and we did not attempt to fit more than three terms into the final model to avoid over-fitting.
Finally, we analysed whether time of day and water-level changes caused by tidal activity had any impact on foraging activity. For this purpose, we used the entire dataset, i.e. including also locations within 300 m of the centre of the colony. Foraging activity (as binomial response variable) was defined as time stamps associated with foraging locations (coded as 1, based on EMbC, as described above); all other time stamps (including all locations within 300 m of the colony) were defined as non-foraging activity (coded as 0). As for the other analyses, we pooled data from all years per site, and once more, for Muddvær and Sklinna, where the breeding stage was unknown for some individuals, we ran models twice: once with the full dataset and once only for chick-rearing birds. The main GAMM thus contained foraging activity as binary response variable, and both hour of the day and water level as explanatory variables. We used thin-plate regression smooth terms for water level, and cyclic cubic regression splines for hour of the day (since hour of the day is circular). For the number of knots, we proceeded as described above. Bird ID was again included as a random effect.
Instead of referring to p-values as cut-offs to significance testing, we used the language of evidence throughout this paper, thus referring to strong or weak evidence for/against differences between groups (Muff et al. 2021).
Results
Foraging trip metrics between sites, breeding stages and sexes
Using all available data from complete foraging trips, including those of potentially non- or failed breeders, there was no evidence that maximum distance from colony or trip duration in black guillemots differed among sites (LMMs, all F2 ≤ 1.87, p ≥ 0.209) or sexes (LMMs, all F1 ≤ 0.76, p ≥ 0.408). There was weak evidence that both maximum distance from colony and trip duration were longer during incubation compared to chick-rearing (LMMs, all F2 ≥ 3.65, p ≤ 0.080), matching with the longest foraging trip being conducted by an incubating bird from Sklinna (33 km). During chick-rearing, birds foraged within 18 km around Vega and Sklinna, and 11 km around Røst, but typically closer to the colonies as reflected by the average trip distances (Table 2; Supplement 2). Using a subset of only chick-rearing birds, we found no evidence for maximum distance from colony or trip duration to differ among sites (LMMs; all F2 ≤ 2.14, p ≥ 0.136) or between sexes (F1 ≤ 0.55, p ≥ 0.472).
Marine habitat preferences
Distance from colony and depth were the two most important environmental variable to explain foraging habitat, whether the full dataset was used, or only data from chick-rearing individuals. Distance from colony explained ≥ 43% of deviance in the models, and distance from colony was the single-most important variable at both Røst and Muddvær (Table 3). At Sklinna, depth was the best predictor of foraging habitat (51% of deviance explained for all birds, and 68% of deviance explained for chick-rearing individuals), while depth was of secondary importance at Røst (27% of deviance explained) and Muddvær (≤ 9% of deviance explained). Kelp presence was of high importance at Sklinna but was the least supported variable in models for both Røst and Muddvær (Table 3, Figs. 1 & S3.1). Slope was of lowest importance at Sklinna and ranked third (before kelp) at Muddvær and Røst (Table 3). Since depth was correlated with distance from colony at Røst, the final composite model for Røst did not include depth, but slope and kelp. At Muddvær, slope was correlated with depth, and therefore, the final composite model for Muddvær did not include slope but kelp. Final GAMMs thus had a different structure for each of the three sites, reflecting somewhat different habitat preferences among the three sites (Table 3). For Muddvær, model structure was identical for the full dataset and chick-rearing individuals. For Sklinna, distance from colony was of higher importance in the models for chick-rearing individuals compared to the full dataset (Table 3).
GAMM response curves for depth, distance from colony and slope showed some variation among sites for birds independent of their breeding status (Fig. 2; Figure S3.2). While black guillemots at Røst and Sklinna showed a clear preference to forage in shallow waters, with foraging probability declining steeply with depth, this pattern was less pronounced for birds from Muddvær, which showed a peak in foraging probability at a water depth of about 120 m (Fig. 2). Foraging probability decreased with distance from colony for birds from all three sites, but in all cases there was a second peak in foraging probability further away, the location of which differed between sites (~ 7 km for Røst, ~ 12 km for Muddvær and ~ 16 km for Sklinna; Fig. 2). Finally, foraging probability in response to seabed slope varied strongly among sites. Birds from Røst foraged over areas that were essentially flat (< 2°), and seabed slope in the available habitat around the colony was never more pronounced than 5°, limiting the importance of this factor. The available habitat for birds from Sklinna and Muddvær showed a larger variation, with slopes down to almost 20°, but foraging activity at both sites was limited to flat or moderately sloped seabed areas (Fig. 2). The response curves of models run on the full dataset for Sklinna and Muddvær, i.e. including birds with unknown breeding status (both sites) and incubating birds (Sklinna only), were similar to those of the chick-rearing individuals (Figure S3.2) with one exception: The second peak in foraging probability for birds from Sklinna was further away (~ 12–20 km) than for chick-rearing individuals, and there was another small increase in foraging probability at 30 km distance from colony (Figure S3.2).
Model response curves (fitted values ± CI) from the GAMMs showing the predicted foraging probability in response to depth (top), distance from colony (middle) and seabed slope (bottom) for all individuals (also those with unknown breeding status) from the different study populations. Fitted values were extracted from the individual models per site (e.g. models RØ1-3 for Røst; see Table 3)
Effects of time of day and water-level changes
There was strong-to-very-strong evidence that water level and hour of the day affected foraging activity of black guillemots (GAMM all chi-square ≥ 15.71, all p ≤ 0.001; Fig. 3). Model results and model responses were very similar when considering only chick-rearing birds (Figure S3.3). The nature of the responses to water level and hour of the day differed, however, markedly among sites. At Røst and Muddvær, foraging activity over the course of the day showed very pronounced but inverse patterns. At Muddvær, foraging activity increased during the afternoon and was highest between 20:00 and 05:00 local time, and thus during several hours of civil twilight, whereas Røst birds were relatively inactive during these hours and foraged mostly during the middle of the day. At Sklinna, foraging activity showed a less pronounced pattern throughout the day than at the other sites, but was somewhat higher from the afternoon and throughout the night (from 15:00 to 05:00 local time), with lower activity between 05:00 and 15:00. At Røst, foraging activity responded almost linearly to water levels, with highest activities coinciding with the lowest water levels. Both Sklinna and Muddvær showed a peak in foraging activity at intermediate water levels, but while foraging activity declined during high tide at Sklinna, it increased further at Muddvær (Fig. 3).
