Abstract
Effective marine ecosystem monitoring is critical for sustainable management. Monitoring seabird diets can convey important information on ecosystem health and seabird–fishery interactions. The diet of breeding black-browed albatross (Thalassarche melanophris) has previously been assessed using stomach content analysis (SCA) or stable isotope analysis (SIA), but not both methods together. Combining dietary sampling approaches reduces biases associated with using single methods. This study combines SCA and SIA to study the diet of black-browed albatross chicks, with a specific focus on fishery discard consumption, at two Falkland Islands colonies (New Island 51°43′S, 61°18′W and Steeple Jason Island 51°01′S, 61°13′W) during two consecutive breeding seasons (2019 and 2020). SCA provided high taxonomic resolution of short-term diet and priors for stable isotope mixing models, with multiple measures of dietary items (e.g. numeric frequency N%, frequency of occurrence FO%). By contrast, SIA of down feathers provided a single and more integrated dietary signal from throughout chick development. Although the two methods disagreed on the dominant prey group (SCA—crustacean; SIA—pelagic fish), the complementary information suggested a chick diet dominated by natural prey (SCA: 74%–93% [FO], 44%–98% [N]; SIA: minimum 87%–95% contribution). Nonetheless, SCA revealed that a high proportion of breeding adults do take discards. We detected consistent colony-specific diets in relation to prey species, but not in relation to higher discard use. Overall, discard consumption was highest in 2020, the year characterised by the poorest foraging conditions. Our results have implications for fisheries management and future dietary studies assessing discard use.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In an era of rapid marine ecosystem change, monitoring is critical for the sustainable management of our oceans. Top predators, such as seabirds, are often regarded as useful indicators of marine ecosystem health (Velarde et al. 2019; Bestley et al. 2020), but indices require careful consideration (Durant et al. 2009). For example, changes in seabird demography, distribution, or breeding biology can reflect environmental change (e.g. Votier et al. 2005; Cury et al. 2011; Sydeman et al. 2021), or impacts of anthropogenic activities such as fishing (e.g. Einoder 2009; Pardo et al. 2017; Sherley et al. 2017). However, seabird populations and distributions alter relatively slowly with time (Nevoux et al. 2010) and non-breeders may buffer the effects of environmental or anthropogenic change (Votier et al. 2008a), making it challenging to detect such impacts (e.g. Sherley et al. 2018). By contrast, dietary variation may be a more sensitive indicator of change.
Seabird diets reflect an individual’s foraging behaviour, including prey preference, foraging distribution and dietary flexibility (e.g. Gaglio et al. 2018; Church et al. 2019), and indicate the quantity, quality and accessibility of prey at the species and population level (Buren et al. 2012; Velarde et al. 2019). In addition, seabird diets may provide information on fishery interactions, including variation in discard use (Votier et al. 2004, 2008b), or bycatch risk (Einoder 2009; Phillips et al. 2016). Methods to accurately study seabird diets can therefore form an integral part of monitoring strategies and ecosystem-based approaches to fisheries management, such as in the Commission for the Conservation of Marine Living Resources (CCAMLR) Ecosystem Monitoring Programme (CEMP) (also see e.g. Scopel et al. 2019).
A range of morphological and biochemical methods exist for assessing diet, and each provide different information and biases (Votier et al. 2003; Barrett et al. 2007). Stomach content analysis (SCA) and stable isotope analysis (SIA) are common approaches for assessing seabird diet (Duffy and Jackson 1986; Barrett et al. 2007; McInnes et al. 2016). SCA provides high taxonomic resolution, but this tends to reflect short time scales (days to weeks), and can underestimate soft-bodied prey (Votier et al. 2003; Inger and Bearhop 2008; McInnes et al. 2017b). Conversely, SIA typically provides dietary information integrated over longer time scales during tissue growth (weeks to months; Inger and Bearhop 2008; Phillips et al. 2014). However, stable isotope mixing models, which are used to quantify prey source contribution to a diet mixture, rely on prior knowledge of diet and are only informative if key prey differ in their isotopic composition (Inger and Bearhop 2008; Phillips et al. 2014). Combining morphological and biochemical analyses can address the pitfalls of individual methods (Karnovsky et al. 2012; Bonin et al. 2020), and a complementary approach is therefore recommended when using seabird diet for monitoring (Bot et al. 2018; Ceia et al. 2022).
The black-browed albatross Thalassarche melanophris (hereafter BBA) is the world’s most abundant species of albatross, and its diet has been well-studied (see McInnes et al. 2017a). It is used as a bio-indicator in the CEMP, as well as a sentinel of the Patagonian Shelf Large Marine Ecosystem (Ventura et al. 2021). The population breeding in the Falkland Islands is of particular interest, because it is the world’s largest (> 70% of breeding populations), and, in contrast to several other populations, is increasing in size (BirdLife International 2018).
BBA breeding in the Falkland Islands forage over the Patagonian Shelf, where large numbers scavenge at fishing vessels for discards, consequently falling victim to bycatch (Granadeiro et al. 2011, 2014; Kuepfer et al. 2022a, b). Dietary studies, however, suggest that they predominantly consume natural prey (Thompson 1992; Kuepfer et al. 2022a (SCA); Granadeiro et al. 2014 (SIA); McInnes et al. 2017a (DNA)), although discards appear important when natural foraging conditions are unfavourable (Kuepfer et al. 2022a). Discard consumption also varies amongst colonies, with larger colonies thought to consume more discards (Thompson 1992; McInnes et al. 2017a). However, the previous diet studies all applied different individual methods (SCA: Thompson 1992; Kuepfer et al. 2022a; DNA: McInnes et al. 2017a, b; SIA: Granadeiro et al. 2014), and (apart from McInnes et al. 2017a) focussed on either individual years or individual colonies. It is therefore difficult to distinguish methodological biases from temporal and spatial variation in diets when comparing results. At a time when discard management in the Falkland Islands is changing (through the introduction of batch discarding by trawlers; Kuepfer and Barton 2018; Kuepfer et al. 2022b), and the climate warming across BBAs’ range (Franco et al. 2022), it is important to gain a more comprehensive dietary understanding for this globally significant population.
Here, we use SCA and SIA to study BBA chick diet at two Falkland Islands colonies from two consecutive breeding seasons. Specifically, we (1) quantify diet, (2) assess the importance of discards and natural prey and (3) determine how diet varies between colonies and years. Considering previous findings, we hypothesise that (1) natural prey will dominate across years and colonies but that (2) discard consumption will be higher at the larger colony.
Materials and methods
Study area and fleet characteristics
Fieldwork took place in the austral summer of 2018/2019 and 2019/2020 (hereafter 2019 and 2020) during mid-chick rearing (~ 6–12 weeks of age) at New Island (NWI, 51°43′S, 61°18′W) and Steeple Jason Island (SJI, 51°01′ S, 61°13′W), located in the west and north-west of the Falkland Islands (Fig. 1). NWI and SJI, respectively, support approximately 16,000 and 210,000 breeding pairs of BBA (Crofts 2020).
