Abstract
High temperatures alter the physiological condition of Octopus maya embryos, juveniles, and adults, and the time of exposure could have a key role in their thermal tolerance. The present study evaluates the effects of temperature and exposure time on octopus juveniles obtained from a thermally stressed female and a control female when exposed to optimal (25 °C) and high temperatures (30 °C) for 20 and 30 days, respectively. The results showed a transgenerational temperature effect that was expressed with low survival, depressed routine resting and high metabolic rates. Moreover, a collapse of antioxidant defense enzymes and high levels of oxidative damage products were detected in juveniles from thermally stressed females. Stress was lethal for animals acclimated at 30 °C, while the performance of juveniles acclimated at optimal temperature (25 °C) was conditioned by high oxidative stress levels and a reduction of the high metabolic rate (HMR) even after 30 days of experiment. In contrast, juveniles from the non-thermally stressed female had an optimal performance when acclimated at 25 °C but at 30 °C, they had a comparatively higher HMR during the first 8 days. These results suggest energy surplus in those animals to escape from warming scenarios before experiencing oxidative damage accumulation. Further studies should confirm if epigenetic alterations could be involved.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The current anthropogenic global warming—estimated in an increment of sea temperature from 1 to 3 °C for the next decades—is expected to generate profound metabolic alterations in marine ectotherm invertebrates, which can have consequences in the distribution and abundance of a wide number of species (Brown et al. 2004; Lefevre 2016; Madeira et al. 2016). Although the physiological processes are enhanced under high temperatures and result in high energy production, they also provoke the increment of deleterious reactive oxygen species (ROS; Johnstone et al. 2019). The mitochondrial respiratory chain is one of the main sources of ROS (e.g., hydroxyl and superoxide radicals, singlet oxygen, etc.) and reactive nitrogen species (RNS; e.g., nitric oxide, peroxynitrite, etc.), produced naturally through physiological and non-physiological processes (Fridovich 1986; Feidantsis et al. 2021).
Oxidative stress produced by ROS consists of deleterious cumulative biomolecular cell damage, mainly oxidation of lipids, proteins, and nucleic acids (Storey 1996; Hulbert et al. 2007). The progressive accumulation of these biomolecules in cells and tissues modifies the function of these structural elements and enzymatic activity, generating damage to the proteasomal system (Beckman and Ames 1998; Grune et al. 2001; Passos and Von Zglinicki 2006). The cell antioxidant defense mechanisms (ANTIOX) consist of a group of enzymes (e.g. catalase, glutathione peroxidase, peroxiredoxin; Di Giulio et al. 1989) complemented by some low-weight non-enzymatic molecules (e.g. vitamins A, C, and E, bilirubin, beta-carotene, uric acid, and flavonoids; Rahal et al. 2014) that progressively reduce ROS into safe compounds (e.g. O2 and H2O; Halliwell 2013) or break the autocatalytic chain of radical reactions (Cadenas 1989), respectively. However, in organisms subjected to heat stress, ROS production can surpass the ANTIOX system, producing a condition named pejus, in which the organism survival depends on the exposure time to heat stress or ability to depress metabolism, and consequently, ROS production (Pörtner 2010; Pörtner et al. 2017; Rodríguez-Fuentes et al. 2017).
Evidence has suggested that thermal tolerance between generations can be enhanced through thermal preconditioning of adults to trigger an epigenetic response that prepares the progeny to effectively cope with challenging temperature increases (Eirin-Lopez and Putnam 2021; Fellous et al. 2015, 2022; Ortiz-Rodríguez et al. 2012). To mention some, the process has been defined as cross-generation plasticity (CGP), when the environment experienced by parents influences offspring phenotype: F0–F1; multigenerational plasticity (MGP) when the environment experienced by previous generations is evident to the F2 and beyond: F1–F2 + ; and carry-over effects (COE) that occur within the development (e.g. embryo to larva; Byrne et al. 2020). However, studies performed on the mussel Mytilus californicus and Octopus maya embryos have also provided evidence for negative parental effects on offspring thermal tolerances (Dominguez-Castanedo et al. 2023); Waite and Sorte 2022), although the involved mechanisms of maladaptation are still unclear.
Octopus maya supports one of the most important octopus fisheries at the world level, with an annual production of around 20,000 Tons and it is considered that their catch has reached the maximum sustainable yield. This species, endemic of the Yucatan Peninsula, is particularly sensitive to thermal stress (Ángeles-Gonzales et al. 2020). It has been observed that temperatures above 27 °C deeply affect the reproductive performance both of males and females. In males, testicular damage and sperm viability, with a reduction of their parental contribution in progeny, has been reported, whereas in females, a reduction in the number of spawned and fertilized eggs, as well as the size, yolk mass, CAT activity and total glutathione (GSH) of embryos, along with higher metabolic rates.
The Yucatán peninsula has two well-differentiated oceanographic areas. One in the northern zone where a seasonal upwelling maintains the shelf floor temperature from 22 to 24 °C in summer, while they can reach more than 30 °C on the floor of the western zone temperatures (Fig. 1; Enriquez et al. 2013).
Temperature variation in the continental shelf of the Yucatán peninsula, Mexico (2010–2020 data). Note: blue color in the zone with the influence of seasonal upwelling (north coast) and green and yellow zones in the western coast where upwelling has no influence. Octopus maya distribution is limited to the isobath of 50 m of the continental Yucatán peninsula shelf (Avendaño et al. 2019)
As a consequence, oceanographic conditions drive the octopus population to have different biological cycles. In the northern zone, reproduction occurs year-round where the upwelling maintains the temperatures below 26 °C even in summer (Fig. 1). In the western zone, the reproductive peak is concentrated in winter, when polar winds chill the platform (Angeles-Gonzalez et al. 2017; Juárez et al. 2018; Markaida et al. 2016). In such circumstances, females that spawn in the western zone incubate the embryos during the winter months (January–March) when temperatures are expected to be maintained from 22 to 26 °C. Considering that in the western zone there is no influence of upwelling is highly probable that this zone, in warming scenarios, experiencing short and hard winters followed by high temperatures that could extend the summer season with high temperatures (IPCC 2021). In such a scenario, hatchlings could be exposed to fast temperature increments and sub-optimal thermal conditions, without the opportunity to make metabolic and energetic adjustments to maintain their homeostasis (Juárez et al. 2016; Pörtner et al. 2017), that could affect their performance ultimately affecting the population (Noyola et al. 2013a, 2013b).
In the past, variations in octopus landings were associated with thermal anomalies resulting from El Niño–Southern Oscillation (ENSO) events (Comisión Nacional de Pesca y Acuacultura 2016). Using that information Ángeles-Gonzalez et al. (2021a; b) found that the octopus fishery production is negatively affected in the western zone of the Yucatan Peninsula when temperature increases 2 °C above average during an ENSO event. This effect has led to the hypothesis that the ENSO anomaly could be causing octopus migration from the western to eastern zone, where temperature increase is limited by seasonal upwelling.
If this hypothesis is true, in warming scenarios or during the ENSO anomalies juveniles feel obligated to migrate to cooler environments before the thermal conditions affect their physiological condition irreversibly. The results obtained in early O. maya juveniles showed that 20 days at 30 °C reduces their growth rate and survival because of the energetic costs associated to maintain the high metabolic rate in high temperatures (Noyola et al. 2013b), indicating that if juveniles do not migrate to low temperature environments their performance could be compromised.