Response curves from the GAMM testing the effect of hour of the day (left) and water level (right) on foraging activity of all individuals (also those with unknown breeding status) at the three sites. For Røst and Sklinna, data from all years were pooled. The model included the smoothed effects for both water level and hour of the day, as well as the interaction of both with site. Chi-square and p-values refer to the summary statistics of the GAMM. Grey areas for the plots on the left-hand side indicate the maximum twilight hours at the sites when birds were tracked. Hour of the day refers to local time (i.e. GMT + 2 h)
Discussion
Foraging range and trip duration
Foraging range and trip duration did not differ statistically among our three study sites. Irrespective of breeding status, most of the foraging activity took place close to the colonies (cf. Figure 1, Figure S3.1) and thus within the typical foraging range of < 10 km around the colonies during chick-rearing reported elsewhere in Europe (Petersen 1981; Sawyer 1999; Owen 2015; Shoji et al. 2015; Johnston et al. 2018; Owen et al. 2019). However, in contrast to these previous studies and the results from Røst, chick-rearing birds from Sklinna and Muddvær travelled up to 18 km away from their colonies to find food. We also found evidence for breeding stage to affect foraging ranges, with chick-rearing birds travelling shorter distances than incubating birds. This has also been observed in Brünnich’s guillemots (Uria lomvia; Ito et al. 2010), yet it does not seem to be a universal pattern in alcids, since it was not found in common guillemots (Uria aalge; Gulka and Davoren 2019) and little auks (Alle alle; Jakubas et al. 2014). A recent study from Caithness, Scotland, also found black guillemots to travel further (up to 26 km) from their colony during the incubation period than during chick-rearing (up to 12 km; Johnston et al. 2021). Maximum foraging ranges of black guillemots from Sklinna (33 km during incubation and 18 km during chick-rearing, respectively) were even longer and are among the longest reported so far for the species during the breeding period.
A number of factors can explain variation in foraging range among breeding sites of black guillemots. Local distribution and patchiness of suitable foraging habitat as well as prey availability seem to be the most important factors (addressed below). In addition, breeding status is clearly important, possibly also chick age and whether parents are provisioning one or two chicks: Being able to carry only one fish at a time in the beak while raising two chicks requires a higher feeding frequency of both parents—and may force them to forage closer to the breeding site (Cairns 1987b; Divoky et al. 2021). Unfortunately, we only had exact data on chick age and brood size for 5 GPS birds at Røst and could not investigate this relationship any further.
GPS loggers in themselves may also affect the behaviour of the tracked birds, due to the added weight and drag (Barron et al. 2010; Vandenabeele et al. 2011, 2012; Evans et al. 2020). Black-legged kittiwakes (Rissa tridactyla) equipped with GPS-loggers performed longer-lasting trips compared to control birds (Heggøy et al. 2015). The same pattern was found in a meta-analysis studying potential impacts of biologging (Bodey et al. 2018). In our study, we used logger types of different sizes, and the added mass (including tape) ranged between 1.5% (Røst 2021) and 5.5% (Sklinna 2013) of the birds’ body mass (Table 1), exceeding the commonly recommended threshold of 3% (Vandenabeele et al. 2012) at both Sklinna and Muddvær. Altogether, we cannot rule out logger effects and would certainly recommend to always use the smallest loggers available (both in size and mass), and not to vary logger sizes across sites and years, when logistically and financially feasible. Finally, since black guillemots are very efficient in removing their loggers (in some cases within less than 24 h), we are confident that the logger attachment had no long-lasting negative impacts on the tracked birds.
Environmental habitat preferences
Matching previous studies on habitat use of black guillemots in the North Atlantic (reviewed in Johnston et al. 2018), we found that shallow (< 50 m) marine habitat near the colony was of high importance for this species also at the three Norwegian study sites. However, there were distinct differences in habitat preferences among sites. Firstly, distance from colony was more important at Røst and Muddvær compared to Sklinna, where depth was the most important variable. Secondly, the presence of kelp forests was strongly associated with foraging habitat around Sklinna, but less so at Røst and Muddvær. Thirdly, while models suggested foraging areas of all three populations were associated with shallow waters, birds from Muddvær appeared to be foraging in areas with a depth of about 120 m. Finally, we found that environmental habitat preferences differed only slightly (and only at Sklinna) between the subset of chick-rearing birds compared to the full dataset including incubating birds and those with an unknown breeding status: The main difference was that distance from colony was more important than the presence of kelp forest for chick-rearing birds, but the final model response curves looked almost identical for both groups (cf. Figure 2 versus Figure S3.2). Our results match somewhat with those for little terns (Sternula albifrons), where failed breeders conducted longer-lasting foraging trips than successful breeders (Perrow et al. 2015). In lesser black-backed gulls (Larus fuscus), however, foraging trip metrics were similar between failed and successful breeders, but use of terrestrial versus marine habitats differed between the two groups (Camphuysen et al. 2015). Although we were unable to determine the breeding status of all the birds we tracked, our results reflect the overall importance of proximity to the breeding site, shallow marine habitat and kelp forests for black guillemots during the breeding period.
The differences in habitat preferences among sites may be explained by differences in the availability of shallow marine habitat, kelp forest distribution and differences in diet. Being a rather small archipelago (about 4 by 4 km) surrounded by deeper waters, Sklinna by itself does not hold much of the shallow marine area preferred by black guillemots (cf. Fig. 1). By contrast, the availability of suitable foraging habitats in the vicinity of the colonies at Røst (lying on a shallow plateau of about 20 by 10 km in size, also surrounded by deeper waters) and Muddvær (surrounded by extensive shallow waters close to the Norwegian mainland) is much larger. These differences in habitat availability and the fact that birds from Sklinna often crossed deeper waters before reaching shallow foraging locations (see Fig. 1), likely explain why depth was a more important variable in the habitat models for Sklinna than distance from colony. Furthermore, the majority of benthic marine habitat of < 50 m depth around Sklinna is covered by kelp forests (cf. Fig. 1d). By contrast, kelp forests overall cover less of the shallow areas around Muddvær and Røst (cf. Fig. 1b and 1c), which also have large patches of sandy bottom as well as beds of dead coralline algae, a substrate that kelp cannot attach to (Bekkby et al. 2009). In addition, not all rocky substrate may hold kelp forest in these areas either, since overgrazing by green sea urchins (Strongylocentrotus droebachiensis) has decimated kelp forests around Vega and other parts of Northern Norway, and kelp forests are only slowly recovering (Christie et al. 2019; Eikrem et al. 2019). Finally, the chick diet of black guillemots at Røst and Sklinna is generally similar, but butterfish is a much more common prey item at Røst (Barrett and Anker-Nilssen 1997; Lorentsen et al. 2010a). This was also the case in the years during which GPS-tracking took place (Fayet and Anker-Nilssen, unpublished data). However, butterfish is not exclusively associated with kelp forests (Koop and Gibson 1991; Shorty and Gannon 2013). This could explain why kelp forests were not an equally important foraging habitat at Røst as at Sklinna. It is further possible that the adults targeted predominately kelp forested areas close to their colony to find food for their chicks, but travelled further away and westwards (cf. Fig. 1b) for self-feeding. That adult diet differs from chick diet is not uncommon in alcids (Davoren and Burger 1999; Wilson 2004; Breed et al. 2009; Myksvoll et al. 2013), including black guillemots (Ewins 1990). Finally, black guillemots are known to show individual specialisation in both foraging area (Johnston 2019; Owen et al. 2019) and diet (Slater and Slater 1972). For example, some individuals may specialise to feed on butterfish, and others on sculpins. We did not record the detailed diet of those individuals equipped with GPS-loggers and thus do not know whether and how many of the individuals tracked specialised on particular diet items.