Chick-rearing adults from NWI and SJI concentrate their foraging on the southern Patagonian Shelf, although they generally segregate by colony whilst at sea (NWI: south-west/west; SJI: north-west/north; Catry et al. 2013). The area is extensively fished, predominantly by bottom trawlers for finfish and jiggers for Argentine shortfin squid (Illex argentinus, hereafter Illex) (Seco Pon et al. 2015; Falkland Islands Government 2021). There is also some longlining (2–4 vessels within the Falklands Conservation Zones (FCZs) and the Argentine Exclusive Economic Zone (AEEZ) combined at < 40° S latitude; Seco Pon et al. 2015; Falkland Islands Government 2021). Discards of demersal and benthopelagic fish are available to seabirds from bottom-trawl vessels throughout the year, including during chick rearing (Fig. S1). Squid discards are limited, as Illex and Patagonian long-finned squid (Doryteuthis gahi, hereafter Loligo) are generally packed whole, and discarding of the latter is prohibited in Falkland Islands waters (Laptikhovsky et al. 2006; Arkhipkin et al. 2015).
Sampling
Stomach content analysis
Stomach contents were collected from chicks using induced regurgitation (after Phillips 2006) during late January through to mid-February at NWI, and in mid-March at SJI (NWI 2019: 29 January–17 February; NWI 2020: 25 January–23 February; SJI 2019 and 2020: 12–16 March). For logistical reasons, it was not possible to sample both colonies simultaneously. Only freshly fed chicks were sampled, and each chick was only sampled once. To obtain a measurement of meal size and stomach oil content, individual stomach samples were weighed whole (total mass, g), and again after stomach oils had been drained (wet mass, g).
Prey were first separated into key prey groups (fish, cephalopod, crustacean, jellyfish and carrion), and subsequently identified to the lowest possible taxonomic level using reference collections (Falkland Islands Fisheries Department (FIFD), unpubl. data; Xavier and Cherel 2009) and with assistance from specialists (B. Lee, Z. Shcherbich). Minimum number of individuals (MNI) was determined through assemblage of whole animals and fresh loose hard structures. MNI for carrion (penguin feathers) and jellyfish within individual samples was always 1, as it was impossible to determine whether parts originated from a single or multiple individuals. Eroded or brittle structures were excluded from all analyses, as these were assumed to have originated from previous meals.
Prey that were identified to species level were further categorised as fishery discards or natural prey based on numerous criteria relating to natural accessibility of prey to albatrosses, fishing and discard practices, and reconstructed prey size (see Kuepfer et al. 2022a for further details; Table S1). Large fish heads in the absence of the bodies were categorised as discards because at-sea catch processing generally involves heading and gutting of fish (see Kuepfer et al. 2022a). For species where reconstructed size was used to assist with classification (hoki Macruronus magellanicus and southern blue whiting Micromesistius australis), maximum sagittal length (mm) was measured from intact otoliths (one from each otolith pair as well as unpaired otoliths) using a binocular microscope equipped with a graticule to reconstruct the original size of the prey from allometric formulae (Table S2). Finally, prey were quantified using four different metrics (see below, Table 1).
Stable isotope analysis
Multiple down feathers from across the body were collected from a random sample of 55–73 chicks at NWI (mid-February, chick age 62 days Catry et al. 2011; Ventura et al. 2021) and at SJI (mid-March, chick age approximately 84 ± 7 days). At this age, given the large increase in body surface area since hatching, the stable isotope composition of down feathers is no longer expected to reflect egg nutrients. Rather, they provide dietary information from the first 2 months of chick development and are therefore directly comparable between NWI and SJI, despite the discrepancy in sampling months.
To characterise stable isotopes of potential prey, we sampled muscle tissue of specimens collected during the FIFD ground-fish surveys in February 2019 and 2020. Sampling focussed south, west and north of the FCZs, where GPS tracked BBA breeding on NWI and SJI forage during chick rearing (Granadeiro et al. 2011; Ventura et al. 2021). Prey species were chosen based on available knowledge of BBA diet in the Falkland Islands (McInnes et al. 2017a, b; Kuepfer et al. 2022a; current study), and included demersal, pelagic and benthopelagic species of fish, cephalopods, crustaceans and jellyfish.
Prey muscle and albatross chick feathers were processed following Meier et al. (2017). To avoid biases in analyses of δ13C related to the presence of lipids in muscle samples (see e.g. Bearhop et al. 2002; Post et al. 2007), a-priori lipid extraction was conducted on lipid-rich samples, defined as species with a C:N > 3.5 (Post et al. 2007). The effervescent test (after e.g. Carabel et al. 2006) confirmed the absence of carbonates from all crustacean samples. Samples were weighed into tin capsules (approx. 0.7 mg) and analysed for δ13C and δ15N values by continuous-flow isotope ratio mass spectrometry at the National Environmental Isotope Facility Stable Isotope Ecology Laboratory, East Kilbride. Samples were analysed using a Elementar vario Pyrocube elemental analyser (2013), coupled with a Thermo Fisher Delta XP Plus Isotope Ratio Mass Spectrometer (IRMS). Ratios were corrected for instrument drift and linearity using interspersed samples of internal laboratory standards (gelatine, glycine and alanine mixtures) with known stable isotope values (for details see Jones et al. 2020). Stable isotope ratios were expressed in δ notation in parts per thousand (‰) relative to Vienna Pee Dee Belemnite (δ13C) or air (δ15N). Precision of the measurements was 0.09‰ for δ13C and 0.17‰ for δ15N, based on the standard deviation of the most common lab standard used (gelatine).
Statistical analysis
All statistical analyses were conducted in R version 4.0.0 (R Core Team 2021). The significance value of frequentist statistical tests was set at α = 0.05, unless stated otherwise.
Stomach content analysis
To assess whether the number of stomach content samples obtained from chicks was sufficient to describe dietary species diversity, samples from individual colony/year combinations were randomised 100 times, and an accumulation curve was constructed as a function of sample size (package vegan::accumcomp, Oksanen et al. 2019). Sample sizes would be considered sufficient to describe the full diversity of the diet if the fitted cumulative prey curves reached an asymptote.
Prey were subsequently described using a range of metrics, after Barrett et al. (2007), and following the method of Kuepfer et al. (2022a) (Table 1). First, key prey groups (fish, crustacean, cephalopod, jellyfish and carrion) were quantified as a percentage index of relative importance (IRI%) as:
where FO% is the percentage of stomach samples (i) in which a particular prey type (p) was present; N% is the number of individuals of a particular prey type present expressed as a percentage of the total prey number at that taxonomic level and M% is the percentage of the total drained sample mass represented by a particular prey type.
Next, individual prey species and categories constructed from identified species (i.e. fish subgroups and prey origin [discards and natural]) were quantified by FO% and N%. We did not obtain M% (and hence IRI%) at these levels, because it was impractical to separate out, and identify, every individual fish bone or loose flesh to obtain species-specific mass (see also Kuepfer et al. 2022a).
Different methods of prey quantification provide complementary dietary information: M% informs on meal size and can indicate approximate nutritional contribution of similar prey; FO% indicates the variability of prey abundance, and N% measures the frequency of prey encounter (Duffy and Jackson 1986; Barrett et al. 2007). The IRI% is considered the most comprehensive approach, as it integrates information from numerous metrics, thereby reducing biases associated with any one measure (Duffy and Jackson 1986; Liao et al. 2001; Mills et al. 2020). The absence of IRI% of prey origin (as explained above) is therefore unfortunate; however, given that crustaceans and cephalopods were categorised as natural prey, and most fish as discards (Table S1), the IRI% of key prey groups can serve as a proxy for prey origin.
