Abstract
Due to their small size, juveniles are more likely to show lower absolute levels of performance leading to a potential competitive disadvantage compared to adults. Therefore, juveniles are expected to compensate by showing a higher relative performance, and/or partitioning resources to overcome this performance disadvantage. Here, we investigate the link between ontogeny and feeding performance in the common cuttlefish Sepia officinalis. We explore the changes in beak shape, wear pattern, mechanical properties, muscular anatomy, and bite force during growth from 3-month post hatching to adults. We show that both upper and lower beaks present important ontogenetic shape variation in the rostrum area that might be due to wear induced by feeding. The mechanical properties of the beaks in juveniles indicate greater resistance compared to adults. Tanning observed on the beaks provides reinforcement to areas under high load during biting. In addition, muscle development and relative bite force were found to differ between cuttlefish of different ages, resulting in juveniles having a similar bite force for their size but with a muscular advantage for opening. Finally, an isometric relation is found in the bite force of S. officinalis, with no sign of feeding performance compensation in juveniles. Feeding performance thus does not reflect the ontogenetic shift from a crustacean-based diet in juveniles to a fish-based diet in adults.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The size of animals affects all aspects of their biology, including locomotor and feeding performance (Herrel and O’Reilly 2006). Due to their smaller dimensions and potential developmental constraints, juveniles likely show lower absolute levels of performance and therefore, may be at a performance disadvantage compared to adults, which increases the strength of intra-specific competition (Schmidt-Nielsen 1984; Werner and Gilliam 1984; Carrier 1996). Therefore, juveniles can be expected to develop strategies to overcome this performance disadvantage. In vertebrates, evidence of compensation has been found for traits related to locomotion and defensive behaviour, leading to higher relative performance in juveniles compared to adults (Herrel and Gibb 2006). Enhanced juvenile performance in relation to that of adult has been reported for example in take-off velocity and acceleration in the jackrabbit Lepus californicus (Carrier 1996), or in escape running speed in the wild guinea pig Cavia aperea (Trillmich et al. 2003). However, a similar compensation pattern was not observed for traits related to feeding and foraging. Rather, positive allometry of bite force relative to body and head dimensions is typically recorded, partly explained by the hypertrophy of the jaw muscles during ontogeny (Herrel and Gibb 2006). In this context, the partitioning of niches and trophic resources appears to be a critical strategy to reduce competition between juveniles and adults (Werner and Gilliam 1984). As feeding behaviour is a key part ecosystem processes, studying the scaling of feeding performance is essential to an understanding of trophic niches, resource repartition, and community structure (Kolmann and Huber 2009). However, whereas studies investigating the link between ontogeny and feeding performance are rare in vertebrates, there are none exploring this question in invertebrates.
Cephalopods provide an ideal model to investigate questions related to the scaling of feeding performance. They are a large and diverse group of marine invertebrates, widely known for their intelligence, unique body plan, and commercial importance. Their feeding system is unique within the animal kingdom, composed of a radula and two beaks moved by a set of masticatory muscles (Boyle et al. 1979; Kear 1994), all embedded within a buccal mass lying in a cavity at the centre of their arms (Fig. 1). The beaks are chitinous structures connected by a “muscle articulation”, a unique type of joint, in which there is no direct contact between the upper and lower part of the beak (Uyeno and Kier 2005). Beaks grow continuously throughout the life of the animal by the addition of chitin layers deposited by specialized cells called beccublasts. These cells are arranged in a thin cell layer located between the aboral side of the beak and the surrounding muscles (Dilly and Nixon 1976). The increments are generally deposited daily (Perales-Raya et al. 2010; Arkhipkin et al. 2018; Guerra-Marrero et al. 2023), and the deposition rate has been validated by experimental data using stress markers visible on the increments (Perales-Raya et al. 2014a, b, 2018). As daily growth was observed in most species investigated, this growth pattern may be widespread among cephalopods, with potential rate variation depending on environmental conditions (Perales-Raya et al. 2018; Schwarz et al. 2019). However, although late stage embryo beaks of Octopus vulgaris and paralarval beaks of some squids and octopus have been described (Franco-Santos and Vidal 2014, 2020; Franco-Santos et al. 2014; Perales-Raya et al. 2018; Xavier et al. 2023), and food intake and growth rate were described in first weeks after hatchling (Koueta and Boucaud-Camou 1999), little is known about the development and growth of the beaks after that stage. Moreover, nothing is known about the growth and development of the buccal mass.