Until now, evidence has demonstrated that high temperatures alter the physiological condition of O. maya embryos, juveniles, and adults and that time of exposure could have a key role in their thermal tolerance. This fact can be explained because animals depend on the duration of energetic reserves and the ability of antioxidant defense mechanisms to neutralize ROS when exposed to high temperature environments (Caamal-Monsreal et al. 2016; Meza-Buendia et al. 2021; Noyola et al. 2013b). In addition to the time of exposure, thermal tolerance has also been observed to depend on the thermal history of the broodstock, which has a key role in the transgenerational thermal tolerance.
Given the concerns and knowledge gaps regarding the effects of global warming on various generations of marine species, the aim of the present study was to evaluate, for the first time, the effects of temperature (25 °C vs. 30 °C) and time of exposure on O. maya juveniles obtained from a thermally stressed female and a control female. The present work integrated biometric, respirometric, and enzymatic activity data to better understand the influence of maternal thermal condition on the ability of animals to make adjustments in their respiratory metabolism (routine and high metabolic rate) and antioxidant defense mechanisms during conditioning time to optimal (25 °C) and high temperatures (30 °C).
This study provides insights on the possible mechanisms involved in temperature stress transgenerational effects and proposes a conceptual model that explains why maternal thermal stress in combination with high temperatures determines the performance of the species and explains also why under warming scenarios Octopus maya could be classified as a species vulnerable to ocean warming.
Materials and methods
Origin of animals
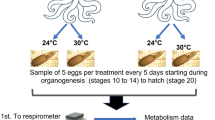
Newly hatched O. maya were obtained from two wild-caught females; one that was thermally stressed (30 °C) and one non-thermally stressed (24 °C) (Fig. 2). Capture and conditioning of the females was performed as described previously (Meza-Buendía et al. 2021). Briefly, ten O. maya males and ten females sexually mature (400–700 g) were captured in the Sisal coast off the Yucatan Peninsula (21°9′55′′N, 90°1′50′′W) by a local drift-fishing method known as ‘Gareteo’. The specimens were caught during one collection trip in March 2019 and transferred to outdoor 6-m-diameter flow-through systems provided with shade mesh, protein skimmers and 500-μm bag filters. Conditioning lasted 10 days (35 ± 1 salinity; dissolved oxygen (DO) ˃ 5.5 mg L−1; 28 ± 1 °C) with a density of 1 animal m−2 and 2-PVC open tubes per animal as refuge. Octopuses were fed twice a day (0900 and 1700 h) with a semi-moist paste made with squid and crab meat, gelatin, a vitamin–mineral premix and ascorbic acid, at a ratio of 8% of its body weight (Tercero et al. 2015).
After the conditioning period, two sexually mature and fertilized females were randomly selected and placed in 80-L individual indoor tanks, one at 30 °C and one at 24 °C. Octopuses were maintained in experimental conditions for 20 days and fed following the same feeding protocol of the conditioning period. A fiberglass box per tank was placed as a refuge and spawn settlement. Each tank was connected to a semi-closed recirculation system coupled with a rapid-rate sand filter. Water parameters showed similar values as above; pH was kept above 8 and photoperiod at 12L/12D with low light intensity (30 Lux m−2). For the 30 °C treatment, seawater temperature was gradually increased at a rate of 2 °C per day and maintained with an 1800-Watt heater connected to an automatic temperature controller, which was placed in the reservoir tank. Temperature of 24 °C was controlled with room air conditioning.
Juveniles
Each female’s clutch was separately incubated at 26 °C for 55–60 days in a 30-L separated tank, both connected to a semi-closed recirculation system (Moguel et al. 2010; Fig. 2). The system was provided with mechanical, biological and ultraviolet (UV) filtration. Then, three-week-of-age hatchlings were randomly exposed to 25 or 30 °C for 30 days and sampling every four days for hatchlings from the stressed female (n = 52) and for 20 days and sampling every 5 days for hatchlings from the non-stressed female (n = 36; Fig. 2). Notice that the control temperature between mothers and progeny differed in 1 °C (24 °C vs. 25 °C). This reflected that for adult O. maya, 24 °C is optimal for growth and reproduction (Meza-Buendia et al. 2021). In contrast, for juvenile development, 24–26 °C, a mean of 25 °C, has been reported to be more appropriate (Noyola et al. 2013a). The different number of replicates between treatments was due to the low fecundity of the stressed female and the low number of hatched individuals. Since juveniles hatched from the stressed female and exposed to 30 °C did not survive more than 15 days, this juvenile group received a thermal treatment of only 15 days.
All organisms were individually kept in 1.41-L plastic containers submerged in 50-L tanks (18H × 72L × 45W cm) with 11–17 containers per tank. Tanks of the same temperature treatment were connected to a 300-L semi-closed recirculatory seawater system equipped with mechanical and UV filtration. Octopus containers were provided with a PVC as a refuge, which had two openings covered with plastic mesh (2 mm) that prevented juveniles from escaping. Air stones and water recirculation in tanks provided current for proper water oxygenation of each container. Water temperature was controlled with CW1000 aquarium chillers and 1800-Watt titanium heaters. Juvenile octopuses were individually fed twice a day (09:00 and 17:00 h) with a heat-dried (40 °C) pelleted diet made of the same ingredients used for the adult diet (Martínez et al. 2014). Every day, feces and uneaten food were siphoned out before feeding. Juvenile survival was recorded daily. Wet weight was assessed by measuring (precision 0.001 g, balance model PMB 53, Adam Equipment, USA) of randomly selected individuals (stressed female, n = 5–8; unstressed female, n = 10–12).
Routine oxygen consumption
Routine oxygen consumption of juvenile O. maya was measured individually in semi-closed respirometric chambers (17 mL) at the corresponding acclimation temperature (25 or 30 °C; n = 5–8 juveniles from the stressed female, n = 6–8 juveniles from the unstressed female). A chamber without octopus was used as a control chamber to evaluate the oxygen consumption of bacteria that could interfere in the final metabolic rate evaluation of the animals. The respirometric trials were run for the time animals consumed 1 mg L−1 which corresponded to approximately 20% of the oxygen in chamber at the beginning of the trial. Trials were performed in an isolated and semi-dark environment to minimize animal stress. Temperature was carefully monitored and controlled with room air conditioning and a 200-W immersion heater attached to an aeration stone to maintain a uniform temperature in the bath during measurements. Dissolved oxygen measurements were recorded for each chamber every second using flow-through oxygen sensors (Loligo systems, Denmark) connected by an optical fiber to Witrox 4 amplifiers (Loligo systems, Denmark). The sensors were calibrated for each experimental temperature using saturated seawater (100% DO) and a 5% sodium sulfite solution (0% DO). The metabolic rate (mg O2 g−1 h−1) was calculated as
where \({\left[{O}_{2}\right]}_{i}\) and \({\left[{O}_{2}\right]}_{f}\) are the oxygen concentration (mg L−1) at the beginning and end of the respirometric trial, respectively; \(\Delta T\) trial period; Vr water volume (L) in the respirometric chamber minus the animal volume (g) and ww wet weight.
High metabolic rate (HMR)
High metabolic rate is a proxy for maximum metabolic rate, and it represents the maximum rate of aerobic metabolism of an organism (Pörtner et al. 2017). This parameter reflects the maximum capacity of transporting oxygen from the environment to the mitochondria. Following a standardized method to induce HMR (Paschke et al. 2018), oxygen consumption was measured when animals were exposed to temperature that induced maximum respiratory rate (TIMR max) defined as 90% of critical thermal maxima (CTMax).
