Abstract
Anthropogenic global warming generates profound metabolic alterations in marine ectotherm invertebrates capable of leading a wide range of these species to extinction. To evaluate the cross-generational effect of thermal stress on the cephalopod Octopus maya, groups of females were exposed at 24 and 30 °C until spawn. After, embryos of each female group were incubated at 24 and 30 °C allowing for evaluating the transgenerational effects on embryos exposed to high and low temperatures. We analyzed the morphology, oxygen consumption, antioxidant mechanisms, and oxidative stress indicators of the embryos. The results demonstrate that thermally stressed females produced smaller eggs with lower yolk content as observed in nonthermally stressed females. Also was observed that embryos from females acclimated at 30 °C had lower body weight and higher respiratory rates when compared with nonthermal stressed females. Embryos from females acclimated at 30 °C showed a collapse of the antioxidant defense system measured as lower both catalase activity and total glutathione concentrations. Additionally, glutathione-s transferase activity increased in embryos incubated at 30 °C and in females maintained at high temperatures in a clear deleterious and cross-generational effect of thermal stress on this octopus species. No changes were observed in the activity of B-esterases in octopus embryos linked with the thermal stress of females. Embryos from thermally stressed females had smaller sizes, less yolk, and higher metabolic rates. Additionally, a collapse in the antioxidant defense system was observed indicating they were unable to control the high load of ROS and oxidative damage, which was partially acquired by maternal inheritance.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
An increment in seawater temperature from 1 to 3 °C is expected for the next three decades (Pörtner et al. 2022). Such increment can generate profound metabolic alterations in marine ectotherm invertebrates, affecting the distribution and abundance of a wide number of species (Gillooly et al. 2001; Lefevre 2016; Madeira et al. 2016). The mitochondrial respiratory chain is one of the main producers of reactive oxygen species (ROS) (e.g., hydroxyl and superoxide radicals, singlet oxygen, etc.) and reactive nitrogen species (RNS) (e.g., nitric oxide, peroxynitrite, etc.) that are produced naturally through physiological and non-physiological processes (Feidantsis et al. 2021; Fridovich 1986). Oxidative stress produced by ROS consists of a set of deleterious cumulative biomolecular cell damage, mainly oxidation of lipids, proteins, and nucleic acids (Storey and Storey 1978; Hulbert et al. 2007). The cell antioxidant defense mechanisms (ANTIOX) consist in groups of enzymes (e.g. catalase, glutathione peroxidase, peroxydoxin, superoxide dismutase; Sillero-Ríos et al. 2018) complemented by some low molecular weight (LMW) non-enzymatic molecules (e.g. glutathione, vitamins A, C, and E, bilirubin, beta-carotene, uric acid, and flavonoids). These LMW non-enzymatic molecules progressively reduce ROS into safe compounds (e.g. O2 and H2O; Halliwell 2013) or break the autocatalytic radical chain reactions (Cadenas 1989), respectively. However, in organisms subjected to thermal stress, ROS production can surpass the ANTIOX system, provoking cell damage and in the end, reducing survival (Pörtner 2010; Pörtner et al. 2017; Rodríguez-Fuentes et al. 2017). The most worrying consequence of global climate change and local thermal anomalies could be that the effect of thermal stress can overpass the capacity of individual generations to neutralize ROS, with profound costs to the ecology of populations and communities (Moreira et al. 2016). Moreover, increasing periods of thermal stress during reproductive phases result in a stronger transgenerational response (Salinas et al. 2013). Evidence suggests that thermal tolerance between generations can be enhanced through thermal preconditioning of adults to trigger an epigenetic response that could increase the thermal tolerance of their offspring (Klosing et al. 2019). That process has been defined as (1) cross-generation plasticity or transgenerational plasticity (CGP and TCP, respectively) (when the environment experienced by parents influences offspring phenotype: F0–F1); (2) multigenerational plasticity (MGP) (when the environment experienced by previous generations is evident to the F2 and beyond: F1–F2 +); and (3) carry-over effects (COE) that occur within the development (e.g. embryo to larva) (Byrne et al. 2019; Ross et al. 2016). Until now, two hypotheses have been used to explain how thermal stress could affect or is transgenerationally affecting ectothermic species. One of those postulates that thermal stress in parents is an effective mechanism for coping with temperature increases because it enhances progeny performance under thermal stress through epigenetic inheritance (Eirin-Lopez and Putnam 2019; Fellous et al. 2015, 2021). Another one, based on results obtained from mussels (Mytilus californicus), provided evidence for adverse parental effects on offspring thermal tolerances suggesting that not all species could benefit from epigenetic alterations in the context of thermal alterations caused by warming (Waite and Sorte 2022).