Wilcoxon rank sum tests were used to assess (1) differences in meal size, and (2) differences in prey species composition between years and colonies. Year or colony were used as our explanatory variables in individual tests, whilst our dependent variables were either (1) the different meal fractions (total/ liquid/drained); (2) counts or presence/absence of prey species from either discards or natural prey. To minimise Type 1 errors, the significance level was adjusted using the Bonferroni correction to α = 0.025. As stomach content samples were not collected simultaneously at the two colonies, inter-colony comparison warrants some caution due to possible seasonal effects (see Discussion).
Stable isotope analysis
To compare isotopic values (δ13C and δ15N) of chicks between colonies, we conducted multivariate analysis of variance (MANOVA) with post hoc ANOVAs, and compared isotopic niche using SIBER (Stable Isotope Bayesian Ellipses in R; Jackson et al. 2011). We did not compare niche space between years due to insufficient certainty around the isotopic baseline for the two years, which makes it difficult to discern potential differences resulting from baseline shifts or diet shifts.
To assess the percentage contribution of different prey, a series of Bayesian mixing models with a multiplicative error structure of residual × process error and uninformative Dirichlet priors were applied using the MixSIAR package (Stock and Semmens 2016). Individual mixing models were run for each colony/year combination (NWI 2019, SJI 2019, NWI 2020, and SJI 2020). Mixing model convergence was assessed using the Gelman–Rubin and Geweke’s diagnostics (Stock and Semmens 2016).
Uncertainty in SIA can be related to isotopic similarity amongst prey types (Phillips and Gregg 2001; Phillips et al. 2014). Further, the discriminatory power of mixing models rapidly deteriorates when more than six prey sources are included, so aggregating isotopically and biologically similar sources is recommended (Phillips et al. 2014). We therefore tested the isotopic differences between prey species using MANOVAs, and based on ecological and isotopic similarities, aggregated them into four a-priori groups: (1) pelagic fish; (2) demersal fish; (3) benthopelagic fish + squid; (4) crustacean + jellyfish.
The decision to include or exclude a particular species as a prey source was informed by our colony/year-specific SCA results (see below, Table 2)—only prey species that comprised > 10% (FO) of the stomach contents in any colony/year were included, as the exclusion of uncommon prey tends to improve mixing model accuracy (Phillips et al. 2014). In all models, we further included potentially important prey which may easily be missed or underestimated in SCA due to soft body parts (i.e. jellyfish and Fuegian sprat (Sprattus fuegensis); McInnes et al. 2017a, b). Where the isotopic values of individual species did not differ significantly between years (MANOVAs), species were pooled from both years to increase sample size (Table S4, Fig. S2). In the absence of stable isotope values for a particular prey species in one year, we used the values available from the other year (Table S4).
Diet-tissue trophic discrimination factors (TDFs) have not been published for Procellariidae, and we therefore used values estimated using Stable Isotope Discrimination Estimation in R (SIDER, Healy et al. 2018): Feathers: δ15N 4.09 ± 1.19, δ13C 2.21 ± 1.25. SIDER is designed to predict TDFs of consumers based on their ecology and phylogenetic relatedness (Healy et al. 2018), and the values determined by SIDER therefore provide for the most up-to-date and biologically justified models (also see e.g. Swan et al. 2020).
Results
Stomach content analysis
Stomach contents were collected from 143 chicks across the 2 years and colonies (NWI 2019 & 2020: n = 40; SJI 2019: n = 32; SJI 2020: n = 31). Meals were significantly heavier in 2020 at NWI due to the higher liquid portion (Mann–Whitney U tests; Total: W = 392, P = 0.004; Liquid: W = 325, P = 0.001; Drained: W = 770, P = 0.924; Fig. 2). At SJI, although the total meal size did not vary between years, liquids were significantly heavier (before Bonferroni correction), and drained samples significantly lighter in 2020 (Mann–Whitney U tests; Total: W = 290, P = 0.138; Liquid: W = 234, P = 0.036; Drained: W = 534, P = 0.019; Fig. 2).
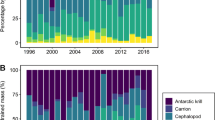
We extracted 9092 prey items from 143 drained regurgitates. The three dominant prey categories were crustacean, fish and cephalopods (Fig. 3a). Jellyfish and carrion (penguin species) were only found in 2020, but their relative importance remained negligible at both colonies (Fig. 3a, Table S3). Crustaceans had the highest relative importance across colonies/years (IRI% = 49–67%), except at NWI 2019, where fish dominated with 80% (IRI). At both colonies, the IRI% of fish was approximately double in 2020 compared to in 2019 (Fig. 3a). Cephalopods, dominated by squid, consistently showed smaller IRI% than fish and crustaceans across colonies and years (IRI% = 4–15%, Fig. 3a).
Black-browed albatross chick diet based on regurgitation from New Island (NWI) and Steeple Jason Island (SJI) in 2019 and 2020, quantified as a percentage index of relative importance (IRI%) of main prey groups; b percentage frequency of occurrence (FO%) and percentage numeric frequency (N%) of fish subgroups; and c percentage frequency of occurrence (FO%) and percentage numeric frequency (N%) of discards and natural prey. Note that the negligible IRI% of jellyfish and carrion renders these practically invisible on the plot. The various symbols indicate which groups are significantly different from one another within each metric
The asymptotes of species accumulation curves were not fully reached; however, the flattening of the species accumulation curves provides confidence that the most important identifiable prey had been captured (Fig. 4). Species richness was higher at SJI than at NWI in 2019, but higher at NWI than SJI in 2020, and was considerably higher in 2020 than in 2019 at both colonies (Fig. 4).
The composition of dominant species differed between colonies but was broadly consistent across years (Table 2). The most frequent and most numerous species encountered at both colonies was lobster krill (Munida gregaria) (Table 2). Individual lobster krill consisted exclusively of adults at NWI, whereas at SJI, both adults and juveniles were present at a ratio of approximately 2:5 in 2019 and 1:50 in 2020. In terms of cephalopods, Loligo squid was the most numerous and abundant species at NWI, whilst Illex squid dominated at SJI (Table 2). In terms of fish, demersal common hake (Merluccius hubbsi) was the most frequent and numerous fish species at SJI in both years, followed by benthopelagic hoki (Fig. 3b; Table 2). At NWI, pelagic Fuegian sprat dominated in 2019, whilst hoki dominated in 2020 (Fig. 3b; Table 2). Ninety-nine percent (n = 134) of all measurable hoki, and one hundred percent of measureable southern blue whiting (n = 6) fell within the size classes caught in the Falkland Islands fishery in 2019 and 2020 during comparable months, and we therefore classified all hoki and southern blue whiting as discards.
Prey origin varied between years at NWI, but not at SJI. Discard consumption at NWI was significantly higher in 2020 compared to in 2019 (Wilcoxon rank sum test; FO%: W = 360, P < 0.001; N%: W = 257, P < 0.001), whilst natural prey declined in terms of N% (Wilcoxon rank sum test; N%: W = 1143, P < 0.001; Fig. 3c). Discards also varied between colonies, being significantly higher at SJI in 2019 (Wilcoxon rank sum test; FO%: W = 366, P < 0.001; N%: W = 66, P < 0.001) but higher at NWI in 2020 (Wilcoxon rank sum test; FO%: W = 772, P = 0.024; N%: W = 825, P = 0.016; Fig. 3c). Natural prey consumption did not differ between colonies regardless of year (Fig. 3c).