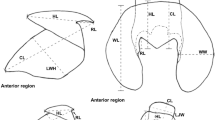
Anatomy of the buccal mass of Sepia officinalis. A, B Basic nomenclature of cephalopods beaks. C, D Dimensions measured on the beaks. E Picture of a buccal mass in vitro with nomenclature of visible anatomical features. F 3D reconstruction of the masticatory muscles. UB = Upper beak. LB = Lower beak. UHL = Upper hood length. URL = Upper rostrum length. UTL = Upper total length. UTH = Upper total height. LRL = Lower rostrum length. LHL = Lower hood length. LTL = Lower total length. LTH = Lower total height. SMM = Superior mandibular muscle. PLM = Postero-lateral muscle. LMMp = Lateral mandibular muscle posterior section. LMMa = Lateral mandibular muscle anterior section. AMM = Anterior mandibular muscle
Here, we studied the common cuttlefish Sepia officinalis, Linnaeus, 1758. Sepia officinalis juveniles resemble the adults in their morphology and lifestyle (Boletzky 1983; Nixon 1985). As they grow, juveniles gradually explore deeper habitats further offshore where subadults prey on larger animals before migrating back into coastal areas as adults for reproduction (Dunn 1999). Juveniles feed mainly on crustaceans (prawns and crabs) while adults incorporate a larger proportion of fish in to their diet (Nixon and Mangold 1998; Alves et al. 2006). Prey items are selected by size, usually similar in dimension to that of the cuttlefish itself. The morphology and performance of the feeding apparatus of juvenile cuttlefish would be expected to be an important aspect constraining access to prey, together with hunting behaviour and the use of the arms. Yet in this species, juveniles appear to have a diet that is mechanically more resistant with prey items showing higher hardness than adults. This then raises the question: does feeding performance in S. officinalis show a negative allometric growth as observed in many vertebrates, or do they, alternatively, compensate for their smaller absolute size to gain access to these mechanically demanding resources?
The aim of this study was to investigate the growth of the feeding structures in S. officinalis and their link with their feeding performance. We aim to decipher whether the change of diet during ontogeny is reflected by their feeding system. To do so we explore changes in beak shape, wear pattern, mechanical properties, muscular anatomy, and bite force. This dataset permits a comprehensive description of the buccal mass growth in S. officinalis and a discussion of its functional consequences on feeding behaviour.
Materials and methods
Sample collection
Sepia officinalis was bred at the Synergie Mer Et Littoral (SMEL) experimental centre in Blainville-sur-Mer (France; permit for animal breeding and experiments including cephalopods: A50058001). Eggs were collected mid-June at a depth of 3 m from buoy ropes installed in April at the Pointe D’Agon site (N 48°59.36–W 1°38.70), and in early September a fresh cluster of eggs was recovered on the beach of Blainville-sur-Mer. Both groups were incubated for 11 to 15 days until hatching in an aquarium filled with seawater maintained at 20 °C. At all times, fifty percent of the total water volume was refreshed daily. After hatching, seawater temperature was maintained at 18.3 ± 1.7 °C and salinity at 33.6 ± 1.3 PSU. From hatching, the cuttlefish measuring approximately 10 mm, living on their yolk reserves, are not fed during the first 3 days. From the fourth day post hatching onwards, juveniles were fed with live shrimps harvested on the foreshore. Each juvenile received a shrimp of its own size every day. The two groups of eggs gave two age groups from which five specimens were sampled for this study. The first age group from eggs sampled in September was composed of the youngest specimens and consisted of three-month-old (93 to 95 days) individuals. The second group from eggs sampled in June was composed of specimens of almost six months old (171 to 176 days). Specimens from this group were occasionally fed small fish and crabs in addition to shrimps. Two adult specimens were caught in early June by local fishermen and maintained in an oxygenised sea water tank at the SMEL for a few days. In vivo bite force was measured in June for adults, a few days after they were caught, and in mid-December for the two groups of juveniles. All individuals were then euthanized by prolonged immersion in Magnesium chloride (MgCl2) following Andrews et al. (2013). Freshly euthanized animals were weighed with a digital scale, and mantle length (ML) was measured with digital callipers (Mitutoyo). Weighing was not possible for the two adult specimens and consequently their total weight was estimated based on the mantle length (Basuyaux and Legrand 2013). Two specimens from each juvenile group, and the two adults, were fixed in a solution of 10% aqueous formaldehyde for two days, rinsed and maintained in a 70% aqueous ethanol solution. All other specimens were freshly frozen. One adult specimen of Sepia officinalis was obtained commercially from a local fishmonger, and its buccal mass was fresh frozen and used for nano-indentation.
Bite force measurement
In vivo bite forces were measured on all live specimens (N = 12) using an isometric Kistler force transducer (type 9203, Kistler Inc., Switzerland) mounted on a purpose-built holder and connected to a Kistler charge amplifier (type 5058 A, Kistler Inc.) following the protocol of Herrel et al. (1999). Each individual was held around the anterior part of the mantle behind the head with one hand and presented with the bite plates (Sup. Figure 1). Animals readily bit the transducer and bite force was recorded at the tip of the rostrum three times for each individual. The single highest value was retained as an estimate of maximal bite force. The bite plates were moved further apart for larger animals, resulting in all animals biting at equivalent gapes. Bite position was measured by inspecting the indentation of the surgical tape wrapped around the bite plates. The distance from the bite point to the pivot point (fulcrum) was measured and bite forces were corrected for bite position.