In contrast to Røst and Sklinna, chick diet at Muddvær was dominated by lesser sandeel (Ammodytes marinus), followed by haddock (Melanogrammus aeglefinus), saithe and butterfish (Supplement 1). Sandeels are strongly associated with sandy bottoms, and due to their high energy-content, they are also considered to be excellent food for seabird chicks (Wanless et al. 2018). The importance of sandeel in the chick diet, and the association of sandeel with sandy substrates, may thus explain the lower importance of kelp forests in habitat models for Muddvær. Sandeels show high annual variability in their presence (Frederiksen et al. 2005) and high sensitivity towards global warming (Lindegren et al. 2018). Unfortunately, fieldwork at Muddvær took place only during one breeding season, and we therefore do not know whether sandeels are of equally high importance in other years and throughout the entire breeding season. Saithe and haddock were also important prey items at Muddvær and may not have been associated exclusively with kelp forests: Based on their size, both haddock and saithe delivered to chicks at Muddvær and Røst were 0-group fish (< 1 year old; Neuheimer et al. 2008; Hillersøy and Lorentsen 2012). Both of these fish species have a pelagic juvenile phase, before settling to the seabed (haddock) and into kelp forests (saithe), respectively (Olsen et al. 2009). As indicated by their silvery colour, at least some of the saithe were still in their pelagic life stage, thus contributing to an explanation for the lower importance of kelp forests as foraging habitat around Muddvær and Røst.
The peak in foraging probability in areas with a depth of ~ 120 m may be explained by the prey selection of birds at Muddvær. This is more than twice the maximum diving depth recorded for black guillemots (50 m; Cairns 1987a; Masden et al. 2013; Johnston et al. 2018), and it is thus highly unlikely they were performing benthic dives in such deep waters. Feeding on juvenile saithe and haddock in their pelagic life stages, however, may well happen over deeper waters, without the need for black guillemots to dive down to the bottom. Also, sandeels occur at water depths down to 120 m, although they prefer depths of 30–70 m (Wright et al. 1998; van der Kooij et al. 2008). In spring and summer, they do, however, exhibit a diurnal change between staying in the sand at night, and feeding in fast-swimming schools in the upper water column, often close to the surface, during daytime (Winslade 1974; van der Kooij et al. 2008). As the black guillemots’ foraging activity over deeper waters (> 30 m, i.e. outside of kelp forests) occurred mostly between midnight and 07:00 local time (Supplement 4; see also below), it still remains unclear what prey were targeted there.
Highly relevant for conservation, the modelled habitat preference of black guillemots for foraging depth (Fig. 2) matches not only the shallow coastal areas, but looks almost identical to the variation in modelled bycatch rates of diving seabirds in response to fishing depth of coastal gillnetting in Norway (cf. Figure 3 in Bærum et al. 2019). In other words, their preference for shallow coastal areas makes black guillemots particularly vulnerable to bycatch. Nevertheless, Bærum et al. (2019) found other species to be even more frequently taken as bycatch in coastal gillnet fisheries.
Another concern is the importance of kelp forest in habitat models, specifically around Sklinna, and to a lesser extent also around Røst and Muddvær. This raises the question to what extent kelp harvesting affects prey availability and, thereby, the breeding success of black guillemots. Kelp harvesting takes place in the same areas as those utilized by foraging black guillemots from Sklinna (c.f. maps in Christensen-Dalsgaard et al. 2020). In 2022, the Norwegian Fishery Directorate extended the harvesting areas northwards, with the northern border put immediately south of Muddvær, without indicating if this will remain a longer-term limitation (Fiskeridirektoratet 2022). Two of the nine birds from Muddvær tracked in this study entered areas that now have been trawled or will be in the coming years.
The issues of kelp harvesting and bycatch highlight the very common management dilemma that most often, even in the case of coastal species, only the seabirds’ breeding sites are protected, but not the areas they rely on to find their food (Yorio 2009; Critchley et al. 2018). In the light of the many declining seabird populations in Norway (Fauchald et al. 2015; Anker-Nilssen et al. 2022), where almost two-thirds of the seabird species are now on the national red list (Stokke et al. 2021), it is high time to implement effective conservation actions and protect important foraging habitats.
Differences in foraging activity with time of day and water level among populations
Rather surprisingly, we found that foraging activity in response to water level and time of day differed distinctly among the study populations. At Røst, foraging activity was highest at low water levels, while at Sklinna, it was highest at intermediate water levels, and lower (but also associated with higher model uncertainty) at very high and low water levels. Finally, birds at Muddvær showed high foraging activity also at high water levels. Tidal range is on average between 1.6 and 1.7 m for all three sites and thus would by itself not lead to differential energetic costs for diving among sites. It therefore seems more likely that differences in habitat and prey availability explain the differential responses to water levels among the three sites.
Black guillemots are known to occur in habitats with strong tidal currents (Masden et al. 2013; Owen 2015; Waggitt et al. 2017). Tidal currents are strongest at intermediate water levels, which is when the foraging activity at Sklinna was highest. Possibly, certain fish species are easier to catch under strong current conditions, e.g. if the accessibility of lower trophic level prey is positively related to tidal currents and the fish change behaviour accordingly (e.g. Hall et al. 1996). Other benthic fish species may be more accessible during high or low tide (Wirjoatmodjo and Pitcher 1984). In addition, black guillemots from Muddvær also foraged over deeper waters, where they may have fed on pelagic sandeels or young gadoids in the upper water column and therefore were less dependent on effects of tidal currents on prey availability in benthic/kelp forest habitats.
We found clear indications for black guillemots from both Sklinna and Muddvær to forage in civil twilight conditions during the night, whereas birds from Røst avoided night-time foraging. A previous study performed in Northern Ireland also reported that diving only occurred during daytime hours, and not between 22:00 and 03:00 GMT (Shoji et al. 2015). We cannot rule out that the EMbC misidentified resting on the water as foraging—testing this would require simultaneous GPS and diving data. Similarly, social displays, which often take place near the colony in the early morning hours, especially at high tides, may have been categorized as foraging. However, it seems unlikely that misidentified foraging behaviour would cause patterns to differ so strongly among populations. Moreover, our data confirm that birds from Sklinna and Muddvær were regularly more than 5 km away from their colony at night-time hours, whereas birds from Røst stayed within a range of about 1 km of the colony at night (Supplement 4). In addition, night-time foraging of black guillemots from Muddvær was identified in waters more than 75 m deep (Supplement 4). This suggests that black guillemots from Sklinna and Muddvær were actively foraging at night, and certainly not engaging in social activities, which is only known to happen near their colonies (i.e. within the 300 m range where all behaviour was coded as “non-foraging”; see Methods). In contrast to the study by Shoji et al. (2015), our more northern study locations are not totally dark in summer. Civil twilight (i.e. when the sun is at an angle of 0–6° under the horizon) prevailed throughout all nights of our study, with exception for the last 5 nights of GPS tracking of one bird at Røst in first half of August 2021, when nautical twilight (sun angle 6–12° under the horizon) prevailed for up to 3 h, but it was still not completely dark. This late tracking event at Røst may thus have had a slight influence on the patterns we found. On the other hand, nocturnal foraging has been observed in both Brünnich’s and common guillemots (Regular et al. 2011; Elliott and Gaston 2015; Dunn et al. 2020; Patterson et al. 2022). It thus seems very likely that black guillemots are able to forage under nautical twilight conditions, especially since the species also winters along the coastline of North Norway and along the ice edge in Svalbard, as well as in other high-Arctic environments (e.g. Divoky et al. 2016). Daylength during winter is short at these latitudes, and other diving seabirds wintering in the Arctic are also known to forage during civil and nautical twilight conditions (e.g. Moe et al. 2021).