Stable isotope analysis
Based on chick down feathers (Table 3), we found a significant difference in the isotopic niche of chicks from NWI and SJI in 2020, but not in 2019 (MANOVA; 2019: Pillai’s Trace = 0.016, F (1, 137) = 1.087, P = 0.340; 2020: Pillai’s Trace = 0.125 F (1, 108) = 7.675, P < 0.001; Fig. 5), with, respectively, 88% and 75% point-estimate of overlap based on the maximum likelihood SEAc (standard ellipse area corrected for small sample size). Post hoc ANOVAs showed that the difference was due to significantly higher δ15N at SJI in 2020 (δ15N: P < 0.001; δ13C: P = 0.049). As the difference of 0.29‰ in δ15N is relatively close to the limit of the machine reading precision of 0.17‰, some caution is warranted for interpretation. The ratio of C/N in down feathers was significantly lower in 2019 compared to in 2020 at both colonies (Mann–Whitney U tests; W = 1700, P < 0.001), but no difference was found between colonies (Mann–Whitney U tests; W = 7493, P = 0.656).
Mixing models estimated pelagic fish as the largest contributor to chick diet across both colonies and years (mean = 69–79%; Fig. 6, Fig. 7). Crustacean + jellyfish were the second most important diet source: 14–19% across years and colonies (Fig. 7). Demersal fish, as well as benthopelagic fish + squid, were estimated to contribute relatively little to the diet (Fig. 7; Table S5), although note that for SJI 2020, the model struggled to discern between the groups of benthopelagic fish + squid and crustacean + jellyfish (Fig. 6). By summing the percentage estimates of prey groups that are categorised as entirely natural prey (pelagic fish; crustacean + jellyfish), we found that natural prey was the dominant diet for all colony/year combinations (mean minimum estimate 91.4–94.6%, excluding squid). Although the differences were relatively small, consumption of fishery discards was higher in 2020 than in 2019 at both colonies, and was higher at SJI than at NWI in both years (Table 4).
Isotopic values of black-browed albatross chick feathers (blue dots) from New Island (NWI) and Steeple Jason Island (SJI) in relation to four prey groups (pelagic fish, demersal fish, benthopelagic prey (fish + squid), crustacean + jellyfish (Crust_Jell)). Chick feather values are presented after correction for diet-tissue isotopic discrimination
Discussion
A complementary approach to diet analyses can help minimise methodological bias, and therefore enable more robust inference (Bot et al. 2018). Using SCA and SIA to assess inter-colony and inter-annual variation in BBA diet, we found that chicks were primarily fed natural prey during mid-chick rearing, although dominant prey sources and species identified varied between methods and colonies. Whilst discards contributed less to the diet than natural prey regardless of sampling method, SCA provided higher discard use estimates compared with SIA. Our results have implications for fisheries management and future dietary studies assessing discard use.
Diet composition
Based on SCA, crustaceans, demersal fish and benthopelagic fish were the dominant prey, whilst SIA suggested that pelagic fish were most important. These results are consistent with SIA studies during early chick rearing at NWI in 2011 (Granadeiro et al. 2014), and DNA metabarcoding of adults and chicks at NWI and SJI in 2014 and 2015 (McInnes et al. 2017a, b). Both SCA and SIA indicate that cephalopods play a relatively minor role in chick diet, as found previously at these colonies (Granadeiro et al. 2014 (SIA); McInnes et al. 2017b (DNA); Kuepfer et al. 2022a (SCA)), as well as at other BBA colonies (McInnes et al. 2017b).
Jellyfish were scarce in SCA but stable isotope mixing models suggest that jellyfish are more common than this (on average 14–19% across colonies/years). Nevertheless, this should be treated with caution given their isotopic similarity to crustaceans. However, previous work using DNA metabarcoding suggests that they are much more prevalent than crustaceans (SJI: up to 80% FO, 50% relative abundance (RA); NWI: up to 20% FO, 8% RA; McInnes et al. 2017b), further highlighting the importance of combining techniques.
SCA identified Fuegian sprat as the dominant pelagic fish, whilst, historically, southern blue whiting was more common in the diet of BBA (Thompson 1992). Increased Fuegian sprat consumption was also found in 2014 and 2015 (McInnes et al. 2017a) and likely reflects a shift in the ecosystem following the collapse of the southern blue whiting stock in 2004–2007 (Laptikhovsky et al. 2013). Unlike southern blue whiting, Fuegian sprat is not targeted by fisheries within the foraging range of NWI and SJI breeding albatross, and discards of this species are rare and patchy (Falkland Islands Government 2021).
Despite the close proximity of NWI and SJI (~ 75 km), SCA revealed inter-colony diet differences, which appear to be stable over time (Thompson 1992; McInnes et al. 2017a). In particular, the dominant fish and squid were hoki and Loligo at NWI, but common hake and Illex at SJI. We cannot entirely exclude an influence of differences in timing between colony visits (NWI: January/February; SJI: March); for example, whilst hoki catches in 2019 and 2020 peaked in January and February, hake catches peaked in March (Ramos and Winter 2019, 2020; Winter and Ramos 2020). However, Thompson (1992) also found increased hake (and Illex) at SJI when sampled in January, compared to at NWI sampled in February/March. Our results may therefore also reflect differences in prey availability at colony-specific foraging sites (see Granadeiro et al. 2011). Indeed, hoki is most abundant in the south-west of the FCZ where NWI birds forage, whereas hake is the dominant catch in the north-west, where SJI birds feed (Catry et al. 2013; Ramos and Winter 2019; Winter and Ramos 2020; Ventura et al. 2021).
Finally, there were differences in trophic niche between colonies. The δ15N values were higher at SJI, although this was only apparent in 2020. This result is suggestive of relatively increased consumption of higher trophic prey (e.g. hake or grenadier).
Relevance of fishery discards
As predicted, albatross chicks were overall fed primarily natural prey; nonetheless, chicks regularly received discards (based on FO%; Table 4). Previous studies have shown limited fishery interactions during early BBA chick rearing (December–early January; Granadeiro et al. 2014)—a period of low fishing effort in Falkland Islands and Argentine waters (see also McInnes et al. 2017a). Some studies suggest discard consumption is more frequent during later chick rearing (from February, McInnes et al. 2017a). Our study supports the high proportion of birds taking discards, but found natural prey to generally remain the main prey source.
Previous multi-colony research suggests higher discard consumption at SJI compared to NWI (Thompson 1992; McInnes et al. 2017a). However, based on SCA, we found that whilst discard consumption was higher at SJI in 2019, the reverse was the case in 2020. Stable isotope mixing model results, which are temporally more comparable, were inconclusive in this regard, because the models cannot distinguish between benthopelagic squid (natural prey) and benthopelagic fish (discards). However, excluding the confounding prey source, discards were higher at SJI, particularly in 2020, which agrees with the increased trophic niche discussed above.