For animals acclimated to 30 °C a TIMR max = 33 °C was used, while a TIMR max = 31 °C was used for animals acclimated to 25 °C considering CTMax data of Noyola et al. (2013a, b). For each trial, acclimated individuals at each experimental temperature (25 °C or 30 °C) were rapidly placed from their acclimation tank in a closed respirometric chamber submerged in a temperature-controlled seawater bath maintained at the corresponding TIMR max temperature for five minutes. An optical oxygen sensor (Loligo systems Denmark) was placed at the internal glass side of the respirometric chamber to allow following the oxygen concentration variations during the 5-min measurements. This time was chosen considering that in such a high temperature, more exposure time could provoke physiological damages, fatigue and anaerobiosis (Rodríguez-Fuentes et al. 2017; Paschke et al. 2018). Changes in oxygen concentration in the respirometric chamber were monitored every second using a flow-through optical oxygen sensor (Loligo system, Denmark) calibrated at the trial temperature (31 or 33 °C). A control measurement (without the animal) was run simultaneously to evaluate the oxygen consumed by microorganisms in seawater. TIMR max was expressed as mg O2 g−1 h−1and calculated as mentioned earlier (Steffensen 1989; Svendsen et al. 2016).
Antioxidant defense system (ANTIOX), oxidative damage, and acetylcholinesterase (AChE) and carboxylesterase (CbE) activities
After routine oxygen consumption and TIRM max measurements, octopuses were weighed, directly snap-frozen in liquid nitrogen, and then stored at − 80 °C for enzyme activity analysis. An average of six and nine, range 3–8 and 8–10, respectively, whole octopuses were analyzed per experimental group, respectively. Samples were homogenized in cold buffer Tris, pH 7.4, at 100 mg tissue ml−1 using a Potter–Elvehjem homogenizer. The homogenate samples used for superoxide dismutase (SOD), Glutathione-S Transferase (GST) and acetylcholinesterase (AChE) and carboxyl esterase (CbE) were centrifuged at 10,000 g at 4 °C for 5 min; the supernatant was separated for analysis; all assays were done in triplicate subsamples.
For the assessment of the antioxidant defense system, SOD activity, total glutathione (GSH), and GST were measured. SOD is an enzyme that alternately catalyzes dismutation (or partitioning) of the superoxide radical into ordinary molecular oxygen and hydrogen peroxide. A Sigma-Aldrich assay kit (19,160) (USA) was used to evaluate SOD, using Dojindo’s highly water-soluble tetrazolium salt, WST-1(2-(4-Iodophenyl)-3-(4-nitrophenyl)-5-(2,4-disulfophenyl)-2H tetrazolium, monosodium salt) that produces a water-soluble formazan dye upon reduction with a superoxide anion. The reduction rate with O2 is linearly related to the xanthine oxidase (XO) activity and inhibited by SOD. GSH was measured with a Sigma-Aldrich Glutathione Assay Kit (CS0260) (USA). This kit utilizes an enzymatic recycling method with glutathione reductase (Baker et al. 1990). The GSH sulfhydryl group reacts with Ellman’s reagent and produces a yellow-colored compound that is read at 405 nm. Glutathione-S-Transferase was measured with a Sigma-Aldrich GST assay Kit (CS0410). This enzyme catalyzes the conjugation of the glutathione thiol group to compounds containing electrophilic centers. The kit utilizes a 1-chloro-2,4-dinitrobenzene substrate (CDNB), which conjugates with the glutathione thiol group, forming a compound that can be read at 340 nm.
To evaluate the oxidative damage caused by ROS, lipid peroxidation (LPO) and carbonyl groups in oxidized proteins (PO) were measured in whole animal samples. LPO was evaluated using Peroxi Detect Kit (PD1, Sigma-Aldrich, USA) following the manufacturer’s instructions. In this assay, peroxide oxidizes Fe2+ ions at acidic pH, forming a colored adduct with xylenol orange that is measured at 560 nm; PO was estimated using the 2,4-dinitrophenylhydrazine alkaline protocols developed by Mesquita et al. (2014) and reported in nmol mg−1 wet weight. For this assay, 200 μL of 2,4 dinitrophenylhydrazine (10 mM in 0.5 M HCL) was incubated with 200 μL of the sample homogenate and 100 μL of NaOH (6 M). Absorbance was read at 450 nm after 10 min of incubation at room temperature against a blank where an equal volume of homogenization buffer substitutes the protein solution.
For this study, two esterases were measured to evaluate physiological condition, AChE and CbE. AChE activity was measured using Ellman et al. (1961) method, adapted to a microplate reader (Rodríguez-Fuentes et al. 2008). Each well contained 10 μL of the enzyme supernatant and 180 μL of 5, 5-dithiobis (2 nitrobenzoic acid; DTNB) 0.5 mM in 0.05 M Tris buffer pH 7.4. The reaction started by adding 10 μL of acetylthiocholine iodide (final concentration 1 mM). The rate of change in absorbance at 405 nm was measured for 120 s. CbE activity was measured using ρ-nitrophenyl-α-arabinofuranoside (ρNPA) substrate, as indicated by Hosokawa and Satoh (2001) with some modifications. Each assay included 25 μL of the supernatant and 200 μL of ρNPA, the reaction was recorded at 405 nm for 5 min. SOD, AChE and CbE activities were reported as mg protein in the sample (Bradford 1976).
Statistical analysis
Changes in wet weight, routine oxygen consumption and high metabolic rate (TIMR max) of juveniles along experimental time were assessed by two-way analysis of variance (ANOVA), with time (days) and juvenile temperature treatment (24 or 30 °C) as explanatory variables, and wet weight, routine oxygen consumption, or TIRM max as response variable. Data that did not meet the ANOVA requirements (linearity, normal distribution and homogeneity of variance) were pre-transformed with square root or natural logarithm. Validation of the ANOVA models was performed by careful inspection of residual distribution according to Zuur et al. (2007). ANOVA procedures were performed with R 4.0.4 (R Core Team 2022).
Variations in the enzymes involved in the ANTIOX system (SOD, GSH and GST), oxidative damage markers (LPO and PO) and the esterases AChE and CbE, through female and juvenile thermal treatments and time (day) were assessed by means of the Principal Coordinate Analyses (PCoA). The PCoA was computed from a resemblance matrix with dissimilarity measures (Euclidian distance) between every pair of samples. Raw data were pre-treated with natural logarithm (Log[X + 1]) and z normalization. In each PCoA, a representation of the relative distances and position of within-group centroids was embedded. Representations were produced reutilizing the corresponding pre-treated resemblance matrix.
Multiple ANOVA with permutations (Anderson 2001) were used to distinguish differences in the multivariate enzyme dynamics between female temperature treatments, and among juvenile temperature and experimental times within each female temperature treatment. The underlying model was a two-way ANOVA per each female (stressed or unstressed) with juvenile thermal treatment as a fixed factor with two levels: 25 °C (n = 23 and 44) and 30 °C (n = 20 and 46); and, time (day) with 4 and 5 levels: 0, 5, 15 and 30 or 0, 4, 8, 12 and 20 (n = 3–11, average 7.9). Unrestricted permutation of raw data and permutations of residuals under the reduced model (999) were used to generate empirical distributions of pseudo-F values under the null hypotheses (Anderson 2017) for the first and latter models, respectively. Post hoc comparisons were applied following a similar procedure after the main test indicated significant differences (p < 0.05) between at least two centroids. Multivariate procedures were carried out using PRIMER 6 and PERMANOVA + for PRIMER. All raw data are available in Data of the study of Maternal temperature stress modulates acclimation and thermal biology in Octopus maya (Cephalopoda: Octopodidae) juvenile progeny|Zenodo.