Digitization and measurements
Pictures of upper and lower beaks were taken using a KEYENCE VHX 7000 Digital Microscope (KEYENCE, UK) for specimens whose beaks were extracted (N = 3 per group). The lateral view illustrated in Fig. 2 was used to measure the jaw angle using ImageJ (Schneider et al. 2012) Pictures of the sagittal sections of beaks embedded in resin were taken before and after indentation under both normal and co-axial light conditions. Beak measurements were taken using a digital calliper.
Upper and lower beak of Sepia officinalis at each growth stage showing size and shape variations. A Outline of the upper beak of adult (top), 6 month old (center) and 3 month old (bottom) individual at the same scale. Picture of upper (top) and lower (bottom) beak of a (B) 3 months old, (C) 6 months old and (D) adult individual. Scale bars = 5 mm. Black arrows indicate the jaw angle
Muscle quantification
For each age group, the buccal mass of one formalin-fixed specimen was extracted by incising the brachial crown ventrally and sectioning the connective tissues and oesophagus. Each mandibular muscle was dissected, weighted, and placed in 30% nitric acid (HNO3) to dissolve connective tissues and isolate the muscles fibres. Once the muscle digestion was complete, the nitric acid was replaced by a 50% aqueous glycerol (C3H8O3) solution. Pictures of the fibres and a scale were taken with the KEYENCE VHX 7000 Digital Microscope, and fibre lengths were measured using ImageJ. The mean fibre length was estimated and used together with muscle mass to estimate the physiological cross-sectional area (PCSA) using the following equation, based on a muscular density estimation of 1.06 g.cm−3 (Denton and Gilpin-Brown 1973):
Material characterization
For each age group, one pair of fresh frozen beaks was embedded in cold cure epoxy resin (Buehler, Germany). The upper and lower beaks were embedded separately to better control the beak orientation. The samples were then polished to create a sagittal section, using silicon carbide paper and aluminium oxide slurries following a protocol previously described (Moazen et al. 2015; Marghoub et al. 2022; final particle size: 0.05 µm). Nanoindentation was then performed at room temperature using an Anton Paar system (UNHT3, Anton Paar GmbH, Switzerland) with a Berkovich diamond tip (see e.g., Ebenstein and Pruitt 2006). Regions of interest were chosen visually to include as much colour and layer diversity as possible. All regions were indented four times under force-controlled linear loading to a force of 50 mN at 100 mN/min, followed by a 10-s hold. A minimum spacing of 30 µm was set between each indent. The elastic modulus was calculated using the standard Oliver-Pharr method (Oliver and Pharr 1992). Here, the Poisson’s ratio of the indented tissue and indenter tip were assumed to be 0.3 and 0.07, respectively, with the elastic modulus of the indenter tip being 1140 GPa (based on the manufacturer’s data).
Statistical analysis
We tested the allometry of variables for which we had more than 5 specimens (i.e. bite force, mantle length, total weight, and beak measurements) with an ordinary least square (OLS) linear regression. All variables were logarithmically transformed (log10) before analyses. We compared the obtained slopes to those expected under isometric growth. This slope depends on the dimensionality of these variables, being one for linear dimensions versus linear dimensions, two for the scaling of bite force versus linear dimensions, and three for the scaling of mass versus linear dimensions. When the predicted slope fell outside the calculated 95% confidence limits, the relationship was deemed allometric.
Results
Beak shape
The overall morphology of the upper and lower beak is similar in all age groups (Fig. 2). It has previously been suggested that juveniles already possess an adult beak shape immediately post-hatching (Boletzky 1983; Nixon 1985). This, in addition to our observations, suggests the general beak shape is conserved throughout the life of the animal from hatching to adult, despite continuous growth. However, some differences can still be observed, especially in the rostrum region (Fig. 2). In lateral view, the principal region of variation of the upper beak is situated at the level of the jaw angle (Figs. 1, 2). In the three-month-old specimens the jaw angle (89°) of the upper beak is rounded, and the oral edge of the rostrum presents a slight curve. In adults, the jaw angle is sharper and more acute (64°), the oral margin of the rostrum is flattened, and the rostrum appears longer. In oral view (Fig. 3), the three-month-old specimens display an upper rostrum/hood complex that is narrow and sharp compared to that of the adults. For the lower beak, a similar and even more striking difference is observed. In the three-month-old specimens the rostrum of the lower beak is short, and the jaw angle is wide (127°), obtuse, and poorly delineated (Fig. 3). The jaw edge is fragmented into two segments (Fig. 2). The six-month-old specimens present a transitional morphology. The adults display a longer rostrum, and a jaw angle that is rounded and acute (83°). The jaw angle gradually deepens, and that the rostrum becomes thicker and blunter over time. In oral view, the growth pattern observed is opposite to that of the upper beak. In the three-month-old specimens, the rostrum region is large, sharp, and has a V-shape in oral view, creating an isosceles triangle (Fig. 3). During growth, the rostrum elongates toward its adult form, which is blunt and U-shaped. These observations are supported by the measurements in Table 1. The fastest growth rate is observed in the rostrum of both the upper and lower beaks, evidenced by the rostrum lengths (URL and LRL), with adult rostra being six times longer. In comparison, the dimension exhibiting the slowest growth is the height of the upper and lower beaks (UTH and LTH), with adult rostra being only four times taller. Rostrum length displays negative allometry against mantle length, and positive allometry of both upper and lower beak height against mantle length was also observed (Table 4). Thus, adults have relatively taller lower and upper beaks with a relatively shorter rostrum.