Octopus maya (an endemic cephalopod adapted to comparatively low environmental temperatures of the Yucatan Peninsula) has been documented as a tropical species relatively sensible to thermal stress (Ángeles-González et al. 2020, 2021). When the temperature was evaluated (above 27 ºC) in adult O. maya females, the number of spawned and fertilized eggs was lower than in females maintained at 24 ºC (Juárez et al. 2015). Essential genes involved in fertilization and egg-laying are downregulated in both optic and oviducal glands of thermally stressed O. maya females (Domínguez-Estrada et al. 2022; Juárez et al. 2022). These and more recent results have indicated that high temperatures affect negatively, not only the capacity of females to produce eggs but also how sperms fertilize the eggs in the oviductal gland (Juárez et al. 2022). In experiments with male octopuses, temperatures of 28 ºC or higher provoked testicular damage, reducing their paternal contribution to progeny (López-Galindo et al. 2018). These results evidence that temperatures from 28 °C to 30 °C deeply affected the reproductive performance of this octopus species.
Transgenerational effects of thermal stress were documented previously in this octopus species (Juárez et al. 2016) where thermally stressed females produced smaller embryos, and metabolic rates 2.5–3.5 fold than those from unstressed females, suggesting that maternal thermal stress can drastically affect the energetic mechanisms of the juveniles. Strong differences were also observed in routine metabolism, suggesting that physiological unbalance observed was at a maintenance metabolism level, where animals from stressed females showed higher metabolic rate than octopuses from unstressed females. The authors suggested that such effects could have epigenetic bases (Juárez et al. 2015, 2016).
In a study conducted on O. mimus embryos, Olivares et al. (2019) hypothesized that negative parental influence on offspring is related to ROS transferred from females to oocytes during oogenesis, causing an ulterior overload for embryos. In this regard, during spawning, female optic glands (located close to the brain) focus on maintaining a balance between energy and ROS production (Ventura-López et al. 2022), but high temperatures drastically affect this process (Domínguez-Estrada et al. 2022). Moreover, at high temperatures, the optic gland in females up-regulates the expression of genes related to stress response and the production of stress molecules like corticosterone (Domínguez-Estrada et al. 2022), which may alter embryonic development (Hayward and Wingfield 2004; Vagnerová et al. 2008).
During the reproductive phase, females use their energy reserves for parental care (Lin et al. 2019; Roumbedakis et al. 2017; Ventura-López et al. 2022). Biological processes like the oxidation–reduction process, respiratory electron transport chain and precursor metabolite, and energy generation are conspicuous during spawning in this octopus species (Ventura-López et al. 2022; Meza-Buendia et al. 2021). However, thermally stressed females showed a lower metabolic scope than females maintained in thermally optimal conditions. Therefore, elevated temperatures could be affecting the ability of females to channel enough energy to all events involved in reproduction (Meza-Buendia et al. 2021).
According to Blount et al. (2016), in mammals and birds, females might, therefore, gain from pre-emptive reduction of oxidative damage in preparedness for reproduction, to reduce the oxidative costs of reproductive effort, or to ensure that levels of oxidative damage do not subsequently exceed some critical thresholds above which it may be impossible to maintain cellular homeostasis. Also, those authors hypothesized that reductions in oxidative damage in reproducing females might also serve to shield gametes and develop healthy offspring. In a study made on O. mimus, Olivares et al. (2019) postulated that under thermal stress, female octopuses fail to reduce oxidative damage transfer to their embryos, provoking an additional charge of ROS that affects embryonic development (Olivares et al. 2019). Additionally, O. vulgaris and O. maya embryos showed limited abilities to neutralize ROS when the maternal charge is summed to ROS produced during their growth. ROS increases as the metabolic rate reach its maximum level at temperatures beyond the thermal optima (Repolho et al. 2014; Sánchez-García et al. 2017).