Discard consumption was higher in 2020, although the extent of this varied between methods. Breeding BBA from the Falkland Islands appear to increase discard consumption during periods of poor natural foraging conditions (Kuepfer et al. 2022a). Indeed, the present study provides some indication of potentially increased nutritional stress in 2020: Prey diversity was higher in 2020, which could result from birds compensating for the scarcity of preferred prey by targeting alternative species (Quillfeldt et al. 2010; van Donk et al. 2017). Significantly higher C/N ratios in 2020 could suggest prey being of inferior protein quality (Robbins et al. 2005). Further, meals contained more stomach oil in 2020, which could be an effect of prolonged foraging trips (supported also by GPS tracking from P. Catry, unpubl. data; also see Warham et al. 1976; Chaurand and Weimerskirch 1994; Connan et al. 2005). The season 2020 also saw reduced chick weight at NWI (on average − 7.3%), and reduced breeding success at both NWI (− 22%) and SJI (− 25%) (P. Catry, unpubl. data; Crofts and Stanworth 2021; Kuepfer et al. 2022a). Combined, these indicators support the argument of increased discard consumption during periods of increased nutritional stress in Falkland Islands BBA.
SCA and SIA—a critical evaluation
Our key objectives were to quantify diet (particularly in the context of fisheries), and test for inter-annual and colony-specific differences. Combining SCA with SIA provided us with complementary information and helped with interpretation of results in several aspects (Table 5).
In contrast to SIA, SCA supplied high taxonomic and morphological details, including information on prey size (age class) and visual confirmation of processed waste from fisheries. This assisted with categorisation and quantification of prey as discards or natural prey. It also enabled us to distinguish between isotopically similar (but from a management perspective, very different) prey (here squid vs benthopelagic fish; crustacean vs jellyfish—although see below). This can be important to complement SIA; if our main prey source was found to be benthopelagic prey, SCA could have helped us determine whether this was dominated by discards (benthopelagic fish) or natural prey (squid). In our case, this confounding prey source only contributed a small amount to the overall diet source of BBA chicks, and therefore bears little influence on the overall result regarding the dominant prey origin.
SCA was also important for selecting mixing model inputs, although our results emphasise that using informative priors from conventional methods in stable isotope mixing models can transfer methodological biases leading to erroneous results (Swan et al. 2020). For example, compared to SCA, SIA suggests the importance of pelagic fish, but more moderate contribution from crustaceans. This difference may reflect differential digestion of prey. Whilst fragile prey like sprat or jellyfish are more easily digested, and hence likely underestimated in SCA, the opposite is true for large or rigid organisms like demersal fish or crustaceans.
We note that differences between methods may in parts also be related to short-term differences in the temporal window they represent, particularly for SJI where down growth and stomach content sampling did not overlap (see also e.g. McCluskey et al. 2021). However, this does not appear to be the case at NWI, where pelagic prey also dominated in both years based on SIA of plasma (reflective of the diet from the previous few days (Phillips et al. 2014)) collected in February 2019 and 2020 (A. Kuepfer unpubl. data).
A further potential issue of using SCA in the absence of a complementary method is the large number of ways this type of diet information can be quantified (e.g. FO%, N%, IRI%). These each have their own biases (see Barrett et al. 2007) and care must be taken when selecting a suitable metric to use based on the study objectives. In our case, a multitude of SCA metrics allowed us to draw more comprehensive conclusions in relation to discard consumption, and also gain insight into the proportion of breeders that interact with vessels (although see e.g. Granadeiro et al. 2011; 2014). Meanwhile, SIA provided a sense check for prey source quantification by providing a single, integrated signal representing multiple meals.
Finally, our SCA was subject to confounding temporal effects due to non-simultaneous sampling. Previous SCA and DNA studies at these colonies suffered from the same limitation (Thompson 1992; McInnes et al. 2017a). The complementary use of feather SIA in the present study assisted with inter-colony comparison due to its more long-term dietary signal. This aspect also makes SIA a suitable candidate for studying diet during the non-breeding period (see e.g. Bugoni et al. 2010; Mariano-Jelicich et al. 2013; Granadeiro et al. 2014), although care must be taken to obtain a meaningful prey field in time and space (Quillfeldt et al. 2015).
Seabird diet in the context of fisheries management
Our dietary results highlight important aspects in terms of fisheries management. First, natural prey are important for BBA chicks, underlining the importance of understanding pelagic fish distribution and abundance in the Falkland Islands (Kuepfer et al. 2022a) and elsewhere (Barrett et al. 2007; Boldt et al. 2022). Second, whilst discards do not contribute substantially to chick diets, a high proportion of adults deliver discards to their young, thus incurring a risk of bycatch. Therefore, we echo previous authors that any management actions aiming to reduce discard availabiliy, and therefore bycatch risk (e.g. batch discharging as currently implemented in the Falkland Islands trawl fleet), will be of conservation benefit (e.g. Granadeiro et al. 2011, 2014; Kuepfer et al. 2022b).
Going forward, seabird diet provides a helpful fishery and ecosystem monitoring tool but requires appropriate methodological consideration. Given climatic changes and continued fishery presence across the Patagonian Shelf (and elsewhere), we recommend the use of stable isotopes to capture broader time scales, but in combination with SCA to allow continued prey species identification in a potentally changing ecosystem. We also recommend multiple SCA metrics be considered (N%, FO%, M%, IRI%), as these can provide complementary information on important prey and the extent of seabird–fishery interaction.
Data availability
The dataset analysed during the current study is available from the corresponding author on reasonable request.
References