Beak mechanical properties
The mechanical properties of the different areas of the beak cross-sections are illustrated in Fig. 4. Low values indicate a more compliant material, while higher values indicate a stiffer material. We observed an anteroposterior stiffness gradient. In Sepia officinalis, hatchling beaks are mostly transparent while adult beaks are entirely tanned with an anteroposterior gradient from a dark rostrum to lighter posterior edges. Given this difference in the degree of tanning, we expected the beaks of S. officinalis juveniles to be mechanically weaker. However, the average elastic modulus of the three-month-old juvenile beaks was already high (1.1 to 6.5 GPa, Fig. 4) compared to that of the adults (3.2 to 8.3 GPa, Fig. 4). The upper beaks were found to be stiffer than the lower ones in the six-month-old specimens and the adults, while both beaks displayed similar values in the three-month-old specimens. The greatest stiffness for all ages was seen in the upper beak rostrum. In both beaks, the hood was always stiffer than the walls.
Sagittal sections of the upper (UB) and lower beak (LB) in the three considered growth stages of Sepia officinalis highlighting the mechanical properties of different regions of the section. Left images were taken in coaxial light to illustrate surface details. White squares represent indentation points. Mean elastic modulus of each region is given in GPa (right side). Scale bar = 1 mm
Muscle architecture
The muscular anatomy is illustrated in Fig. 1F, and quantitative data for each muscle are given in Table 2 and Fig. 5. The general anatomy and insertion points of the masticatory muscles are conserved during growth. Five main masticatory muscles are recognized (Kear 1994; Uyeno and Kier 2005): (1) Superior Mandibular Muscle (SMM): a large dorsal muscle divided into three parts, the central one originating from the crest of the upper beak and the ventral face of the hood, and right and left parts originating on the interior face of the lower beak wings and connected to the central part. (2) Lateral Mandibular Muscle (LMM): symmetrical left and right muscles originating on the lateral walls of the upper beak and inserting on surrounding connective tissues. Each can be separated into an anterior and a posterior part that are connected but presenting a different fibre orientation. (3) Anterior Mandibular Muscle (AMM): a ventral muscle originating on the anterior part of the lateral walls and the crest of the lower beak, firmly attached to the overlying buccal sheath, and connected in its dorsal part to the SMM. (4) Posterior Mandibular Muscle (PMM, not shown in Fig. 1): a superficial muscle that originates on the crest of the lower beak and inserts on the lateral wall of the upper beak, inferior to the crest, and surrounding the oesophagus. (5) Postero-Lateral Muscle (PLM): a pair of symmetrical muscles originating from the posterior margin of the lateral walls of the upper beak, with insertions on the lower beak lateral walls and connected to the SMM, AMM, LMM, and radular apparatus. The muscle presenting the highest proportion of the total muscle mass at each growth stage is the LMM (43% three-month-old; 30% six-month-old; 32% adults, Table. 2, Fig. 5). This muscle also presents the largest PCSA in adults, and therefore the highest theoretical muscle force (Table. 2, Fig. 5). This is due to the LMM having the slowest growth in fibre length (adult fibres 2.5 times longer than the 3-month-old specimens) combined with a moderate weight gain. Therefore, the LMM grows faster in width than in length, leading to a larger PCSA and associated theoretical maximal muscle force. However, in juveniles, the muscle presenting the highest PCSA is the PMM (11 N three-month-old specimen; 37 N in the six-month-old specimen; Table. 2, Fig. 5). The decrease in its relative importance during growth is due to the fast growth of its muscle fibres, in combination with a low volume increase. The PMM grows in length but not much in thickness, leading to a lower PCSA gain compared to other muscles. The fastest weight gain is observed in the PLM and AMM. Adults have a PLM mass that is 90 times greater and an AMM mass that is 89 times greater than the same muscles in the three-month-old individuals. However, the PLM remains the muscle with the lowest proportion of the total muscle mass, despite this fast growth. The AMM displays the highest gain in PCSA. The SMM exhibits an average growth for all parameters compared to other muscles.