Based on the above, this research study addresses four questions to know the effect of maternal thermal stress transferred to O. maya embryos as follows: (i) Do embryos from thermally stressed females show alterations in their development (e.g. smaller amount of yolk), regardless of their incubation temperature? (ii) Does maternal thermal stress before spawning affect the metabolic response of embryos (e.g. faster respiratory rates)? (iii) Does maternal thermal stress and/or high incubation temperature increase oxidative stress in embryos at a specific developmental stage? And (iv) is the antioxidant system of embryos from thermally stressed females subjected to thermal stress during their incubation able to cope with oxidative stress? Based on these questions and the fact that females under thermal stress may not be able to regulate ROS or oxidized biomolecule transfer to their embryos, as previously suggested by the studies done in octopus (Olivares et al. 2019; Meza-Buendia et al. 2021), their embryos are expected to show deleterious morphophysiological differences due to a decreased antioxidant system because of overwhelming oxidative stress. Moreover, those embryos incubated under thermal stress, will not be able to metabolically compensate for it.
To answer these questions, this research was directed to experimentally test in the cephalopod Octopus maya, the effect of thermal maternal stress on embryonic morphology, oxygen consumption, oxidative damage, and antioxidant defense mechanisms in embryos exposed to thermal and optimal temperatures during their development.
Material and methods
Animals and experimental design
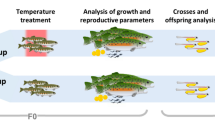
Wild mature males (754 ± 43 g ww) and females (682 ± 59 g ww) were collected on the coast of Sisal, Yucatán by the local capture method called “gareteo”, which comes from adrift (“al garete” in Spanish, Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación 2014). The octopuses were then transferred to the experimental cephalopod production unit at the Teaching and Research Multidisciplinary Unity (UMDI-UNAM) at Universidad Autónoma de México located approximately 1–2 km far from the sampling zone. The octopuses were conditioned for 2 weeks in 6000 L open-air tanks, allowing them to copulate. In the tanks, animals were maintained from 26 to 28 °C. During that time, brooders were fed ad libitum twice a day with a paste made with squid (Dosidicus gigas), and crab (Callinectes spp) bound gelatin (Tercero-Iglesias et al. 2015). Then, six females were acclimated into individual dark tanks connected to a seawater recirculation system with continuous seawater flow at 24 ºC (n = 3) or 30 ºC (n = 3) for 30 days. Seawater in tanks was kept in a semi-closed recirculation system coupled with a rapid-rate sand filter and 36 ± 1 part per thousand (ppt) salinity, dissolved oxygen higher than 5 mg/L, pH above 8, photoperiod of 12L/12D, and a light intensity of 30 Lux in the tank surface. Seawater temperature was gradually increased by 1ºC/day until the experimental temperature (30 ºC) was reached and maintained with two 1800 Watt heaters connected to automatic temperature controllers. The temperature of 24 ºC was controlled with a titanium chiller (Pentair; United States of America) and room air conditioner. Octopus females were maintained until spawning, with fiberglass boxes (30 × 15 × 20 cm; length, wide, high) that served as a nest. The animals were fed twice a day with the previously described paste (Tercero-Iglesias et al. 2015) during the conditioning period and maintained in their experimental temperatures. Embryos of each female were obtained after eight days, just when spawning ends. After, 80 eggs per spawn (240 embryos per experimental temperature) were randomly sampled and artificially incubated at 24 ºC or 30 °C (Fig. 1). This study was performed following the European directive related to the use of cephalopods as experimental organisms (DIRECTIVA 2010/63/UE) and approved by the Bioethical Commission at UNAM (Universidad Nacional Autónoma de México) Faculty of Sciences (CEARC/Bioética/25102021).