Prey choice may also have had an influence on the feeding patterns. However, we do not know what type of prey the GPS birds were feeding on, nor if foraging at night was more associated with self-feeding than providing for chicks.
In the larger perspective, and independent of the birds’ foraging strategies, the fact that black guillemots in northern and central Norway during the breeding season also forage at night-time may be important information for conservation actions. As discussed above, one of the main threats for black guillemots is bycatch in gillnet fisheries (Fangel et al. 2015; Bærum et al. 2019; Christensen-Dalsgaard et al. 2019). Diel restrictions on gillnet-fishing have been considered in some fisheries as a measure to reduce bycatch (Melvin et al. 1999). For example, a recent study from the UK suggested night-only gillnet fishing as a potential mitigation measure to reduce bycatch of razorbills (Alca torda), common guillemots and European shags (Cleasby et al. 2022). Considering our results, such a policy would likely have limited effect on black guillemot bycatch in central and northern parts of Norway.
Conclusions
Our study confirmed that shallow marine habitat within 10–30 km of the breeding site is the most important foraging habitat for black guillemots. The presence of kelp forests was also important, but not equally important at all sites. Differences among sites in foraging habitat preferences as well as diurnal foraging patterns, including also night-time foraging at Sklinna and Muddvær, are likely associated with differences in habitat availability and bathymetry as well as different availability of local fish prey. Our study is the first to provide information about the fine-scale foraging behaviour of black guillemots in Norway, and thereby provides important knowledge to inform and improve conservation actions.
In 2015, the UN formulated the global goal to protect 30% of the marine environment, including coastal zones, by 2030 (Maestro et al. 2019). As of 2021, Norway had only protected 3.6% of its territorial waters (Statistics Norway 2022). Based on our results and parallel work on other species (e.g. Dehnhard et al. 2022), protection of shallow marine areas and kelp forests in the vicinity of important breeding sites of coastal, fish-eating seabirds appears to be a good strategy for conservation of marine biodiversity in a changing world with increasing anthropogenic pressures.
Data availability
The datasets generated by and/or analysed in the current study are available from the corresponding author on reasonable request.
References
Aarts G, MacKenzie M, McConnell B, Fedak M, Matthiopoulos J (2008) Estimating space-use and habitat preference from wildlife telemetry data. Ecography 31:140–160. https://doi.org/10.1111/j.2007.0906-7590.05236.x
Anker-Nilssen T (2009) Ornithological values of the Lofoten Islands in a world heritage perspective: An update as of (2009) Norwegian institute for nature research (NINA). Trondheim, Norway
Anker-Nilssen T, Hanssen SA, Moe B, Systad GHR, Barrett RT, Bustnes JO, Chrisensen-Dalsgaard S, Dehnhard N, Descamps S, Erikstad KE, Follestad A, Langset M, Layton-Matthews K, Lorentsen S-H, Lorentzen E, Reiertsen T, Strøm H (2022) Key-site monitoring in Norway 2021, including Svalbard and Jan Mayen. SEAPOP, Trondheim
Baak JE, Leonard ML, Gjerdrum C, Dodds MD, Ronconi RA (2021) Non-breeding movements and foraging ecology of the black guillemot Cepphus grylle in Atlantic Canada. Mar Ornithol 49:57–70
Bærum KM, Anker-Nilssen T, Christensen-Dalsgaard S, Fangel K, Williams T, Vølstad JH (2019) Spatial and temporal variations in seabird bycatch: Incidental bycatch in the Norwegian coastal gillnet-fishery. PLoS ONE 14:e0212786. https://doi.org/10.1371/journal.pone.0212786
Bakken V, Runde O, Tjørve E (2003) Norsk ringmerkingsatlas, vol 1. Stavanger Museum, Stavanger, Norway
Barrett R, Anker-Nilssen T (1997) Egg-laying, chick growth and food of black guillemots Cepphus grylle in North Norway. Fauna Norvegica, Series C 20:69–79
Barrett R, Lorentsen S-H, Anker-Nilssen T (2006) The status of seabirds breeding in mainland Norway. Atlantic Seabirds 8:97–126
Barrett R, Christensen-Dalsgaard S, Anker-Nilssen T, Langset M, Fangel K (2016) Diet of adult and immature black guillemots Cepphus grylle. Seabird 29:1–14
Barron DG, Brawn JD, Weatherhead PJ (2010) Meta-analysis of transmitter effects on avian behaviour and ecology. Meth Ecol Evol 1:180–187. https://doi.org/10.1111/j.2041-210X.2010.00013.x
Bates D, Maechler M, Bolker B (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67:1–48. https://doi.org/10.18637/jss.v067.i01
Bekkby T, Rinde E, Erikstad L, Bakkestuen V (2009) Spatial predictive distribution modelling of the kelp species Laminaria hyperborea. ICES J Mar Sci 66:2106–2115. https://doi.org/10.1093/icesjms/fsp195
Bekkby T, Rinde E, Espeland SH, Olsen HA, Thormar J, Grefsrud ES, Bøe R, Freitas C, Moy FE (2020) Nasjonal kartlegging - kyst 2019. Ny revisjon av kriterier for verdisetting av marine naturtyper og nøkkelområder for arter. Norwegian Institute for Water Research (NIVA), Oslo
Bell WJ (1990) Central place foraging searching behaviour: the behavioural ecology of finding resources. Springer, Netherlands, Dordrecht, pp 171–187
Benjamins S, Masden E, Collu M (2020) Integrating wind turbines and fish farms: an evaluation of potential risks to marine and coastal bird species. J Mar Sci Engin 8:414. https://doi.org/10.3390/jmse8060414
Bodey TW, Cleasby IR, Bell F, Parr N, Schultz A, Votier SC, Bearhop S (2018) A phylogenetically controlled meta-analysis of biologging device effects on birds: deleterious effects and a call for more standardized reporting of study data. Meth Ecol Evol 9:946–955. https://doi.org/10.1111/2041-210X.12934
Boyce MS, McDonald LL (1999) Relating populations to habitats using resource selection functions. Trends Ecol Evol 14:268–272. https://doi.org/10.1016/S0169-5347(99)01593-1