Masticatory muscles quantification. A Muscle weight proportion (% total muscular mass) at each age. B Physical cross section area (PCSA, in cm2) of each muscle at each age. PMM: Posterior Mandibular Muscle. AMM: Anterior mandibular muscle. SMM: Superior mandibular muscle. LMM: Lateral mandibular muscle. PLM = Postero-lateral muscle
Wear pattern
The wear patterns on the upper and lower beaks are illustrated in Fig. 3. Clear traces of wear are visible on both beaks in all age groups. The wear is the result of mechanical damage to the chitinous beak tissues during feeding. The damage on the beaks is too significant to allow characterization of microwear pattern. However, the macrowear pattern can be described and is informative with respect to the occlusal pattern and food intake behaviour. On all specimens, the most extreme wear is observed at the jaw angle and on the sides of the oral margin of the rostrum. Surprisingly, the anterior extremity of the rostrum presents minimal damage compared to other areas. However, we noted that the rostral tip of one three-month-old specimen (illustrated in Fig. 3) was broken. The breakage had been smoothed over time, indicating that the fracture was not recent. Overall, beaks of the three-month-old specimens presented visible but moderate wear, while older specimens exhibited more severe damage. Scratches are present on the aboral face of the rostrum hood of the upper beak, and, to a lesser extent, on the lower beak in the six-month-old specimens. In adults, antero-posterior scratches were visible on the oral part of the lower beak. The oral face of the upper beak is little worn compared to the other areas.
Bite force
Bite force for each specimen is given in Table 3. Bite force increase with age, with mean maximal bite force of 2.2 ± 0.59 N (n = 5) in the three-month-old specimens, 18.9 ± 5.02 N (n = 5) in the six-month-old, and 46.74 ± 0.17 N (n = 2) in adults. Bite force is strongly correlated with mantle length (R2 = 0.95, P < 0.001, Fig. 6) but remains isometric (Table 4).
Discussion
Is the feeding system of juveniles the same as in adults?
Previous research has shown that the buccal mass of newly hatched cuttlefish is relatively large, with all the main features of adults already present (Nixon 1985; Nixon and Mangold 1998). Upper and lower beaks are described as partially tanned (Guerra-Marrero et al. 2023), with a well-developed rostrum and large mandibular muscles. Nonetheless, the growth-related variation in the buccal mass observed in this study was greater than expected from published literature. Although the overall shape of the juvenile beak is similar to that of the adult with an isometric growth of most of the beak features relative to mantle length, we observed significant shape differences in the hood/rostrum region, mainly related to rostrum size and shape, and the jaw angle. The rostrum length is the only feature showing negative allometry against mantle length, meaning that the rostrum grows slower than the rest of the beak. Moreover, muscle development and relative bite force were found to differ between ages, with some variation in mechanical properties as well. However, these anatomical differences do not impact the biting abilities of young cuttlefish, with an isometric relationship between bite force and the mantle length. Therefore, although anatomical differences were observed in the buccal mass between juveniles and adults, bite force shows no performance compensation during early life.
Are mechanical properties of the beak related to the bite force?
In three-month-old specimens, the Young’s moduli of the beak were relatively high (max value: 6.5 GPa) compared to those of adults (max value: 8.3 GPa), but also compared to those of other cephalopod species. In the octopods Adelieledone polymorpha and Pareledone turqueti, mean Young’s modulus values for the upper beak were, respectively, 4.69 GPa and 4.99 GPa (Matias et al. 2019). Octopods have a diet composed of stiff prey including crustaceans and bivalves and have a theoretical bite force that is significantly higher than that of cuttlefish (Roscian 2021). Therefore, our results suggest that the mechanical properties of the beak are not directly related to bite force. Matias et al. (2019) reported a correlation between mechanical properties and isotopic composition in the two octopus species they studied. However, as the difference between the species was minor compared to the within species ontogenetic differences observed in this study, the disparity observed in octopods might be explained by age variability in the individuals studied, or intraspecific variation. An anteroposterior gradient of stiffness (from a stiff rostrum to a compliant posterior edge) was evidenced by Miserez et al. (2008) in the jumbo squid Dosidicus gigas, and by Matias et al. (2019) in the Antarctic knobbed octopus Adelieledone polymorpha and Turquet’s octopus Pareledone turqueti. Together with our results, this suggests that the tanning pattern is the main determinant of the beak mechanical properties. However, the heavily tanned hood and rostrum are the stiffest parts of the beak and are also those most exposed to external mechanical loading during food intake. Therefore, tanning is likely to provide reinforcement of beak regions exposed to feeding loads. The fact that the lower beak is always more elastic than the upper beak, and that its mechanical properties differ little during growth, suggests that the lower beak undergoes less mechanical loading during feeding. This supports the suggestion (Altman and Nixon 1970; Uyeno and Kier 2005) that the upper beak is the main “tool” used during the bite, experiencing more loading, while the lower beak is used to hold the prey. Alternatively, the longer rostrum of the upper beak in adults could induce a higher levels of stress and strain during loading, hence requiring more resistance.
What is the connection between beak size and shape, muscle size, and bite force during growth?