Results
Wet weight and survival
Water temperature influenced the survival of juveniles hatched from the stressed female; juveniles exposed to 30 °C did not survive more than 15 days, while those exposed to 25 °C showed 80% survival at the end of the experiment. In contrast, all juveniles hatched from the unstressed female survived until the end of the experiment. Wet weight of octopus juveniles of both treatments was similar along the experimental time (ANOVA, F (1, 24–27) = 0.24, p > 0.05; Table 1; Fig S1).
Routine oxygen consumption
Although no statistical differences were observed between the experimental temperatures, a tendency to obtain higher respiratory metabolism was observed in animals maintained at 30 °C (Fig. 3A; ANOVA, F (2,4–7) = 1.30, p = 0.28; Table S1). After 15 days at 30 °C, the octopus maintained at 30 °C died, while those at 25 °C showed a metabolic rate like the ones obtained at the beginning of the experiment (Fig. 3A). In the case of juveniles hatched from the unstressed female (Fig. 3B), a significantly and fluctuating respiratory metabolism was observed with high values recorded in those maintained at 25 °C on day 8 and high values in animals maintained at 30 °C on day 12 (ANOVA, F (4,5–7) = 3.10, p = 0.02). At the end of the experiment, the routine metabolic rate of O. maya juveniles maintained at 25 and 30 °C was similar to those obtained at the beginning of the experiment (Fig. 3B; Table S1).
Routine oxygen consumption rate (mg O2 g−1 h−1) of Octopus maya juveniles hatched from a thermally stressed female (30 °C; A; n = 5–8) and a non-thermally stressed female (24 °C; B; n = 6–8), acclimated at either 25 or 30 °C. Solid points and tendency lines represent mean values, whereas open points represent the raw data. Cages represent ± standard deviation (SD)
High metabolic rate (HMR)
High metabolic rate (HMR) of juveniles from the stressed female maintained at 25 and 30 °C both showed a significantly higher oxygen consumption on day 15 of the experiment, than recorded in the rest of the sampling times (Fig. 4A; ANOVA, F (3,4–6) = 6.22, p = 0.004; Table S2). The HMR of animals maintained at 25 °C and measured on day 30 was similar to those obtained at the beginning of the experiment (Fig. 4A). The HMR of juveniles from unstressed female showed a higher metabolic rate of animals maintained at 30 than 25 °C at least during the first eight days of the experiment (ANOVA, F (1,22) = 4.3, p = 0.04). After that time, a reduction on metabolic rate of the animals acclimated at 30 °C was recorded, which at the end of the experiment were similar to those obtained in animals acclimated at 25 °C and those recorded at the beginning of the experiment (Fig. 4B; Table S2).
Temperature-induced maximum metabolic rate (TIMR max; mg O2 g−1 h−1) of Octopus maya juveniles hatched from a thermally stressed female (30 °C; A; n = 2–5) and a non-thermally stressed female (24 °C; B; n = 4–6), acclimated at either 25 or 30 °C. Solid points and tendency lines represent mean values, whereas open points show the raw data. Cages represent ± standard deviation (SD)
ANTIOX system, oxidative damage, and AChE and CbE activities
PCOs showing ANTIOX system enzymes (SOD, GST and GSH), oxidative damage markers (LPO and PO) and esterases AChE and CbE of juvenile octopuses, display 54.3 (Fig. 5A), 56 (Fig. 5B) and 100% (Fig. 6 A and B) of the total variation in the data (Tables S3 to S7).
Principal coordinate analysis split by maternal treatment (stressed female (A) and unstressed female (B)), showing the effect of juvenile temperature and experimental time (days) on enzymes involved in the ANTIOX system (superoxide dismutase [SOD], glutathione (GSH) and Glutathione-S Transferase GST) and oxidative damage markers lipid peroxidation (LPO) and oxidized proteins (PO) of the juvenile octopus progenies. A 2-D representation of the relative distance and location of within-group centroids is embedded in each figure. Note: solid shapes 25 °C juveniles and open shapes 30 °C juveniles
Principal coordinate analysis (PCoA) split by maternal treatment (stressed female (A) and unstressed female (B), showing the effect of juvenile temperature and experimental time (days) on the esterase AChE and CbE of the juvenile octopus progenies. A 2-D representation of the relative distance and location of within-group centroids is embedded in each figure. Note: solid shapes 25 °C juveniles and open shapes 30 °C juveniles
PCO of juveniles from the stressed female (Fig. 5A) shows a significant increment of LPO and SOD in juveniles acclimated at 30 °C on day 5 (Fig. 5A, blue dash line), followed by a collapse of all the ANTIOX defense enzymes on day 15 (Fig. 5A, red dash line; pair-wise t test, t = 1.71, p = 0.022, 759 unique permutations; Table S3). In addition, animals acclimated to 25 °C formed a cluster on the left-bottom side of the ordination at the end of the experiment (day 30), in correlation with GSH on the first coordinate and with PO on the second one. This cluster can be better appreciated in the embedded figure which shows the relative distance and position of centroids (Fig. 5A; Table S3). These observations were supported by significant time–temperature interaction differences (PERMANOVA, Pseudo-F (2,3–8) = 1.91; p = 0.034, 999 unique permutations).
Regarding juveniles from the unstressed female (Fig. 5B), at the beginning of the experiment (day 0 and 4) both juvenile temperature samples (25 and 30 °C) showed similar positions (levels), in positive correlation with LPO on the first coordinate and with GST on the second one. Then, a progressive reduction on such concentrations (LPO and GST) and an increase in PO were recorded in juveniles at 30 °C (Fig. 5B; Red dash line). These trends were supported by PERMANOVA significant interactions (PERMANOVA, pseudo-F (4,8–11) = 2.22, p = 0.005, 999 unique permutations; Table S4).
Esterases of juveniles from the stressed female were similar along the sampling times and temperatures, and no cluster formation was evident (Fig. 5A; Table S5). Similar results were observed in juveniles from the unstressed female (Fig. 6B), except for day 8 samples of juveniles exposed to 30 °C that moved to the right side of the ordination with respect to 25 °C, indicating higher levels of AChE and CbE in this sampling time (Table S6). This result was supported by significant differences in the juvenile temperature-experimental day interaction term (PERMANOVA, pseudo-F (4,8–11) = 2.62; p = 0.042, 999 unique permutations).
Discussion
It is important to note that in the present study, only one female per experimental group was evaluated. Therefore, more female replicates are needed to represent the population’s genetic diversity. Thus, caution must be taken in interpreting the present results. Also, it should be taken into account that in one female, also are sperms of several males that help us to have a more wide genetic representation of the population. Results obtained in our laboratory demonstrated that in one female, there are sperms of between 8 and 11 males indicating that in each female, there is a diverse mix of genomes (López-Galindo et al. 2019).