Breed GA, Jonsen ID, Myers RA, Bowen WD, Leonard ML (2009) Sex-specific, seasonal foraging tactics of adult grey seals (Halichoerus grypus) revealed by state–space analysis. Ecology 90:3209–3221. https://doi.org/10.1890/07-1483.1
Brown EJ, Vasconcelos RP, Wennhage H, Bergström U, Støttrup JG, van de Wolfshaar K, Millisenda G, Colloca F, Le Pape O (2018) Conflicts in the coastal zone: human impacts on commercially important fish species utilizing coastal habitat. ICES J Mar Sci 75:1203–1213. https://doi.org/10.1093/icesjms/fsx237
Buchadas ARC, Hof AR (2017) Future breeding and foraging sites of a southern edge population of the locally endangered black guillemot Cepphus grylle. Bird Study 64:306–316. https://doi.org/10.1080/00063657.2017.1358251
Byers T, Smith A, Mallory ML (2010) Diet of black guillemots and northern fulmars breeding beside a high arctic polynya. Polar Biol 33:457–467. https://doi.org/10.1007/s00300-009-0720-4
Cairns DK (1987a) Diet and foraging ecology of black guillemots in northeastern Hudson Bay. Can J Zool 65:1257–1263. https://doi.org/10.1139/z87-196
Cairns DK (1987b) The ecology and energetics of chick provisioning by black guillemots. Condor 89:627–635. https://doi.org/10.2307/1368652
Camphuysen KCJ, Shamoun-Baranes J, van Loon EE, Bouten W (2015) Sexually distinct foraging strategies in an omnivorous seabird. Mar Biol 162:1417–1428. https://doi.org/10.1007/s00227-015-2678-9
Cecere JG, De Pascalis F, Imperio S, Ménard D, Catoni C, Griggio M, Rubolini D (2020) Inter-individual differences in foraging tactics of a colonial raptor: consistency, weather effects, and fitness correlates. Mov Ecol 8:28. https://doi.org/10.1186/s40462-020-00206-w
Christensen-Dalsgaard S, Mattisson J, Bekkby T, Gundersen H, May R, Rinde E, Lorentsen S-H (2017) Habitat selection of foraging chick-rearing European shags in contrasting marine environments. Mar Biol 164:196. https://doi.org/10.1007/s00227-017-3227-5
Christensen-Dalsgaard S, Anker-Nilssen T, Crawford R, Bond A, Sigurðsson GM, Glemarec G, Hansen ES, Kadin M, Kindt-Larsen L, Mallory M, Merkel FR, Petersen A, Provencher J, Bærum KM (2019) What’s the catch with lumpsuckers? A North Atlantic study of seabird bycatch in lumpsucker gillnet fisheries. Biol Conserv 240:108278. https://doi.org/10.1016/j.biocon.2019.108278
Christensen-Dalsgaard S, Mattisson J, Norderhaug KM, Lorentsen S-H (2020) Sharing the neighbourhood: assessing the impact of kelp harvest on foraging behaviour of the European shag. Mar Biol 167:136. https://doi.org/10.1007/s00227-020-03739-1
Christie H, Gundersen H, Rinde E, Filbee-Dexter K, Norderhaug KM, Pedersen T, Bekkby T, Gitmark JK, Fagerli CW (2019) Can multitrophic interactions and ocean warming influence large-scale kelp recovery? Ecol Evol 9:2847–2862. https://doi.org/10.1002/ece3.4963
Cleasby IR, Wilson LJ, Crawford R, Owen E, Rouxel Y, Bolton M (2022) Assessing bycatch risk from gillnet fisheries for three species of diving seabird in the UK. Mar Ecol Prog Ser 684:157–179
Crain CM, Kroeker K, Halpern BS (2008) Interactive and cumulative effects of multiple human stressors in marine systems. Ecol Lett 11:1304–1315. https://doi.org/10.1111/j.1461-0248.2008.01253.x
Critchley EJ, Grecian WJ, Kane A, Jessopp MJ, Quinn JL (2018) Marine protected areas show low overlap with projected distributions of seabird populations in Britain and Ireland. Biol Conserv 224:309–317. https://doi.org/10.1016/j.biocon.2018.06.007
Croxall JP, Butchart SHM, Lascelles B, Stattersfield AJ, Sullivan B, Symes A, Taylor P (2012) Seabird conservation status, threats and priority actions: a global assessment. Bird Conserv Internat 22:1–34
Davoren GK, Burger AE (1999) Differences in prey selection and behaviour during self-feeding and chick provisioning in rhinoceros auklets. Anim Behav 58:853–863. https://doi.org/10.1006/anbe.1999.1209
De Pascalis F, Imperio S, Benvenuti A, Catoni C, Rubolini D, Cecere JG (2020) Sex-specific foraging behaviour is affected by wind conditions in a sexually size dimorphic seabird. Anim Behav 166:207–218. https://doi.org/10.1016/j.anbehav.2020.05.014
Dehnhard N, Achurch H, Clarke J, Michel LN, Southwell C, Sumner MD, Eens M, Emmerson L (2020a) High inter- and intraspecific niche overlap among three sympatrically breeding, closely related seabird species: Generalist foraging as an adaptation to a highly variable environment? J Anim Ecol 89:104–119. https://doi.org/10.1111/1365-2656.13078
Dehnhard N, Skei J, Christensen-Dalsgaard S, May R, Halley D, Ringsby TH, Lorentsen S-H (2020b) Boat disturbance effects on moulting common eiders Somateria mollissima. Mar Biol 167:12. https://doi.org/10.1007/s00227-019-3624-z
Dehnhard N, Mattisson J, Tarroux A, Anker-Nilssen T, Lorentsen S-H, Christensen-Dalsgaard S (2022) Predicting foraging habitat of European shags - a multi-year and multi-colony tracking approach to identify important areas for marine conservation. Front Mar Sci 9:852033. https://doi.org/10.3389/fmars.2022.852033
Dias MP, Martin R, Pearmain EJ, Burfield IJ, Small C, Phillips RA, Yates O, Lascelles B, Borboroglu PG, Croxall JP (2019) Threats to seabirds: a global assessment. Biol Conserv 237:525–537. https://doi.org/10.1016/j.biocon.2019.06.033
Divoky GJ, Lukacs PM, Druckenmiller ML (2015) Effects of recent decreases in arctic sea ice on an ice-associated marine bird. Prog Oceanog 136:151–161. https://doi.org/10.1016/j.pocean.2015.05.010
Divoky GJ, Douglas DC, Stenhouse IJ (2016) Arctic sea ice a major determinant in Mandt’s black guillemot movement and distribution during non-breeding season. Biol Lett 12:20160275. https://doi.org/10.1098/rsbl.2016.0275
Divoky GJ, Brown E, Elliott KH (2021) Reduced seasonal sea ice and increased sea surface temperature change prey and foraging behaviour in an ice-obligate Arctic seabird, Mandt’s black guillemot (Cepphus grylle mandtii). Polar Biol 44:701–715. https://doi.org/10.1007/s00300-021-02826-3