Arkhipkin AI, Rodhouse PGK, Pierce GJ, Sauer W, Sakai M, Allcock L, Arguelles J, Bower JR, Castillo G, Ceriola L, Chen C-S, Chen X, Diaz-Santana M, Nicola Downey AFG, Amores JG, Green CP, Guerra A, Hendrickson LC, Ibáñez C, Ito K, Jereb P, Kato Y, Katugin ON, Kawano M, Kidokoro H, Kulik VV, Laptikhovsky VV, Lipinski MR, Liu B, Mariátegui L, Marin W, Medina A, Miki K, Miyahara K, Moltschaniwskyj N, Moustahfid H, Nabhitabhata J, Nanjo N, Nigmatullin CM, Ohtani T, Gretta Pecl J, Perez AA, Piatkowski U, Saikliang P, Salinas-Zavala CA, Steer M, Tian Y, Ueta Y, Vijai D, Wakabayashi T, Yamaguchi T, Yamashiro C, Yamashita N, Zeidberg LD (2015) World squid fisheries. Rev Fish Sci Aquac 23:92–252. https://doi.org/10.1080/23308249.2015.1026226
Barrett RT, Camphuysen K, Anker-Nilssen T, Chardine JW, Furness RW, Garthe S, Hüppop O, Leopold MF, Montevecchi WA, Veit RR (2007) Diet studies of seabirds: a review and recommendations. ICES J Mar Sci 64:1675–1691. https://doi.org/10.1093/icesjms/fsm152
Bearhop S, Waldron S, Votier SC, Furness RW (2002) Factors that influence assimilation rates and fractionation of nitrogen and carbon stable isotopes in avian blood and feathers. Physiol Biochem Zool 75:451–458. https://doi.org/10.1086/342800
Bestley S, Ropert-Coudert Y, Bengtson Nash S, Brooks CM, Cotté C, Dewar M, Friedlaender AS, Jackson JA, Labrousse S, Lowther AD, McMahon CR, Phillips RA, Pistorius P, Puskic PS, de Reis Ao A, Reisinger RR, Santos M, Tarszisz E, Tixier P, Trathan PN, Wege M, Wienecke B (2020) Marine ecosystem assessment for the southern ocean: birds and marine mammals in a changing climate. Front Ecol Evol 8:566936. https://doi.org/10.3389/fevo.2020.566936
BirdLife International (2018) Thalassarche melanophris. The IUCN Red List of Threatened Species 2018: e.T22698375A132643647. In: 2018. http://dx.doi.org/10.2305/IUCN.UK.2018%0A-2.RLTS.T22698375A132643647.en. Accessed 25 Nov 2021
Boldt JL, Murphy HM, Chamberland J, Debertin A, Gauthier S (2022) Canada’s forage fish: an important but poorly understood component of marine ecosystems. Can J Fish Aquat Sci 79:1911–1933. https://doi.org/10.1139/cjfas-2022-0060
Bonin M, Dussault C, Taillon J, Lecomte N, Côté SD (2020) Combining stable isotopes, morphological, and molecular analyses to reconstruct the diet of free-ranging consumers. Ecol Evol 10:6664–6676. https://doi.org/10.1002/ece3.6397
Bugoni L, McGill RAR, Furness RW (2010) The importance of pelagic longline fishery discards for a seabird community determined through stable isotope analysis. J Exp Mar Bio Ecol 391:190–200. https://doi.org/10.1016/j.jembe.2010.06.027
Buren AD, Koen-Alonso M, Montevecchi WA (2012) Linking predator diet and prey availability: common murres and capelin in the Northwest Atlantic. Mar Ecol Prog Ser 445:25–35. https://doi.org/10.3354/meps09465
Carabel S, Godínez-Domínguez E, Verísimo P, Fernández L, Freire J (2006) An assessment of sample processing methods for stable isotope analyses of marine food webs. J Exp Mar Bio Ecol 336:254–261. https://doi.org/10.1016/j.jembe.2006.06.001
Catry P, Forcada J, Almeida A (2011) Demographic parameters of black-browed albatrosses Thalassarche melanophris from the Falkland Islands. Polar Biol 34:1221–1229. https://doi.org/10.1007/s00300-011-0984-3
Catry P, Lemos RT, Brickle P, Phillips RA, Matias R, Granadeiro JP (2013) Predicting the distribution of a threatened albatross: the importance of competition, fisheries and annual variability. Prog Oceanogr 110:1–10. https://doi.org/10.1016/j.pocean.2013.01.005
Ceia FR, Xavier JC, Carreiro AR, dos Santos I, Cherel Y (2022) Conventional and modern approaches to study seabird trophic ecology and diet. In: Ramos JA, Pereira L (eds) Seabird biodiversity and human activities, 1st edn. CRC Press, Boca Raton, pp 19–35
Chaurand T, Weimerskirch H (1994) The regular alternation of short and long foraging trips in the blue petrel Halobaena caerulea: a previously undescribed strategy of food provisioning in a pelagic seabird. J Anim Ecol 63:275–282. https://doi.org/10.2307/5546
Church GE, Furness RW, Tyler G, Gilbert L, Votier SC (2019) Change in the North Sea ecosystem from the 1970s to the 2010s: Great skua diets reflect changing forage fish, seabirds, and fisheries. ICES J Mar Sci 76:925–937. https://doi.org/10.1093/icesjms/fsy165
Connan M, Mayzaud P, Boutoute M, Weimerskirch H, Cherel Y (2005) Lipid composition of stomach oil in a procellariiform seabird Puffinus tenuirostris: implications for food web studies. Mar Ecol Prog Ser 290:277–290. https://doi.org/10.3354/meps290277
Crofts S (2020) Notes on population census of black-browed albatrosses in the Falkland Islands in 2017. Stanley, Falkland Islands
Crofts S, Stanworth A (2021) Falkland Islands Seabird monitoring programme, annual report 2020/2021 (SMP28). Stanley, Falkland Islands
Cury PM, Boyd IL, Bonhommeau S, Anker-Nilssen T, Crawford RJM, Furness RW, Mills JA, Murphy EJ, Österblom H, Paleczny M, Piatt JF, Roux JP, Shannon L, Sydeman WJ (2011) Global seabird response to forage fish depletion—one-third for the birds. Science 334:1703–1706. https://doi.org/10.1126/science.1212928
Duffy DC, Jackson S (1986) Diet studies of seabirds: a review of methods. Colon Waterbirds 9:1–17. https://doi.org/10.2307/1521138
Durant JM, Hjermann DO, Frederiksen M, Charrassin J, Le MY, Sabarros PS, Crawford RJM, Stenseth NC (2009) Pros and cons of using seabirds as ecological indicators. Clim Res Inter Res 39:115–129. https://doi.org/10.3354/cr00798
Einoder LD (2009) A review of the use of seabirds as indicators in fisheries and ecosystem management. Fish Res 95:6–13. https://doi.org/10.1016/j.fishres.2008.09.024
Falkland Islands Government (2021) Fisheries Department Fisheries Statistics 2020. Stanley, Falkland Islands
Franco B, Ruiz-Etcheverry L, Marrari M, Piola A, Matano R (2022) Climate change impacts on the Patagonian shelf break front. Geophys Res Lett 49:e2021GL096513. https://doi.org/10.1029/2021GL096513
Gaglio D, Cook TR, McInnes A, Sherley RB, Ryan PG (2018) Foraging plasticity in seabirds: a non-invasive study of the diet of greater crested terns breeding in the Benguela region. PLoS ONE 13:e0190444. https://doi.org/10.1371/journal.pone.0190444
Granadeiro JP, Phillips RA, Brickle P, Catry P (2011) Albatrosses following fishing vessels: how badly hooked are they on an easy meal? PLoS ONE 6:e17467. https://doi.org/10.1371/journal.pone.0017467
Granadeiro JP, Brickle P, Catry P (2014) Do individual seabirds specialize in fisheries’ waste? The case of black-browed albatrosses foraging over the Patagonian shelf. Anim Conserv 17:19–26. https://doi.org/10.1111/acv.12050
Healy K, Guillerme T, Kelly SBA, Inger R, Bearhop S, Jackson AL (2018) SIDER: an R package for predicting trophic discrimination factors of consumers based on their ecology and phylogenetic relatedness. Ecography (cop) 41:1393–1400. https://doi.org/10.1111/ecog.03371
Inger R, Bearhop S (2008) Applications of stable isotope analyses to avian ecology. Ibis 150:447–461. https://doi.org/10.1111/j.1474-919X.2008.00839.x
Jackson AL, Inger R, Parnell AC, Bearhop S (2011) Comparing isotopic niche widths among and within communities: SIBER—stable isotope Bayesian ellipses in R. J Anim Ecol 80:595–602. https://doi.org/10.1111/j.1365-2656.2011.01806.x