The results obtained in this study indicate that time may have a role on the mechanisms and strategies involved in metabolic repair in response of maternal thermal stress. If juveniles from non-thermally stressed females are exposed to fast temperature increments after hatch (i.e., 30 °C), they may have a time-limited opportunity to cope with oxidative stress to avoid irreversible and even fatal consequences. In contrast, if adult females are exposed to temperatures around 30 °C it is highly probable that juveniles experience a transgenerational effect, which could limit survival of the next generation in few days after hatching without any chances to surpass that environmental condition.
In another scenario where hatchlings are from thermally stressed females but hatch in 26 °C environment, results obtained in the present study suggest that those animals survived for long time possibly due to their capacity to neutralize the ROS. Previous studies hypothesized that octopus females inhere ROS to octopus embryos, which are neutralized during the growth phase of the embryo (Olivares et al. 2019). That study also proposed that in warming scenarios, the high metabolism of females could provoke an increment of ROS in the ovary, provoking an additional ROS load to embryos during the egg synthesis. More recent results showed that thermally stressed octopus females inherited an extra load of ROS which affected embryo development (Dominguez-Castanedo et al. 2023) indicating that high temperatures (30 °C) have a clear and transgenerational effect in this species. This result means that embryos that hatch at 25 °C should have physiological mechanisms that allow neutralizing the excess ROS coming from their embryo life. According to our results, these mechanisms could be taking more than 30 day, where hatchlings could require an environment with low (around 25 °C) and stable temperature.
In aquatic ectotherms, a decreased capacity to perform aerobically at higher temperatures is hypothesized to be the key physiological mechanism that may determine the response of many species to climate change (Nilsson et al. 2009; Pörtner 2001; Pörtner and Knust 2007). Previous studies have demonstrated that the limited capacity of the circulatory and ventilatory systems of cephalopods to keep pace with increased oxygen demand at higher temperatures causes a reduction in the aerobic scope and sets the boundaries of the whole-organism thermal tolerance. For that reason, a reduction of the HMR could be an auxiliary mechanism to be used by animals when ROS inherited from thermal stressed females takes a long time to be neutralized (Melzner et al. 2007; Meza-Buendia et al. 2021; Pörtner et al. 2017; Fig. 7).
The flow diagram shows the hypothesis to explain the negative transgenerational effect of maternal thermal stress in Octopus maya embryos and juveniles. Less yolk in embryos, high metabolic rates, and high reactive oxygen species (ROS) levels were inherited to juveniles of thermally stressed females. Juveniles with inherited high ROS levels, as assessed by the oxidative damage markers PO and LPO, were not able to neutralize them when exposed at 30 °C. Those juveniles died at day 15. Juveniles from thermally stressed females and maintained at 25 °C had also high ROS levels too compromise their performance, reducing their metabolism presumably to neutralize ROS excess. AS Aerobic scope, SMR Standard metabolic rate
Results obtained in the present study suggest that metabolic depression could act as a mechanism to reduce ROS production, allowing the animals to move to a colder environment (i.e., 25 °C; Sobrino et al. 2002). In an environment of 25 °C octopus juveniles will produce less ROS and, at the same time, give the animals the time to neutralize ROS inherited from females, those accumulated since embryonic life (Dominguez-Castanedo et al. 2023; Olivarez et al. 2019), and those produced after hatching.
In this study, juveniles from the temperature-stressed female died after 15 days of exposure to 30 °C, presumably due to the limited capacity to neutralize ROS production. High levels of GSH and PO were observed in those organisms. Between non-enzymatic antioxidant is the endogenous reduced glutathione (GSH) that contains a thiol group which acts as a reducing agent. Glutathione reductase (GR) reduces glutathione disulfide (GSSG) to glutathione (GSH), contributing to the maintenance of the cellular redox status (Fassiano et al. 2017; Moreira et al. 2016; Regoli and Giuliani 2014). Although high levels of GSH could indicate a high intensity of antioxidant defense mechanisms, when a metabolic depression is observed (as provoked by 30 °C), it could be showing that animals are in preparation for oxidative stress (POS) in a similar way as observed when animals are exposed to hypoxia levels (Hermes-Lima and Storey 1998). Moreover, high levels of PO suggest cellular damage that in O. maya hatchlings from the thermally stressed female and maintained at 30 °C resulted lethal, because at such high temperature the metabolic depression cannot control the PO production.
Hatchlings from the non-stressed female but exposed at 30 °C showed a higher HMR during the first eight days of the experiment compared to HMR values obtained in animals acclimated at 25 °C. This result means that animals exposed to 30 °C but from the non-stressed female had a temporal surplus of energy as a consequence of acceleration of biochemical pathways linked with aerobic energy production. Furthermore, after eight days, the HMR of juveniles maintained at 30 °C was reduced and maintained similarly to that observed in octopus held at 25 °C. Interestingly, this change coincided with the beginning of a progressive increase in PO (Fig. 5A, red dash line). These results suggest that the first days surplus energy was reduced as a strategy that affected the octopus performance (less physiological energy) but acted as a mechanism to reduce the ROS accumulation, which avoided compromising their survival (Noyola et al. 2013a, b). The ability of organisms to depress metabolic rates is one of a suite of mechanisms available for the animals to cope with high temperatures, including overexpression of heat shock proteins (HSP) to protect cells from protein denaturation, nutrient allocation, digestive enzymes activity, an increase of polyunsaturated fatty acids to maintain cell membrane integrity, between others. Many biochemical studies have demonstrated the effectiveness of mechanisms that aquatic animals have to respond to extreme temperatures. Although the comprehension of the relationship between temperature and aerobic metabolism has many processes involved, in the present study, the relationship between exposure time, metabolic rate, antioxidant defense mechanisms, and oxidant damage was evaluated in an attempt to include the time scale into the complexity of mechanisms involved in the thermal adaptation of aquatic organisms (Schulte 2015). In O. maya, the results obtained in this study suggest that the ability of the octopus juveniles to maintain a high aerobic capacity is time limited and, in consequence, its ability to tolerate temperatures close to 30 °C as previously shown in embryos (Dominguez-Castanedo et al. 2023; Caamal-Monsreal et al. 2016) and adults (López-Galindo et al. 2019; Meza-Buendía et al. 2021; Fig. 7).
A previous study in O. maya embryos postulated the hypothesis that explains the negative effect of maternal thermal stress in this octopus species. It proposed that thermally stressed females provoke epigenetic alterations that modify the energetic metabolism of embryos, with the consequence of high metabolic rates (Dominguez-Castanedo et al. 2023). It also demonstrated that thermally stressed females transfer less yolk to the egg, which added to the high metabolic rate of the embryos resulted in embryos with a smaller size than those laid by non-stressed females. Embryos from thermally stressed females were also observed with high ROS levels and a limited ability of the antioxidant defense mechanisms to neutralize the oxidative damage indicating an excess of ROS at the end of the embryo development. In such circumstances, hatchlings from thermally stressed females could hatch with an extra load of ROS, which could provoke irreversible cellular damages if it is not neutralized fast. In this sense, the results of this study suggest that besides the metabolic wear that 30 °C provoked in juveniles from the two experimental groups, animals from thermally stressed females could have the additional load of ROS inherited by females to embryos. In such conditions, juveniles from stressed females, and exposed at 30 °C had two ROS sources: one probably inherited by females and the other from its own production, both enhanced by high temperature (Dominguez-Castanedo et al. 2023; Fig. 7).