Dunn RE, Wanless S, Daunt F, Harris MP, Green JA (2020) A year in the life of a North Atlantic seabird: behavioural and energetic adjustments during the annual cycle. Sci Rep 10:5993. https://doi.org/10.1038/s41598-020-62842-x
Eikrem W, Golmen L, Fagerli CW, Kristiansen T, Staalstrøm A, Engesmo A (2019) ØKOKYST - delprogram Norskehavet Sør (II) Årsraport 2018. Norwegian Institute for Water Research (NIVA)
Elliott KH, Gaston AJ (2015) Diel vertical migration of prey and light availability constrain foraging in an Arctic seabird. Mar Biol 162:1739–1748. https://doi.org/10.1007/s00227-015-2701-1
Enstipp MR, Grémillet D, Jones DR (2006) The effects of depth, temperature and food ingestion on the foraging energetics of a diving endotherm, the double-crested cormorant (Phalacrocorax auritus). J Exp Biol 209:845–859. https://doi.org/10.1242/jeb.02064
Evans TJ, Young RC, Watson H, Olsson O, Åkesson S (2020) Effects of back-mounted biologgers on condition, diving and flight performance in a breeding seabird. J Avian Biol. https://doi.org/10.1111/jav.02509
Ewins PJ (1988) An analysis of ringing recoveries of black guillemots cepphus grylle in britain and ireland. Ring Migr 9:95–102. https://doi.org/10.1080/03078698.1988.9673932
Ewins PJ (1990) The diet of black guillemots Cepphus grylle in Shetland. Ecography 13:90–97. https://doi.org/10.1111/j.1600-0587.1990.tb00593.x
Ewins PJ, Kirk DA (1988) The distribution of Shetland black guillemots Cepphus grylle outside the breeding season. Seabird 11:50–61
Fangel K, Aas Ø, Vølstad JH, Bærum KM, Christensen-Dalsgaard S, Nedreaas K, Overvik M, Wold LC, Anker-Nilssen T (2015) Assessing incidental bycatch of seabirds in Norwegian coastal commercial fisheries: empirical and methodological lessons. Glob Ecol Conserv 4:127–136. https://doi.org/10.1016/j.gecco.2015.06.001
Fauchald P, Anker-Nilssen T, Barrett RT, Bustnes JO, Bårdsen BJ, Christensen-Dalsgaard S, Descamps S, Engen S, Erikstad KE, Hanssen SA, Lorentsen S-H, Moe B, Reiertsen TK, Strøm H, Systad GH (2015) The status and trends of seabirds breeding in Norway and Svalbard. Norwegian Institute for Nature Research, Tromsø
Fiskeridirektoratet (2022) Government Document J-131–2022: Forskrift om høsting av tare i Nordland sør av Vegaøyan verdensarvområde. https://www.fiskeridir.no/Yrkesfiske/Regelverk-og-reguleringer/J-meldinger/Gjeldende-J-meldinger/j-131-2022. Accessed 24th Feb 2023
Fjeld PE, Lindseth JH, Soglo E (2020) Utvikling i teistbestand etter uttak av mink i Færder nasjonalpark. Vår Fuglefauna 43:92–95
Fliessbach KL, Borkenhagen K, Guse N, Markones N, Schwemmer P, Garthe S (2019) A ship traffic disturbance vulnerability index for Northwest European seabirds as a tool for marine spatial planning. Front Mar Sci 6:192. https://doi.org/10.3389/fmars.2019.00192
Frederiksen M, Wright PJ, Harris MP, Mavor RA, Heubeck M, Wanless S (2005) Regional patterns of kittiwake Rissa tridactyla breeding success are related to variability in sandeel recruitment. Mar Ecol Prog Ser 300:201–211. https://doi.org/10.3354/meps300201
Gaglio D, Cook TR, McInnes A, Sherley RB, Ryan PG (2018) Foraging plasticity in seabirds: a non-invasive study of the diet of greater crested terns breeding in the Benguela region. PLoS ONE 13:e0190444. https://doi.org/10.1371/journal.pone.0190444
Garriga J, Palmer JRB, Oltra A, Bartumeus F (2016b) Expectation-maximization binary clustering for behavioural annotation. PLoS ONE 11:e0151984. https://doi.org/10.1371/journal.pone.0151984
Garriga J, Palmer JRB, Oltra A, Bartumeus F (2016a) EMbC: Expectation-Maximization binary Clustering. R package version 1.9.4. https://CRAN.R-project.org/package=EMbC
Gulka J, Davoren GK (2019) High individual flexibility in the foraging behavior of a marine predator, the common murre. Mar Biol 166:83. https://doi.org/10.1007/s00227-019-3530-4
Hall SJ, Tuck ID, Robertson MR, Basford DJ, Heaney SD (1996) Patch exploitation by small gadoids: evidence for an asymmetric tidal effect. J Fish Biol 48:996–1005. https://doi.org/10.1111/j.1095-8649.1996.tb01493.x
Heggøy O, Christensen-Dalsgaard S, Ranke PS, Chastel O, Bech C (2015) GPS-loggers influence behaviour and physiology in the black-legged kittiwake Rissa tridactyla. Mar Ecol Prog Ser 521:237–248. https://doi.org/10.3354/meps11140
Hijmans RJ (2021) terra: Spatial data analysis. R package version 1.4–1. https://rspatial.org/terra
Hillersøy G, Lorentsen S-H (2012) Annual variation in the diet of breeding European shag (Phalacrocorax aristotelis) in Central Norway. Waterbirds 35:420–430. https://doi.org/10.1675/063.035.0306
Ito M, Takahashi A, Kokubun N, Kitaysky AS, Watanuki Y (2010) Foraging behavior of incubating and chick-rearing thick-billed murres Uria lomvia. Aquat Biol 8:279–287. https://doi.org/10.3354/ab00229
Jakubas D, Wojczulanis-Jakubas K, Iliszko L, Darecki M, Stempniewicz L (2014) Foraging strategy of the little auk Alle alle throughout breeding season - switch from unimodal to bimodal pattern. J Avian Biol 45:1–10. https://doi.org/10.1111/jav.00303
Johnston DT, Furness RW, Robbins AMC, Tyler G, Taggart MA, Masden EA (2018) Black guillemot ecology in relation to tidal stream energy generation: an evaluation of current knowledge and information gaps. Mar Environ Res 134:121–129. https://doi.org/10.1016/j.marenvres.2018.01.007
Johnston DT, Furness RW, Robbins AMC, Tyler GA, McIlvenny J, Masden EA (2021) Tidal stream use by black guillemots Cepphus grylle in relation to a marine renewable energy development. Mar Ecol Prog Ser 669:201–212. https://doi.org/10.3354/meps13724
Johnston DT (2019) Investigating the foraging ecology of black guillemots Cepphus grylle in relation to tidal stream turbines and marine protected areas. PhD Thesis. University of the Higlands and Islands, Environmental Research Institute, Inverness, Scotland.