Three-month-old juvenile specimens feed on relatively hard prey items like prawns whereas adults feed mostly on fishes. However, this diet shift is not reflected by feeding performance as bite force increases isometrically with the mantle length. Whereas the AMM and SMM induce a closing motion, the LMM, PMM and PLM are described as responsible for opening the beak (Kear 1994; Uyeno and Kier 2005, 2007). The fact that PMM exhibits the highest force in juveniles could indicate an advantage for beak opening, whereas the high LMM force in adults might indicate an advantage in closing. Why would beak opening need to be more powerful than beak closure in juveniles? One possible explanation could be that juveniles need more force to create a gape large enough to take in prey items as big as themselves. A large gape could be combined with a more efficient bite, aided by a short and sharp rostrum allowing to pierce crustaceans more easily than the larger, blunter rostrum of adults. Alternatively, this muscle arrangement could be the result of developmental plasticity of the buccal mass during growth. In either case, the beak closing muscles grow gradually during development, resulting in a progressive increase in bite force relative to beak size, explaining the observed changes between juveniles and adults.
What can the wear pattern tell us about the feeding behaviour?
We observed wear on the jaw angle area in all specimens studied. In contrast, the tip of the rostrum appeared little worn. We propose two alternative hypotheses to explain this pattern. First, the rostrum may be more resistant to wear due to its increased stiffness and reinforcement from tanning. If the rostrum is the main biting tool, the wear observed on the jaw angle could simply be collateral damage to these more elastic tissues. Alternatively, the wear could be concentrated on the jaw angle because it plays an active role in the feeding motion and the breakdown of prey items during food intake. This would support the hypothesis proposed by Uyeno and Kier (2007), that the beak has a shearing scissor-like motion. The contact between the edges of the upper and lower beaks could cut skin and flesh more easily. This beak action may therefore be an adaptation in adults to allow the incorporation of a larger quantity of fish into their diet. The progressive elongation of the rostrum highlighted in this study could thus make it possible to increase the shearing surface between the two beaks. However, these two hypotheses are not mutually exclusive. The beak may provide two different “tools”, as in the mammalian dentition, with cutting incisors and crushing molars. In cephalopods, the rostrum could be reinforced for percussion and piercing to allow an initial bite through the prey's defences, especially important in juveniles which have a more durophagous diet, followed by a shearing motion of the posterior parts of the rostrum to cut through the softer tissues exposed.
Does beak wear impact beak morphology during growth?
The highly worn jaw angle also displays the greatest morphological variation between juveniles and adults, with this angle deepening progressively during growth. Perales-Raya et al. (2010) showed that the rostrum of Octopus vulgaris was worn on the anterior extremity and the oral face, leading to loss of growth increments in the chitinous beak material in this region. Consequently, beak wear has an important role in the morphological evolution of the hood/rostrum complex during ontogeny. As the most anterior and superficial layers of the adult beak are the first deposited during growth, this region should retain the shape of the juvenile beak. However, the adult rostrum is longer than the entire beak of three-month-old individuals, suggesting that the wear contributes to sculpting the rostrum and the jaw angle to fit the adult morphology by removing material through wear.
Conclusion
An isometric relationship is found in the feeding performance of S. officinalis, with no sign of performance compensation in juveniles, consistent with what has been previously described in vertebrates (Herrel and Gibb 2006; Herrel and O’Reilly 2006). Juveniles of S. officinalis seem to escape intraspecific competition by selecting small prey that may be less profitable in terms of energy content but are highly abundant in their environment. Surprisingly, feeding performance does not reflect the diet shift observed later in ontogeny with incorporation of a higher proportion of fish. The results presented here lay the foundations for future studies focusing on evolutionary dynamics and trophic ecology of cephalopods in a fragile and changing environment.
Data availability
The datasets generated and analysed during the current study are available from the corresponding author on reasonable request.
References
Altman JS, Nixon M (1970) Use of the beaks and radula by Octopus vulgaris in feeding. J Zool 161:25–38. https://doi.org/10.1111/j.1469-7998.1970.tb02167.x