Although to date, it is still not known what kind of biochemical pathways are modified as a consequence of parental thermal stress, as in embryos, it may be related with the epigenetic alterations produced by parental thermal stress. This hypothesis lies on the fact that molecular mechanisms exist by which intermediary metabolites involved in different routes for obtaining cellular energy, such as α-ketoglutarate (α-CG) and cofactors, such as acetyl-CoA, S-adenosyl methionine (SAMe) and nicotinamide adenine dinucleotide (NAD +), are affected by epigenetic modifications (Thomas and David 2001).
Moreover, the hypothesis is supported by a toxicological study that showed how microcystin tolerance was enhanced by maternal factors transferred to the progeny of the freshwater crustacean Daphnia magna (Ortiz-Rodríguez et al. 2012). These factors are involved in the modulation of the GST-mediated detox system (also relevant in the ANTIOX system), which is energetically costly. As a result, increased survival in the crustaceans with the maternal factor was at the expense of a reduction in growth rates, presumably due to energy allocations that accelerate metabolism and prepare the organisms to cope with future toxic challenges. It is still unknown which epigenetic mechanisms could be involved in temperature stress tolerance in octopus. Further studies should be performed in octopus to know both if in juveniles from stressed females epigenetic alterations exist and if this condition affects their growth.
Conclusion
The results obtained so far show that O. maya has a thermal history linked to the environmental conditions in which it lives, with all phases of the life cycle occurring in a temperature range from 24 to 26 °C (Enriquez et al. 2013). In line with this, the results obtained in the present study suggest that females exposed to a temperature beyond the natural range could produce progenies with physiological alterations that limit their metabolic performance and probably their growth and survival. Although more female replicates are needed to confirm this fact, females studied had not only their genomes represented in the progeny but also the genetic information from many males represented in the sperm cells stored in the oviducal gland. In the case of O. maya females, we have detected between 8 to 11 males represented in one spawn, indicating that multiple paternity is presented in this species (López-Galindo et al. 2019) and, in consequence, a wider variability between juveniles beyond the variability that each female exposed to experimental conditions could have. Consequently, the results obtained in the present study could have a wider representativity if it is considered that juveniles in the experiments were half-brothers.
Until now, the temperature of the northern coast of the Yucatán peninsula is regulated by upwelling. However, other sites on the Yucatán continental shelf are not thermally controlled. Those zones can experience temperatures far from the thermal tolerance of O. maya could affect the structure and abundance of marine communities on which the octopus population depends (Juárez et al. 2015).
Therefore, if environmental changes exceed the capacity of octopuses to respond via their plasticity (migration to the coolest environments, increase in oxygen consumption, alteration of critical temperature limits, etc.), the population would be exposed to damage that could be irreversible, affecting the abundance of this species in the coastal environment of the Yucatán Peninsula where thousands of families depend on this valuable marine resources.
Data availability
The datasets generated during and/or analysed during the current study are available in the ZENODO repository, “Data of the study of Maternal temperature stress modulates acclimation and thermal biology in Octopus maya (Cephalopoda: Octopodidae) juvenile progeny|Zenodo”.
References
Anderson MJ (2001) A new method for non-parametric multivariate analysis of variance. Austral Ecol 26:32–46. https://doi.org/10.1111/j.1442-9993.2001.01070.pp.x
Anderson MJ (2017) Permutational multivariate analysis of variance (PERMANOVA). Wiley StatsRef Stat Ref Online Major Ref Works. https://doi.org/10.1002/9781118445112.stat07841
Angeles-Gonzalez LE, Calva R, Santos-Valencia J, Avila-Poveda OH, Olivares A, Díaz F, Rosas C (2017) Temperature modulates spatio-temporal variability of the functional reproductive maturation of Octopus maya (Cephalopoda) on the shelf of the Yucatan Peninsula. Mexico J of Mollusc Stud. https://doi.org/10.1093/mollus/eyx013,1-9
Ángeles-González LE, Lima FD, Caamal-Monsreal C, Díaz F, Rosas C (2020) Exploring the effects of warming seas by using the optimal and pejus temperatures of the embryo of three Octopoda species in the Gulf of Mexico. J Therm Biol. https://doi.org/10.1016/j.jtherbio.2020.102753
Ángeles-González LE, Martínez-Meyer E, Rosas C, Guarneros-Narváez PV, López-Rocha JA, Escamilla-Aké Á, Osorio-Olvera L, Yáñez-Arena C (2021a) Long-term environmental data explain better the abundance of the red octopus (Octopus maya) when testing the niche centroid hypothesis. J Exp Mar Bio Ecol 544:151609. https://doi.org/10.1016/j.jembe.2021.151609
Ángeles-González LE, Martínez-Meyer E, Yañez-Arenas C, Velázquez-Abunader I, López-Rocha J, Torrejón-Magallanes J, Rosas C (2021b) Climate change effect on Octopus maya (Voss and Solís-Ramírez, 1966) suitability and distribution in the Yucatan Peninsula, Gulf of Mexico: a correlative and mechanistic approach. Estuar Coast and Shelf Sci. https://doi.org/10.1016/j.ecss.102021.107502
Avendaño O, Velázquez-Abunder I, Fernández-Jardón CM, Ángeles-González LE, Hernández-Flores A, Guerra A (2019) Biomass and distribution of the red octopus (Octopus maya) in the north-east of the Campeche Bank. J Mar Biol Ass UK. https://doi.org/10.1017/S0025315419000419
Baker MA, Cerniglia GJ, Zaman A (1990) Microtiter plate assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem 190:360–365. https://doi.org/10.1016/0003-2697(90)90208-Q
Beckman KB, Ames BN (1998) The free radical theory of aging matures. Physiol Rev 78:547–581. https://doi.org/10.1152/physrev.1998.78.2.547
Bradford MM (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248–254. https://doi.org/10.1016/0003-2697(76)90527-3
Brown JH, Gillooly JF, Allen AP, Savage VM, West GB (2004) Toward a metabolic theory of ecology. Ecol 85:1771–1789. https://doi.org/10.1890/03-9000
Byrne M, Foo SA, Ross PM, Putnam HM (2020) Limitations of cross- and multigenerational plasticity for marine invertebrates faced with global climate change. Glob Chang Biol 26:80–102. https://doi.org/10.1111/gcb.14882
Caamal-Monsreal C, Uriarte I, Farias A, Díaz F, Sánchez A, Re D, Rosas C (2016) Effects of temperature on embryo development and metabolism of O. maya. Aquacul 451:156–162. https://doi.org/10.1016/j.aquaculture.2015.09.011
Cadenas E (1989) Biochemistry of oxygen toxicity. Annu Rev Biochem 58:79–110. https://doi.org/10.1146/annurev.bi.58.070189.000455