Koop JH, Gibson RN (1991) Distribution and movements of intertidal butterfish Pholis gunnellus. J Mar Biol Assoc UK 71:127–136. https://doi.org/10.1017/S0025315400037449
Kuznetsova A, Brockhoff B, Christensen HB (2014) lmerTest: Tests for random and fixed effects for linear mixed effect models (lmer objects of lme4 package) R package version 20–11
Lindegren M, Van Deurs M, MacKenzie BR, Worsoe Clausen L, Christensen A, Rindorf A (2018) Productivity and recovery of forage fish under climate change and fishing: North Sea sandeel as a case study. Fish Oceanog 27:212–221. https://doi.org/10.1111/fog.12246
Lønne OJ, Gabrielsen GW (1992) Summer diet of seabirds feeding in sea-ice-covered waters near Svalbard. Polar Biol 12:685–692. https://doi.org/10.1007/BF00238868
Lorentsen S-H, Moe T, Stübner E (2010a) Key-site monitoring in Sklinna in 2009, SEAPOP short report 9–2010. Norwegian Polar Institute and University of Tromsø, Norway, Norweigan Institute for Nature Research
Lorentsen S-H, Sjøtun K, Grémillet D (2010b) Multi-trophic consequences of kelp harvest. Biol Conserv 143:2054–2062. https://doi.org/10.1016/j.biocon.2010.05.013
Maestro M, Pérez-Cayeiro ML, Chica-Ruiz JA, Reyes H (2019) Marine protected areas in the 21st century: current situation and trends. Ocean Coast Manag 171:28–36. https://doi.org/10.1016/j.ocecoaman.2019.01.008
Masden EA, Foster S, Jackson AC (2013) Diving behaviour of black guillemots Cepphus grylle in the Pentland Firth, UK: potential for interactions with tidal stream energy developments. Bird Study 60:547–549. https://doi.org/10.1080/00063657.2013.842538
McLeay LJ, Page B, Goldsworthy SD, Ward TM, Paton DC (2009) Size matters: variation in the diet of chick and adult crested terns. Mar Biol 156:1765–1780. https://doi.org/10.1007/s00227-009-1211-4
Melvin EF, Parrish JK, Conquest LL (1999) Novel tools to reduce seabird bycatch in coastal gillnet fisheries. Conserv Biol 13:1386–1397. https://doi.org/10.1046/j.1523-1739.1999.98426.x
Moe B, Daunt F, Bråthen VS, Barrett RT, Ballesteros M, Bjørnstad O, Bogdanova MI, Dehnhard N, Erikstad KE, Follestad A, Gíslason S, Hallgrimsson GT, Lorentsen SH, Newell M, Petersen A, Phillips RA, Ragnarsdóttir SB, Reiertsen TK, Åström J, Wanless S, Anker-Nilssen T (2021) Twilight foraging enables European shags to survive the winter across their latitudinal range. Mar Ecol Prog Ser 676:145–157. https://doi.org/10.3354/meps13697
Monaghan P, Walton P, Wanless S, Uttley JD, Burns MD (1994) Effects of prey abundance on the foraging behaviour, diving efficiency and time allocation of breeding guillemots Uria aalge. Ibis 136:214–222. https://doi.org/10.1111/j.1474-919X.1994.tb01087.x
Muff S, Nilsen EB, O’Hara RB, Nater CR (2021) Rewriting results sections in the language of evidence. Trends Ecol Evol 37:203–210. https://doi.org/10.1016/j.tree.2021.10.009
Myksvoll MS, Erikstad KE, Barrett RT, Sandvik H, Vikebø F (2013) Climate-driven Ichthyoplankton drift model predicts growth of top predator young. PLoS ONE 8:e79225. https://doi.org/10.1371/journal.pone.0079225
Neuheimer AB, Taggart CT, Frank KT (2008) Size-at-age in haddock (Melanogrammus aeglefinus): application of the growing degree-day (GDD) metric. Resiliency of gadid stocks to fishing and climate change Alaska Sea Grant College Program AK-SG-08–01: 111–124
Norderhaug KM, Filbee-Dexter K, Freitas C, Birkely SR, Christensen L, Mellerud I, Thormar J, van Son T, Moy F, Vázquez Alonso M, Steen H (2020) Ecosystem-level effects of large-scale disturbance in kelp forests. Mar Ecol Prog Ser 656:163–180. https://doi.org/10.3354/meps13426
Nordström M, Högmander J, Laine J, Nummelin J, Laanetu N, Korpimäki E (2003) Effects of feral mink removal on seabirds, waders and passerines on small islands in the Baltic Sea. Biol Conserv 109:359–368. https://doi.org/10.1016/S0006-3207(02)00162-3
Northrup JM, Vander Wal E, Bonar M, Fieberg J, Laforge MP, Leclerc M, Prokopenko CM, Gerber BD (2022) Conceptual and methodological advances in habitat-selection modeling: guidelines for ecology and evolution. Ecol Appl 32:e02470. https://doi.org/10.1002/eap.2470
Olsen E, Aanes S, Mehl S, Holst JC, Aglen A, Gjøsæter H (2009) Cod, haddock, saithe, herring, and capelin in the Barents Sea and adjacent waters: a review of the biological value of the area. ICES J Mar Sci 67:87–101. https://doi.org/10.1093/icesjms/fsp229
Owen E (2015) Black guillemot (Cepphus grylle) tracking in Orkney, 2013 and 2014, Scottish Natural Heritage Commisionned Report no. 903
Owen E, Wakefield E, Hollinrake P, Leitch A, Steel L, Bolton M (2019) Breeding together, feeding apart: sympatrically breeding seabirds forage in individually distinct locations. Mar Ecol Prog Ser 620:173–183
Patterson A, Gilchrist HG, Robertson GJ, Hedd A, Fifield DA, Elliott KH (2022) Behavioural flexibility in an Arctic seabird using two distinct marine habitats to survive the energetic constraints of winter. Mov Ecol 10:45. https://doi.org/10.1186/s40462-022-00344-3
Pebesma E (2018) Simple features for r: standardized support for spatial vector data. The R Journal 10:439–446. https://doi.org/10.32614/RJ-2018-009
Péron C, Authier M, Grémillet D (2018) Testing the transferability of track-based habitat models for sound marine spatial planning. Div Distrib 24:1772–1787. https://doi.org/10.1111/ddi.12832
Perrow MR, Harwood AJP, Skeate ER, Praca E, Eglington SM (2015) Use of multiple data sources and analytical approaches to derive a marine protected area for a breeding seabird. Biol Conserv 191:729–738. https://doi.org/10.1016/j.biocon.2015.08.031
Peschko V, Mendel B, Mercker M, Dierschke J, Garthe S (2020a) Northern gannets (Morus bassanus) are strongly affected by operating offshore wind farms during the breeding season. J Environ Manag. https://doi.org/10.1016/j.jenvman.2020.111509
Peschko V, Mercker M, Garthe S (2020b) Telemetry reveals strong effects of offshore wind farms on behaviour and habitat use of common guillemots (Uria aalge) during the breeding season. Mar Biol 167:118. https://doi.org/10.1007/s00227-020-03735-5
Petersen A (1981) Breeding biology and feeding ecology of black guillemots. PhD Thesis. University of Oxford
Piatt JF, Harding AM, Shultz M, Speckman SG, van Pelt TI, Drew GS, Kettle AB (2008) Seabirds as indicators of marine food supplies: Cairns revisited. Mar Ecol Prog Ser. https://doi.org/10.3354/meps07078
Ponchon A, Grémillet D, Christensen-Dalsgaard S, Erikstad KE, Barrett RT, Reiertsen TK, McCoy KD, Tveraa T, Boulinier T (2014) When things go wrong: intra-season dynamics of breeding failure in a seabird. Ecosphere 5:4. https://doi.org/10.1890/ES13-00233.1
Quick NJ, Middlemas SJ, Armstrong JD (2004) A survey of antipredator controls at marine salmon farms in Scotland. Aquaculture 230:169–180
R Core Team (2022) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna. http://www.R-project.org/