Jones KA, Baylis AMM, Orben RA, Ratcliffe N, Votier SC, Newton J, Staniland IJ (2020) Stable isotope values in South American fur seal pup whiskers as proxies of year-round maternal foraging ecology. Mar Biol 167:148. https://doi.org/10.1007/s00227-020-03760-4
Karnovsky NJ, Hobson KA, Iverson SJ (2012) From lavage to lipids: estimating diets of seabirds. Mar Ecol Prog Ser 451:263–284. https://doi.org/10.3354/meps09713
Kuepfer A, Barton J (2018) Discard management for Falkland Islands trawlers as a long-term seabird bycatch mitigation measure. Stanley, Falkland Islands
Kuepfer A, Votier SC, Sherley RB, Ventura F, Matias R, Anderson O, Brickle P, Arkhipkin A, Catry P (2022a) Prey-switching to fishery discards does not compensate for poor natural foraging conditions in breeding albatross. ICES J Mar Sci. fsac069. https://doi.org/10.1093/icesjms/fsac069
Kuepfer A, Sherley RB, Brickle P, Arkhipkin A, Votier SC (2022b) Strategic discarding reduces seabird numbers and contact rates with trawl fishery gears in the Southwest Atlantic. Biol Conserv 266:109462. https://doi.org/10.1016/j.biocon.2022.109462
Laptikhovsky V, Pompert J, Brickle P (2006) Fishery discards management and environmental impact in Falkland Islands fisheries. Stanley, Falkland Islands
Laptikhovsky V, Arkhipkin A, Brickle P (2013) From small bycatch to main commercial species: explosion of stocks of rock cod Patagonotothen ramsayi (Regan) in the Southwest Atlantic. Fish Res 147:399–403. https://doi.org/10.1016/j.fishres.2013.05.006
Bot TL, Lescroël A, Grémillet D (2018) A toolkit to study seabird—fishery interactions. ICES J Mar Sci 75:1513–1525. https://doi.org/10.1093/icesjms/fsy038
Liao H, Pierce C, Larscheid J (2001) Empirical assessment of indices of prey importance in the diets of predacious fish. Am Fish Soc 130:583–591
Mariano-Jelicich R, Copello S, Seco Pon JP, Favero M (2013) Contribution of fishery discards to the diet of the black-browed albatross (Thalassarche melanophris) during the non-breeding season: an assessment through stable isotope analysis. Mar Biol 161:119–129. https://doi.org/10.1007/s00227-013-2320-7
McCluskey SM, Sprogis KR, London JM, Bejder L (2021) Foraging preferences of an apex marine predator revealed through stomach content and stable isotope analyses. Glob Ecol Conserv 25:e01396. https://doi.org/10.1016/j.gecco.2020.e01396
McInnes JC, Raymond B, Phillips RA, Jarman SN, Lea MA, Alderman R (2016) A review of methods used to analyse albatross diets—assessing priorities across their range. ICES J Mar Sci 73:2125–2137. https://doi.org/10.1093/icesjms/fsw105
McInnes JC, Jarman SN, Lea M-A, Raymond B, Deagle BE, Phillips RA, Catry P, Stanworth A, Weimerskirch H, Kusch A, Gras M, Cherel Y, Maschette D, Alderman R (2017a) DNA metabarcoding as a marine conservation and management tool: a circumpolar examination of fishery discards in the diet of threatened albatrosses. Front Mar Sci 4:277. https://doi.org/10.3389/fmars.2017.00277
McInnes JC, Alderman R, Lea MA, Raymond B, Deagle BE, Phillips RA, Stanworth A, Thompson DR, Catry P, Weimerskirch H, Suazo CG, Gras M, Jarman SN (2017b) High occurrence of jellyfish predation by black-browed and Campbell albatross identified by DNA metabarcoding. Mol Ecol 26:4831–4845. https://doi.org/10.1111/mec.14245
Meier RE, Votier SC, Wynn RB, Guilford T, McMinn Grivé M, Rodríguez A, Newton J, Maurice L, Chouvelon T, Dessier A, Trueman CN (2017) Tracking, feather moult and stable isotopes reveal foraging behaviour of a critically endangered seabird during the non-breeding season. Divers Distrib 23:130–145. https://doi.org/10.1111/ddi.12509
Mills WF, Xavier JC, Bearhop S, Cherel Y, Votier SC, Waluda CM, Phillips RA (2020) Long-term trends in albatross diets in relation to prey availability and breeding success. Mar Biol 167:29. https://doi.org/10.1007/s00227-019-3630-1
Nevoux M, Forcada J, Barbraud C, Croxall J, Weimerskirch H (2010) Bet-hedging response to environmental variability, an intraspecific comparison. Ecology 91:2416–2427. https://doi.org/10.1890/09-0143.1
Oksanen J, Blanchet F, Friendly M, Kindt R, Legendre P, McGlinn D, Minchin PR, O’Hara RB, Simpson GL, Solymos P, Henry M, Stevens H, Szoecs E, Wagner H (2019) vegan: Community Ecology Package. In: R Packag. version 2.5–6. https://www.cran.r-project.org/package=vegan. Accessed 6 Jan 2022
Pardo D, Forcada J, Wood AG, Tuck GN, Ireland L, Pradel R, Croxall JP, Phillips RA (2017) Additive effects of climate and fisheries drive ongoing declines in multiple albatross species. Proc Natl Acad Sci USA 114:10829–10837. https://doi.org/10.1073/pnas.1618819114
Phillips RA (2006) Efficacy and effects of diet sampling of albatross chicks. Emu 106:305–308. https://doi.org/10.1071/MU06035
Phillips DL, Gregg JW (2001) Uncertainty in source partitioning using stable isotopes. Oecologia 127:171–179. https://doi.org/10.1007/s004420000578
Phillips DL, Inger R, Bearhop S, Jackson AL, Moore JW, Parnell AC, Semmens BX, Ward EJ (2014) Best practices for use of stable isotope mixing models in food-web studies. Can J Zool 92:823–835. https://doi.org/10.1139/cjz-2014-0127
Phillips RA, Gales R, Baker GB, Double MC, Favero M, Quintana F, Tasker ML, Weimerskirch H, Uhart M, Wolfaardt AC (2016) The conservation status and priorities for albatrosses and large petrels. Biol Conserv 201:169–183. https://doi.org/10.1016/j.biocon.2016.06.017
Post DM, Layman CA, Arrington DA, Takimoto G, Quattrochi J, Montaña CG (2007) Getting to the fat of the matter: models, methods and assumptions for dealing with lipids in stable isotope analyses. Oecologia 152:179–189. https://doi.org/10.1007/s00442-006-0630-x
Quillfeldt P, Michalik A, Veit-Köhler G, Strange IJ, Masello JF (2010) Inter-annual changes in diet and foraging trip lengths in a small pelagic seabird, the thin-billed prion Pachyptila belcheri. Mar Biol 157:2043–2050. https://doi.org/10.1007/s00227-010-1472-y
Quillfeldt P, Ekschmitt K, Brickle P, McGill RAR, Wolters V, Dehnhard N, Masello JF (2015) Variability of higher trophic level stable isotope data in space and time—a case study in a marine ecosystem. Rapid Commun Mass Spectrom 29:667–674. https://doi.org/10.1002/rcm.7145
R Core Team (2021) R—a language and environment for statistical computing
Ramos J, Winter A (2019) Stock assessment of hoki (Macruronus magellanicus) in the Falkland Islands. Stanley, Falkland Islands
Ramos J, Winter A (2020) February trawl survey biomasses of fishery species in Falkland Islands waters, 2010–2020. Stanley, Falkland Islands
Robbins CT, Felicetti LA, Sponheimer M (2005) The effect of dietary protein quality on nitrogen isotope discrimination in mammals and birds. Oecologia 144:534–540. https://doi.org/10.1007/s00442-005-0021-8