Comisión Nacional de Pesca y Acuacultura C (2016) Información Estadistica por Especie y Entidad. Comisión Nacional De Pesca y Acuacultura, SAGARPA, México. http://www.conapesca.gob.mx/wb/cona/informacion_estadistica_por_especie_y_entidad
Di Giulio RT, Washburn PC, Wenning RJ, Winston GW, Jewell CS (1989) Biochemical responses in aquatic animals: a review of determinants of oxidative stress. Environ Toxicol Chem 8:1103–1123. https://doi.org/10.1002/etc.5620081203
Dominguez-Castanedo O, Palomino-Cruz D, Mascaró M, Rodíguez-Fuentes G, Juárez O, Galind-Sánchez CE, Caamal-Monsreal C, Galindo-Torres P, Díaz F, Rosas C (2023) Trans-generational physiological condition of embryos is conditioned by maternal thermal stress in Octopus maya. Mar Biol 170:41. https://doi.org/10.1007/s00227-023-04183-7
Eirin-Lopez JM, Putnam H (2021) Marine environmental epigenetics. Front Mar Sci. https://doi.org/10.3389/fmars.2021.685075
Ellman GL, Courtney KD, Andres V, Featherstone RM (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95. https://doi.org/10.1016/0006-2952(61)90145-9
Enriquez C, Mariño-Tapia I, Jeronimo G, Capurro-Filograsso L (2013) Thermohaline processes in a tropical coastal zone. Cont Shelf Res 69:101–109. https://doi.org/10.1016/j.csr.2013.08.018
Fassiano AV, Ortiz N, de Molina MCR (2017) Reproductive status, antioxidant defences and lipid peroxidation in Octopus tehuelchus (Cephalopoda: Octopodidae) females. J Nat Hist 51:2645–2660. https://doi.org/10.1080/00222933.00222017.01329460
Feidantsis K, Georgoulis I, Giantsis IA, Michaelidis B (2021) Treatment with ascorbic acid normalizes the aerobic capacity, antioxidant defence, and cell death pathways in thermally stressed Mytilus galloprovincialis. Comp Biochem Physiol Part B Biochem Mol Biol 255:110611. https://doi.org/10.1016/j.cbpb.2021.110611
Fellous A, Favrel P, Riviere G (2015) Temperature influences histone methylation and mRNA expression of the Jmj-C histone-demethylase orthologues during the early development of the oyster Crassostrea gigas. Mar Genomics 19:23–30. https://doi.org/10.1016/j.margen.2014.09.002
Fellous A, Wegner KM, John U, Mark FC, Shama LNS (2022) Windows of opportunity: ocean warming shapes temperature-sensitive epigenetic reprogramming and gene expression across gametogenesis and embryogenesis in marine stickleback. Glob Change Biol 28:54–71. https://doi.org/10.1111/gcb.15942
Fridovich I (1986) Biological effects of the superoxide radical. Arch Biochem Biophys 247:1–11. https://doi.org/10.1016/0003-9861(86)90526-6
Grune T, Shringarpure R, Sitte N, Davies K (2001) Age-related changes in protein oxidation and proteolysis in mammalian cells. J Gerontol Ser A 56:B459–B467. https://doi.org/10.1093/gerona/56.11.B459
Halliwell B (2013) The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol 75:637–644. https://doi.org/10.1111/j.1365-2125.2012.04272.x
Hermes-Lima M, Storey KB (1998) Role of antioxidant defenses in the tolerance of severe dehydration by anurans. The case of the leopard frog Rana pipiens. Mol Cell Biochem 189:79–89. https://doi.org/10.1023/A:1006868208476
Hosokawa M, Satoh T (2001) Measurement of carboxylesterase (CES) activities. Curr Protoc Toxicol 10:4.7.1-4.7.14. https://doi.org/10.1002/0471140856.tx0407s10
Hulbert AJ, Pamplona R, Buffenstein R, Buttemer WA (2007) Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213. https://doi.org/10.1152/physrev.00047.2006
IPCC (2021) Summary for policymakers. In: Masson-Delmotte V, Zhai P, Pirani A, Connors SL, Péan C, Berger S, Caud N, Chen Y, Goldfarb L, Gomis MI, Huang M, Leitzell K, Lonnoy E, Matthews JBR, Maycock TK, Waterfield T, Yelekçi O, Yu R, Zhou B (eds) Climate change 2021: the physical science basis. Contribution of working group I to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press
Johnstone J, Nash S, Hernandez E, Rahman MS (2019) Effects of elevated temperature on gonadal functions, cellular apoptosis, and oxidative stress in Atlantic sea urchin Arbacia punculata. Mar Environ Res 149:40–49. https://doi.org/10.1016/j.marenvres.2019.05.017
Juárez OE, Galindo-Sánchez CE, Díaz F, Re D, Sánchez-García AM, Camaal-Monsreal C, Rosas C (2015) Is temperature conditioning Octopus maya fitness? J Exp Mar Bio Ecol 467:71–76. https://doi.org/10.1016/j.jembe.2015.02.020
Juárez OE, Hau V, Caamal-Monsreal C, Galindo-Sánchez CE, Díaz F, Re D, Rosas C (2016) Effect of maternal temperature stress before spawning over the energetic balance of Octopus maya juveniles exposed to a gradual temperature change. J Exp Mar Bio Ecol 474:39–45. https://doi.org/10.1016/j.jembe.2015.10.002
Juárez OE, Enriquez L, Camarena F, Arena L, Galindo-Sánchez C, Lafarga-De la Cruz F, López-Galindo L, Nambo K, Rosas C (2018) Genetic monitoring of the Mexican four-eyed octopus Octopus maya population: new insights and perspectives for the fishery management. Fish Res 206:109–114. https://doi.org/10.1016/j.fishres.2018.1005.1002
Lefevre S (2016) Are global warming and ocean acidification conspiring against marine ectotherms? A meta-analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv Physiol 4:009. https://doi.org/10.1093/conphys/cow009
López-Galindo L, Galindo-Sánchez C, Olivares A, Avila-Poveda OH, Díaz F, Juárez OE, Lafarga F, Pantoja-Pérez J, Caamal-Monsreal C, Rosas C (2019) Reproductive performance of Octopus maya males conditioned by thermal stress. Ecol Ind 96:437–447. https://doi.org/10.1016/j.ecolind.2018.09.036
Madeira D, Costa PM, Vinagre C, Diniz MS (2016) When warming hits harder: survival, cellular stress and thermal limits of Sparus aurata larvae under global change. Mar Biol 163:91. https://doi.org/10.1007/s00227-016-2856-4
Markaida U, Méndez-Loeza I, Rosales-Raya M (2016) Seasonal and spatial trends of Mayan Octopus, Octopus maya, population dynamics from Campeche, México. J Mar Biol Ass UK 97(1663–1673):16. https://doi.org/10.1017/S0025315416001132
Martínez R, Gallardo P, Pascual C, Navarro J, Sánchez A, Caamal-Monsreal C, Rosas C (2014) Growth, survival and physiological condition of Octopus maya when fed a successful formulated diet. Aquacul 426–427:310–317. https://doi.org/10.1016/j.aquaculture.2014.02.005
Melzner F, Mark FC, Pörtner HO (2007) Role of blood-oxygen transport in thermal tolerance of the cuttlefish Sepia officinalis. Integr Compar Biol 47:645–655. https://doi.org/10.1093/icb/icm1074
Mesquita CS, Oliveira R, Bento F, Geraldo D, Rodrigues JV, Marcos JC (2014) Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem 458:69–71. https://doi.org/10.1016/j.ab.2014.04.034
Meza-Buendía AK, Trejo-Escamilla I, Piu M, Caamal-Monsreal C, Rodríguez-Fuentes G, Diaz F, Re D, Galindo-Sánchez CE, Rosas C (2021) Why high temperatures limit reproduction in cephalopods? The case of Octopus maya. Aquac Res. https://doi.org/10.1111/are.15387
Moguel C, Mascaró M, Avila-Poveda OH, Caamal-Monsreal C, Sanchez A, Pascual C, Rosas C (2010) Morphological, physiological and behavioral changes during post-hatching development of Octopus maya (Mollusca: Cephalopoda) with special focus on the digestive system. Aquat Biol 9:35–48. https://doi.org/10.3354/ab00234