Alves DM, Cristo M, Sendão J, Borges TC (2006) Diet of the cuttlefish Sepia officinalis (Cephalopoda: Sepiidae) off the south coast of portugal (eastern Algarve). J Mar Biol Assoc UK 86:429–436. https://doi.org/10.1017/S0025315406013312
Andrews PLR, Darmaillacq A-S, Dennison N, Gleadall IG, Hawkins P, Messenger JB, Osorio D, Smith VJ, Smith JA (2013) The identification and management of pain, suffering and distress in cephalopods, including anaesthesia, analgesia and humane killing. J Exp Mar Biol Ecol 447:46–64. https://doi.org/10.1016/j.jembe.2013.02.010
Arkhipkin AI, Bizikov VA, Doubleday ZA, Laptikhovsky VV, Lishchenko FV, Perales-Raya C, Hollyman PR (2018) Techniques for estimating the age and growth of molluscs: Cephalopoda. J Shellfish Res 37:783–792. https://doi.org/10.2983/035.037.0409
Basuyaux O, Legrand V (2013) La seiche sur la côte ouest du cotentin – De la ponte à la capture. Rapport D’étude 2012–2013:37
Boyle PR, Mangold K, Froesch D (1979) The organisation of beak movements in octopus. Malacologia 18:423–430
Carrier DR (1996) Ontogenetic limits on locomotor performance. Physiol Zool 69(3):467–488
Denton E, Gilpin-Brown J (1973) Floatation mechanisms in modern and fossil cephalopods. Advances in Marine Biology 11. Elsevier, pp 197–268
Dilly PN, Nixon M (1976) The cells that secrete the beaks in octopods and squids (Mollusca, Cephalopoda). Cell Tissue Res 167:229–241. https://doi.org/10.1007/BF00224330
Dunn MR (1999) Aspects of the stock dynamics and exploitation of cuttlefish, Sepia officinalis (Linnaeus, 1758), in the English channel. Fish Res 40:277–293. https://doi.org/10.1016/S0165-7836(98)00223-9
Ebenstein DM, Pruitt LA (2006) Nanoindentation of biological materials. Nanotoday 1:26–33
Franco-Santos RM, Vidal EAG (2014) Beak development of early squid paralarvae (Cephalopoda: Teuthoidea) may reflect an adaptation to a specialized feeding mode. Hydrobiologia 725:85–103. https://doi.org/10.1007/s10750-013-1715-2
Franco-Santos RM, Vidal EAG (2020) Tied hands: synchronism between beak development and feeding-related morphological changes in ommastrephid squid paralarvae. Hydrobiologia 847:1943–1960. https://doi.org/10.1007/s10750-020-04223-z
Franco-Santos RM, Iglesias J, Domingues PM, Vidal EAG (2014) Early beak development in Argonauta nodosa and Octopus vulgaris (Cephalopoda: Incirrata) paralarvae suggests adaptation to different feeding mechanisms. Hydrobiologia 725:69–83. https://doi.org/10.1007/s10750-013-1721-4
Guerra-Marrero A, Perales-Raya C, Lishchenko F, Espino-Ruano A, Jiménez-Alvarado D, Lorena C-M, Castro JJ (2023) Age validation in early stages of Sepia officinalis from beak microstructure. Mar Biol 170:24. https://doi.org/10.1007/s00227-022-04165-1
Herrel A, Gibb AC (2006) Ontogeny of performance in vertebrates. Physiol Biochem Zool 79(1):1–6
Herrel A, O’Reilly JC (2006) Ontogenetic scaling of bite force in lizards and turtles. Physiol Biochem Zool 79(1):31–42. https://doi.org/10.1086/498193
Herrel A, Spithoven L, Van Damme R, Vree DE, F, (1999) Sexual dimorphism of head size in Gallotia galloti: testing the niche divergence hypothesis by functional analyses. Funct Ecol 13:289–297. https://doi.org/10.1046/j.1365-2435.1999.00305.x
Kear AJ (1994) Morphology and function of the mandibular muscles in some coleoid cephalopods. J Mar Biol Assoc UK 74:801–822. https://doi.org/10.1017/S0025315400090068
Kolmann MA, Huber DR (2009) Scaling of feeding biomechanics in the horn shark Heterodontus francisci: ontogenetic constraints on durophagy. Zoology 112(5):351–361
Koueta N, Boucaud-Camou E (1999) Food intake and growth in reared early juvenile cuttlefish Sepia officinalis L. (Mollusca Cephalopoda). J Exp Mar Biol Ecol 240(1):93–109. https://doi.org/10.1016/s0022-0981(99)00054-4
Marghoub A, Williams CJA, Vasco Leite J, Kirby AC, Kever L, Porro LB, Barrett PM, Bertazzo S, Abzhanov A, Vickaryous M, Herrel A, Evans SE, Moazen M (2022) Unravelling the structural variation of lizard osteoderms. Acta Biomater 146:306–316
Matias RS, Gregory S, Ceia FR, Baeta A, Seco J, Rocha MS, Fernandes EM, Reis RL, Silva TH, Pereira E, Piatkowski U, Ramos JA, Xavier JC (2019) Show your beaks and we tell you what you eat: different ecology in sympatric Antarctic benthic octopods under a climate change context. Mar Environ Res 150:104757. https://doi.org/10.1016/j.marenvres.2019.104757
Miserez A, Schneberk T, Sun C, Zok FW, Waite JH (2008) The transition from stiff to compliant materials in squid beaks. Science 319:1816–1819. https://doi.org/10.1126/science.1154117
Moazen M, Peskett E, Babbs C, Pauws E, Fagan MJ (2015) Mechanical properties of calvarial bones in a mouse model for craniosynostosis. PLoS ONE 12(10):e0125757
Nixon M (1985) Capture of prey, diet and feeding of Sepia officinalis and Octopus vulgaris (Mollusca: Cephalopoda) from hatchling to adult. Vie Et Milieu/life Environ 35(3):255–261
Nixon M, Mangold K (1998) The early life of Sepia officinalis, and the contrast with that of Octopus vulgaris (Cephalopoda). J Zool 245:407–421. https://doi.org/10.1017/S0952836998008048