Regular PM, Hedd A, Montevecchi WA (2011) Fishing in the dark: a pursuit-diving seabird modifies foraging behaviour in response to nocturnal light levels. PLoS ONE 6:e26763. https://doi.org/10.1371/journal.pone.0026763
Ronconi RA, St. Clair CC, (2002) Management options to reduce boat disturbance on foraging black guillemots (Cepphus grylle) in the Bay of Fundy. Biol Conserv 108:265–271. https://doi.org/10.1016/S0006-3207(02)00126-X
Sawyer TR (1999) Habitat use and breeding performance in an inshore foraging seabird, the black guillemot Cepphus grylle. University of Glasgow (United Kingdom)
Shoji A, Elliott KH, Greenwood JG, McClean L, Leonard K, Perrins CM, Fayet A, Guilford T (2015) Diving behaviour of benthic feeding black guillemots. Bird Study 62:217–222. https://doi.org/10.1080/00063657.2015.1017800
Shorty JT, Gannon DP (2013) Habitat selection by the rock gunnel, Pholis gunnellus L. (Pholidae). Northeastern Naturalist 20: 155–170, 116
Signer J, Fieberg J, Avgar T (2019) Animal movement tools (amt): R package for managing tracking data and conducting habitat selection analyses. Ecol Evol 9:880–890. https://doi.org/10.1002/ece3.4823
Slater PJB, Slater EP (1972) Behaviour of the Tystie during feeding of the young. Bird Study 19:105–113. https://doi.org/10.1080/00063657209476332
Smale DA (2020) Impacts of ocean warming on kelp forest ecosystems. New Phytol 225:1447–1454. https://doi.org/10.1111/nph.16107
Smith KE, Burrows MT, Hobday AJ, King NG, Moore PJ, Gupta AS, Thomsen MS, Wernberg T, Smale DA (2023) Biological impacts of marine heatwaves. Ann Rev Mar Sci 15:119–145. https://doi.org/10.1146/annurev-marine-032122-121437
Statistics Norway (2022) Protected areas URL: https://www.ssb.no/en/natur-og-miljo/areal/statistikk/vernede-omrader. Accessed 25th of Feb 2023
Stokke BG, Jacobsen K-O, Liselevand T, Solvang R, Strøm H (2021) Artsgruppeomtale fugler (Aves). Norsk rødliste for arter 2021. Norwegian Biodiversity Information Centre. https://artsdatabanken.no/lister/rodlisteforarter/2021/ Accessed 22nd of Feb 2023
Sydeman WJ, Thompson SA, Anker-Nilssen T, Arimitsu M, Bennison A, Bertrand S, Boersch-Supan P, Boyd C, Bransome NC, Crawford RJM, Daunt F, Furness RW, Gianuca D, Gladics A, Koehn L, Lang JW, Logerwell E, Morris TL, Phillips EM, Provencher J, Punt AE, Saraux C, Shannon L, Sherley RB, Simeone A, Wanless RM, Wanless S, Zador S (2017) Best practices for assessing forage fish fisheries-seabird resource competition. Fish Res 194:209–221. https://doi.org/10.1016/j.fishres.2017.05.018
van der Kooij J, Scott BE, Mackinson S (2008) The effects of environmental factors on daytime sandeel distribution and abundance on the Dogger Bank. J Sea Res 60:201–209. https://doi.org/10.1016/j.seares.2008.07.003
Vandenabeele SP, Wilson RP, Grogan A (2011) Tags on seabirds: how seriously are instrument-induced behaviours considered? Anim Welfare 20:559–571
Vandenabeele SP, Shepard E, Grogan A, Wilson R (2012) When three per cent may not be three per cent; device-equipped seabirds experience variable flight constraints. Mar Biol 159:1–14
Waggitt JJ, Robbins AMC, Wade HM, Masden EA, Furness RW, Jackson AC, Scott BE (2017) Comparative studies reveal variability in the use of tidal stream environments by seabirds. Mar Pol 81:143–152. https://doi.org/10.1016/j.marpol.2017.03.023
Wanless S, Harris MP, Redman P, Speakman JR (2005) Low energy values of fish as a probable cause of a major seabird breeding failure in the North Sea. Mar Ecol Prog Ser 294:1–8. https://doi.org/10.3354/meps294001
Wanless S, Harris MP, Newell MA, Speakman JR, Daunt F (2018) Community-wide decline in the occurrence of lesser sandeels Ammodytes marinus in seabird chick diets at a North Sea colony. Mar Ecol Prog Ser 600:193–206. https://doi.org/10.3354/meps12679
Wilson LJ (2004) Self-feeding and chick provisioning diet differ in the common guillemot Uria aalge. Ardea 92:197–208
Winslade P (1974) Behavioural studies on the lesser sandeel Ammodytes marinus (Raitt) II. The effect of light intensity on activity. J Fish Biol 6:577–586. https://doi.org/10.1111/j.1095-8649.1974.tb05101.x
Wirjoatmodjo S, Pitcher TJ (1984) Flounders follow the tides to feed: evidence from ultrasonic tracking in an estuary. Est Coast Shelf Sci 19:231–241. https://doi.org/10.1016/0272-7714(84)90067-2
Wood SN (2017) Generalized additive models. CRC Press Boca Raton, Florida, USA, An Introduction with R
Wright P, Pedersen S, Donald L, Anderson C, Lewy P, Proctor R (1998) The influence of physical factors on distribution of lesser sandeel, Ammodytes marinus, and its relevance to fishing pressure in the North Sea. ICES CM 1998 AA: 3: 1–9
Yorio P (2009) Marine protected areas, spatial scales, and governance: implications for the conservation of breeding seabirds. Conserv Lett 2:171–178. https://doi.org/10.1111/j.1755-263X.2009.00062.x
Acknowledgements
Thanks to the many field assistants for helping to catch black guillemots and deploy GPS loggers, especially Natalie Isaksson, Hanne Bjørnås Krogstie, Christoffer Høyvik Hilde and Terje Lorentzen Landsem. Thanks also to Jannike Wika, Rita Johansen and Oddleif Dahlen for local support at Vega. Thanks to the Norwegian Coastal Administration for facilitating extended stays at Sklinna through a rental agreement for the lighthouse station. We would like to thank three anonymous reviewers and the editor for constructive comments to an earlier draft of the manuscript.
Funding
Open access funding provided by Norwegian institute for nature research.
Author information
Authors and Affiliations
Contributions
SCD, SHL and EAM conceived and designed the study. ND, TAN, SHL, SCD and DJ carried out the fieldwork. ND analysed the data. All authors contributed to the writing of the paper and gave the final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
This study was part of the SEAPOP programme (www.seapop.no/en), which is financed by the Norwegian Ministry of Climate and Environment via the Norwegian Environment Agency, the Norwegian Ministry of Petroleum and Energy via the Norwegian Research Council (grant 192141), and the Norwegian Oil and Gas Association (now Offshore Norge). The work at Muddvær, Vega, was co-funded by Stiftelsen Vegaøyan Verdensarv, Vega Verneområdestyre and the County Governor in Nordland through the Norwegian Environment Agency. There were no conflicts of interest. We obtained permits to work in the colonies from the local County governors (for Sklinna and Røst) and the local landowners at Muddvær, Vega. Ethical permits to catch birds and attach loggers were granted by the Norwegian Environment Agency and The Norwegian Food Safety Authority (FOTS ID 5148, 12163, 19570, 27473).
Additional information
Responsible Editor: T.A. Clay .
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Dehnhard, N., Anker-Nilssen, T., Johnston, D. et al. Foraging behaviour of black guillemots at three Norwegian sites during the breeding season. Mar Biol 170, 87 (2023). https://doi.org/10.1007/s00227-023-04228-x
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04228-x