Moreira DS, Venancio LPR, Sabino MACT, Hermes-Lima M (2016) How widespread is preparation for oxidative stress in the animal kingdom? Comp Biochem Physiol Part A. https://doi.org/10.1016/j.cbpa.2016.1001.1023
Nilsson GE, Crawley N, Lunde IG, Munday PL (2009) Elevated temperature reduces the respiratory scope of coral reef fishes. Glob Chan Biol 15:1405–1412
Noyola J, Caamal-Monsreal C, Díaz F, Re D, Sánchez A, Rosas C (2013a) Thermopreference, tolerance and metabolic rate of early stages juvenile Octopus maya acclimated to different temperatures. J Therm Biol 38:14–19. https://doi.org/10.1016/j.jtherbio.2012.09.001
Noyola J, Mascaró M, Caamal C, Noreña-Barroso E, Díaz F, Re AD, Sanchez A, Rosas C (2013b) Effect of temperature on energetic balance and fatty acid composition of early juveniles of Octopus maya. J Exp Mar Biol Ecol 445:156–165
Olivares A, Rodríguez-Fuentes G, Mascaró M, Arteaga AS, Ortega K, Monsreal CC, Tremblay N, Rosas C (2019) Maturation trade-offs in octopus females and their progeny: energy, digestion and defence indicators. PeerJ. https://doi.org/10.7717/peerj.6618
Ortiz-Rodríguez R, Son Dao T, Wiegand C (2012) Transgenerational effects of microcystin-LR on Daphnia magna. J Exp Biol 215:2795–2805. https://doi.org/10.1242/jeb.069211
Paschke K, Agüero J, Gebauer P, Díaz F, Mascaró M, López-Ripoll E, Re D, Caamal-Monsreal C, Tremblay N, Pörtner H-O, Rosas C (2018) Comparison of aerobic scope for metabolic activity in aquatic ectotherms with temperature related metabolic stimulation: a novel approach for aerobic power budget. Front Physiol. https://doi.org/10.3389/fphys.2018.01438
Passos JF, Von Zglinicki T (2006) Oxygen free radicals in cell senescence: are they signal transducers? Free Radic Res 40:1277–1283. https://doi.org/10.1080/10715760600917151
Pörtner HO (2001) Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften 88:137–146
Pörtner H-O (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–893. https://doi.org/10.1242/jeb.037523
Pörtner HO, Knust R (2007) Climate change affects marine fishes through the oxygen limitation of thermal tolerance. Science 315:95–97
Pörtner HO, Bock C, Mark FC (2017) Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J Exp Biol 220:2685–2696. https://doi.org/10.1242/jeb.134585
R Core Team (2022) R: a language and environment for statistical computing (Version 4.0.4)
Rahal A, Kumar A, Singh V, Yadav B, Tiwari R, Chakraborty S, Dhama K (2014) Oxidative stress, prooxidants, and antioxidants: the interplay. Biomed Res Int 2014:761264. https://doi.org/10.1155/2014/761264
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Env Res 93:106–117. https://doi.org/10.1016/j.marenvres.2013.07.006
Rodríguez-Fuentes G, Armstrong J, Schlenk D (2008) Characterization of muscle cholinesterases from two demersal flatfish collected near a municipal wastewater outfall in Southern California. Ecotoxicol Environ Saf 69:466–471. https://doi.org/10.1016/j.ecoenv.2007.06.008
Rodríguez-Fuentes G, Murúa-Castillo M, Díaz F, Rosas C, Caamal-Monsreal C, Sánchez A, Paschke K, Pascual C (2017) Ecophysiological biomarkers defining the thermal biology of the Caribbean lobster Panulirus argus. Ecol Indic 78:192–204. https://doi.org/10.1016/j.ecolind.2017.03.011
Schulte PM (2015) The effects of temperature on aerobic metabolism: towards a mechanistic understanding of the responses of ectotherms to changing environment. J Exp Biol 218:1856–1866. https://doi.org/10.1242/jeb.118851
Sobrino I, Silva L, Bellido JM, Ramos F (2002) Rainfall, river discharges and sea temperature as factors affecting abundance of two coastal benthic cephalopod species in the Gulf of Cadiz (SW Spain). Bull of Mar Sci 71(2):851–865
Steffensen JF (1989) Some errors in respirometry of aquatic breathers: how to avoid and correct for them. Fish Physiol Biochem 6:49–59. https://doi.org/10.1007/BF02995809
Storey KB (1996) Oxidative stress: animal adaptations in nature. Brazil Med J Biol Res 29:1715–1733
Svendsen MBS, Bushnell PG, Steffensen JF (2016) Design and setup of intermittent-flow respirometry system for aquatic organisms. J Fish Biol 88:26–50. https://doi.org/10.1111/jfb.12797
Tercero JF, Rosas C, Mascaro M, Poot G, Domingues P, Noreña E, Caamal-Monsreal C, Pascual C, Estefanell J, Gallardo P (2015) Effects of parental diets supplemented with different lipid sources on Octopus maya embryo and hatching quality. Aquacul 448:234–242. https://doi.org/10.1016/j.aquaculture.2015.05.023
Thomas J, David AC (2001) Translating the histone code. Science 293:1074–1080. https://doi.org/10.1126/science.1063127
Waite HR, Sorte CJB (2022) Negative carry-over effects on larval thermal tolerances across a natural thermal gradient. Ecol 103:e03565. https://doi.org/10.1002/ecy.3565
Zuur A, Leno E, Smith G (2007) Analyzing ecological data. Springer-Verlag, New York. https://doi.org/10.1007/978-0-387-45972-1
Acknowledgements
This work was supported by Consejo Nacional de Ciencia y Tecnología for funding the project CONACYT 61503 and PAPIIT IN203022 to CR; D. Fischer provided English Edition. Vargas-Abúndez JA was supported by “Programa de becas posdoctorales en la UNAM” (DGAPA), UNAM. Plata-Díaz A (PCMyL-UNAM) was supported by CONACyT scholarship.
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript. Consejo Nacional de Ciencia y Tenología (MX), 615503, Carlos Rosas, Dirección General de Asuntos del Personal Académico, Universidad Nacional Autónoma de México, IN203022, Carlos Rosas.
Author information
Authors and Affiliations
Contributions
APD, CCM, and CR conducted the experiments and make the preliminary analysis of data and the write the draft of the manuscript. MM and GRF make the analysis of the data and wrote the manuscript. CCM, provided the octopus juveniles, and pictures for the elaboration of the figure, and contributed to the writing of the manuscript. FD and CR contributed to the design of experiments, the writing of the final version of the manuscript, and to administrate the project.
Corresponding author
Ethics declarations
Conflict of interest
No competing interest declared in consequence, the authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed following the European directive related to the use of cephalopods as experimental organisms (DIRECTIVA 2010/63/UE) and approved by the Bioethical Commission at UNAM (Universidad Nacional Autónoma de México) Faculty of Sciences (CEARC/Bioética/25102021).
Additional information
Responsible Editor: E. A. G. Vidal.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Vargas-Abúndez, J.A., Plata-Díaz, A., Mascaró, M. et al. Maternal temperature stress modulates acclimation and thermal biology in Octopus maya (Cephalopoda: Octopodidae) juvenile progeny. Mar Biol 170, 56 (2023). https://doi.org/10.1007/s00227-023-04200-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04200-9