Oliver WC, Pharr GM (1992) An improved technique for determining hardness and elastic modulus using load and displacement sensing indentation experiments. J Mater Res 7(6):1564–1583
Perales-Raya C, Bartolomé A, García-Santamaría MT, Pascual-Alayón P, Almansa E (2010) Age estimation obtained from analysis of octopus (Octopus vulgaris Cuvier, 1797) beaks: improvements and comparisons. Fish Res 106(2):171–176. https://doi.org/10.1016/j.fishres.2010.05.003
Perales-Raya C, Jurado-Ruzafa A, Bartolomé A, Duque V, Carrasco MN, Fraile-Nuez E (2014a) Age of spent Octopus vulgaris and stress mark analysis using beaks of wild individuals. Hydrobiologia 725:105–114. https://doi.org/10.1007/s10750-013-1602-x
Perales-Raya C, Almansa E, Bartolomé A, Felipe BC, Iglesias J, Sánchez FJ, Carrasco JF, Rodríguez C (2014b) Age validation in Octopus vulgaris beaks across the full ontogenetic range: beaks as recorders of life events in octopuses. J Shellfish Res 32(2):481–493. https://doi.org/10.2983/035.033.0217
Perales-Raya C, Nande M, Roura A, Bartolomé A, Gestal C, Otero JJ, García-Fernández P, Almansa E (2018) Comparative study of age estimation in wild and cultured Octopus vulgaris paralarvae: effect of temperature and diet. Mar Ecol Prog Ser 598:247–259. https://doi.org/10.3354/meps12218
Roscian M (2021) Morphologie fonctionnelle et adaptations des mâchoires des céphalopodes actuels : implications chez les fossiles. PhD thesis, Muséum National d’Histoire Naturelle, Paris, France
Schmidt-Nielsen K (1984) Scaling: why is animal size so important? Cambridge University Press
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH Image to ImageJ: 25 years of image analysis. Nat Methods 9(7):671–675. https://doi.org/10.1038/nmeth.2089
Schwarz R, Hoving H-J, Noever C, Piatkowski U (2019) Life histories of Antarctic incirrate octopods (Cephalopoda: Octopoda). PLoS ONE 14(7):e0219694. https://doi.org/10.1371/journal.pone.0219694
Trillmich F, Bieneck M, Geissler E, Bischof HJ (2003) Ontogeny of running performance in the wild guinea pig (Cavia aperea). Mamm Biol 68:214–223. https://doi.org/10.1078/1616-5047-00087
Uyeno TA, Kier WM (2005) Functional morphology of the cephalopod buccal mass: a novel joint type. J Morphol 264:211–222. https://doi.org/10.1002/jmor.10330
Uyeno TA, Kier WM (2007) Electromyography of the buccal musculature of octopus (Octopus bimaculoides): a test of the function of the muscle articulation in support and movement. J Exp Biol 210:118–128. https://doi.org/10.1242/jeb.02600
von Boletzky S (1983) Sepia officinalis. Cephalopod life cycles. Academic Press, pp 31–52
Werner EE, Gilliam JF (1984) The ontogenetic niche and species interactions in size-structured populations. Annu Rev Ecol Syst 15:393–425
Xavier JC, Golikov AV, Queirós JP, Perales-Raya C, Rosas-Luis R, Abreu J, Bello G, Bustamante P, Capaz JC, Dimkovikj VH, González ÁF, Guímaro H, Guerra-Marrero A, Gomes-Pereira JN, Hernández-Urcera J, Kubodera T, Laptikhovsky V, Lefkaditou E, Lishchenko F, Luna A, Liu B, Pierce GJ, Pissarra V, Reveillac E, Romanov EV, Rosa R, Roscian M, Rose-Mann L, Rouget I, Sánchez P, Sánchez-Márquez A, Seixas S, Souquet L, Varela J, Vidal EAG, Cherel Y (2023) Corrigendum: the significance of cephalopod beaks as a research tool: an update. Front Physiol 14:1140110. https://doi.org/10.3389/fphys.2023.1140110
Acknowledgements
This work was supported by a Human Frontier Science Program Long-Term fellowship awarded to LS (LT000476/2021-L). We warmly thank José Quiero, Erica Vidal and José Xavier for enlightening discussion about cephalopod beak growth. Special thanks to Marjorie Roscian for her support and constructive conversations during this research process.
Funding
Human Frontier Science Program, LT000476/2021-L, Louise Souquet
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. OB and GG collected the eggs, bred and provided the Sepia officinalis specimens. Bite force recording were performed by AH with the help of OB, IR and LS. Material preparation, data collection and analysis were performed by LS. The first draft of the manuscript was written by LS and all authors commented on early versions of the manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of inerest
This work was supported by a Human Frontier Science Program Long-Term fellowship awarded to LS. The authors declare no conflict of interest. Authors declare that all applicable international, national and/or institutional guidelines for sampling, care and experimental use of organisms for the study have been followed, and all necessary approvals have been obtained.
Additional information
Responsible Editor: E.A.G. Vidal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Souquet, L., Basuyaux, O., Guichard, G. et al. The growth of the buccal mass in Sepia officinalis: functional changes throughout ontogeny. Mar Biol 170, 82 (2023). https://doi.org/10.1007/s00227-023-04224-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04224-1