Scopel L, Diamond A, Kress S, Shannon P (2019) Varied breeding responses of seabirds to a regime shift in prey base in the Gulf of Maine. Mar Ecol Prog Ser 626:177–196. https://doi.org/10.3354/meps13048
Seco Pon PJ, Copello S, Tamini L, Mariano-Jelicich R, Paz J, Blanco G, Favero M, Nacional U, Mar D, Plata M, Nacional U, Mar D, Plata M (2015) Seabird conservation in fisheries: current state of knowledge and conservation needs for Argentine high-seas fleets. In: Mahala G (ed) Seabirds and songbirds: habitat preferences, conservation and migratory behavior, 1st edn. Nova Science Publishers Inc., New York, pp 45–88
Sherley RB, Ludynia K, Dyer BM, Lamont T, Makhado AB, Roux JP, Scales KL, Underhill LG, Votier SC (2017) Metapopulation tracking juvenile penguins reveals an ecosystem-wide ecological trap. Curr Biol 27:563–568. https://doi.org/10.1016/j.cub.2016.12.054
Sherley RB, Barham BJ, Barham PJ, Campbell KJ, Crawford RJM, Grigg J, Horswill C, McInnes A, Morris TL, Pichegru L, Steinfurth A, Weller F, Winker H, Votier SC (2018) Bayesian inference reveals positive but subtle effects of experimental fishery closures on marine predator demographics. Proc R Soc B Biol Sci 285:20172443. https://doi.org/10.1098/rspb.2017.2443
Stock BC, Semmens BX (2016) MixSIAR GUI User Manual Version 3.1. San Diego, USA
Swan GJF, Bearhop S, Redpath SM, Silk MJ, Goodwin CED, Inger R, McDonald RA (2020) Evaluating Bayesian stable isotope mixing models of wild animal diet and the effects of trophic discrimination factors and informative priors. Methods Ecol Evol 11:139–149. https://doi.org/10.1111/2041-210X.13311
Sydeman WJ, Schoeman DS, Thompson SA, Hoover BA, García-Reyes M, Daunt F, Agnew P, Anker-Nilssen T, Barbraud C, Barrett R, Becker PH, Bell E, Boersma PD, Bouwhuis S, Cannell B, Crawford RJM, Dann P, Delord K, Elliott G, Erikstad KE, Flint E, Furness RW, Harris MP, Hatch S, Hilwig K, Hinke JT, Jahncke J, Mills JA, Reiertsen TK, Renner H, Sherley RB, Surman C, Taylor G, Thayer JA, Trathan PN, Velarde E, Walker K, Wanless S, Warzybok P, Watanuki Y (2021) Hemispheric asymmetry in ocean change and the productivity of ecosystem sentinels. Science 372:980–983. https://doi.org/10.1126/science.abf1772
Thompson KR (1992) Quantitative analysis of the use of discards from squid trawlers by black-browed albatrosses Diomedea melanophris in the vicinity of the Falkland Islands. Ibis 134:11–21. https://doi.org/10.1111/j.1474-919X.1992.tb07223.x
van Donk S, Camphuysen KCJ, Shamoun-Baranes J, van der Meer J (2017) The most common diet results in low reproduction in a generalist seabird. Ecol Evol 7:4620–4629. https://doi.org/10.1002/ece3.3018
Velarde E, Anderson DW, Ezcurra E (2019) Seabird clues to ecosystem health. Science 365:116–117. https://doi.org/10.1126/science.aaw9999
Ventura F, Lukacs P, Granadeiro JP, Matano R, Catry P (2021) Demographic responses to environmental change of the black-browed albatross, sentinel of the Patagonian Shelf Large Marine ecosystem. Mar Ecol Prog Ser 668:107–120. https://doi.org/10.3354/meps13743
Votier SC, Bearhop S, MacCormick A, Ratcliffe N, Furness RW (2003) Assessing the diet of great skuas, Catharacta skua, using five different techniques. Polar Biol 26:20–26. https://doi.org/10.1007/s00300-002-0446-z
Votier SC, Furness RW, Bearhop S, Crane JE, Caldow RWG, Catry P, Ensor K, Hamer KC, Hudson AV, Kalmbach E, Klomp NI, Pfeiffer S, Phillips RA, Prieto I, Thompson DR (2004) Changes in fisheries discard rates and seabird communities. Nature 427:727–730. https://doi.org/10.1038/nature02251.1
Votier SC, Hatchwell BJ, Beckerman A, McCleery RH, Hunter FM, Pellatt J, Trinder M, Birkhead TR (2005) Oil pollution and climate have wide-scale impacts on seabird demographics. Ecol Lett 8:1157–1164. https://doi.org/10.1111/j.1461-0248.2005.00818.x
Votier SC, Birkhead TR, Oro D, Trinder M, Grantham MJ, Clark JA, McCleery RH, Hatchwell BJ (2008a) Recruitment and survival of immature seabirds in relation to oil spills and climate variability. J Anim Ecol 77:974–983. https://doi.org/10.1111/j.1365-2656.2008.01421.x
Votier SC, Bearhop S, Fyfe R, Furness RW (2008b) Temporal and spatial variation in the diet of a marine top predator-links with commercial fisheries. Mar Ecol Prog Ser 367:223–232. https://doi.org/10.3354/meps07621
Warham J, Watts R, Dainty RJ (1976) The composition, energy content and function of the stomach oils of petrels (Order, Procellariiformes). J Exp Mar Bio Ecol 23:1–13
Winter A, Ramos J (2020) Stock assessment hake (Merluccius hubbsi, Merluccius australis). Stanley, Falkland Islands
Xavier JC, Cherel Y (2009) Cephalopod beak guide for the Southern Ocean. British Antarctic Survey, Cambridge, UK
Acknowledgements
We are thankful to Ludovic Goyot, Brendon Lee, and Rebecca Piontek of the Falkland Islands Fisheries Department for collection of prey samples and assistance with sample processing. We are grateful for generous support from Falklands Conservation who enabled data collection at Steeple Jason Island, and to the New Island Conservation Trust and Wildlife Conservation Society for granting us site access.
Funding
This study had the support of the Fundação para a Ciência e a Tecnologia (FCT) I.P. through the strategic project UIDB/04292/2020, UIDP/04292/2020 and DivRestore/0012/2020 awarded to MARE and ISPA, and LA/P/0069/2020 granted to the Associate Laboratory ARNET. Further funding was provided by Fortuna Ltd., the Falkland Islands Government through the Environmental Studies Budget, as well as by the National Environmental Research Committee (NERC) through grants-in-kind (EK316-03/19, 2264.0420).
Author information
Authors and Affiliations
Contributions
AK, PC, SCV and SB contributed to the study conception, design and methodology; material preparation and data collection was undertaken by AK with support from PC, PB and AA; data processing was performed by AK, OB and JN; data analysis was conducted by AK with support from SCV, SB, RBS, JN, PC and OB. The first draft of the manuscript was written by AK, and all authors commented on the manuscript. Resources for this project were provided by PB, AA, SCV and JN; Supervision: SCV, RBS, SB, PC, PB and AA.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no competing interests to declare that are relevant to the content of this article.
Ethical approval
Diet samples were collected under research licence issued by the Falkland Islands Government Environmental Department (R29/2017) and with approval from the University of Exeter College of Life and Environmental Sciences Penryn Research Ethics Committee.
Additional information
Responsible Editor: T.A. Clay.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kuepfer, A., Catry, P., Bearhop, S. et al. Inter-colony and inter-annual variation in discard use by albatross chicks revealed using isotopes and regurgitates. Mar Biol 170, 46 (2023). https://doi.org/10.1007/s00227-023-04191-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04191-7