Experimental design and sample procedures to evaluate the effect of transgenerational thermal stress on the physiological condition of Octupus maya embryos. Please note that females were exposed for 30 days at each experimental temperature and that the embryos sampled every 5 days were used to evaluate oxygen consumption, morphological changes during development, antioxidant defense mechanisms (ANTIOX) and oxidant damage (OXD)
Routine oxygen consumption in vivo
Every five days, respiratory metabolism was measured individually: embryos were placed in micro-plate clear glass vials with integrated sensor spots (750 μL volume, Loligo Systems, Copenhagen, DK) and maintained in seawater in experimental incubation temperature (Fig. 1). Simultaneously, oxygen consumption of five control chambers (vials without an embryo) was also measured. Prior to each trial, the flow-through cell O2 sensor was calibrated at each acclimation temperature using air-saturated seawater (100%) and with 1–1.5% anhydrous sodium sulphite (at 0%). Vials were submerged in a transparent glass container with temperature-controlled seawater maintained at 24 or 30 °C. The container was placed on a Sensor Dish Reader (Presens, Regensburg, DE) that recorded oxygen concentration measurements every 15 s. The measurement time was adjusted according to the oxygen concentration in the vial, which was never lower than 80% of the saturation level. All measurements were graphed according to time and embryo oxygen consumption (MO2) was calculated as follows:
where \(M{O}_{2}\) is respiration rate (mg O2 h−1 mg WW−1); \({O}_{2(A)}\) is initial oxygen concentration in the chamber (mg O2 L−1); \({O}_{2(B)}\) is final oxygen concentration in the chamber (mg O2 L−1); \(V\) is water volume in the chamber minus water volume displaced by the embryo (expressed as L); \(t\) is time elapsed during measurement (h); and M is egg body mass containing the embryo (mg WW).
Embryonic development
Immediately after respiration rate measurements, eggs, and their corresponding embryos were photographed, (Fig. 1) maintaining eggs at the same experimental temperature. Morphological parameters of O. maya eggs (eye diameter, mm; arm length, mm; yolk length, mm; egg length, mm; egg wide, mm; and wet weight, g) were measured. Each egg was observed and photographed using Leica EZ4 HD (Wetzlar, DE) stereoscopic microscope whose software (Leica LAS EZ, Wetzlar, DE) allows identification of embryonic stage and size. The developmental stages were identified (Caamal-Monsreal et al. 2016; Naef 1928) and grouped as organogenesis (before heart activity started; stages X–XIV), activation (when the heart starts its activity; stages XV–XVI), and growth (stages XVII–XIX). Afterward, the weight (g) of each embryo was obtained (± = 0.001 g), and immediately stored in liquid nitrogen on Eppendorf tubes, and stored at -80ºC until analysis.
Antioxidant defense mechanisms and oxidative damage
The frozen embryos, at activation and growth phases, were individually homogenized in cold buffer 0.05 M Tris pH 7.4 at 100 mg tissue/mL using a Potter–Elvehjem homogenizer. Homogenate samples used for Catalase (CAT), glutathione-s-transferase (GST), and esterases were centrifuged at 10,000 g for 5 min at 4 °C, and the supernatant was separated for analysis. All samples were stored at − 80 °C until analysis; all assays were done in duplicates. Catalase activity was measured according to Góth (1991) modified by Hadwan and Abed (2016). In this method, undecomposed H2O2 is measured with ammonium molybdate after three minutes to produce a yellowish color with maximum absorbance at 374 nm. Total glutathione (GSH) was measured with Sigma-Aldrich Glutathione Assay Kit (CS0260) (St. Louis MO, US). This kit utilizes an enzymatic recycling method with glutathione reductase (Baker et al. 1990). The GHS sulfhydryl group reacts with Ellman’s reagent and produces a yellow-colored compound read at 405 nm. The glutathione-s-transferase (GST) activity was determined from the reaction between reduced glutathione and 1-chloro-2.4-dinitrobenzene at 340 nm (Habig and Jakoby 1981). Proteins were analyzed in supernatant according to Bradford (1976) and used to normalize enzyme activities. To evaluate oxidative damage (OXD) caused by radical oxygen species (ROS), carbonyl groups in oxidized proteins (PO) were measured in the sampled embryos, estimating PO by using the 2,4-dinitrophenylhydrazine alkaline protocols developed by Mesquita et al. (2014) and reported in nmol/mg wet weight. For this assay, 200 µl of 2,4 dinitrophenylhydrazine (10 mM in 0.5 M HCL) were incubated with 200 µl of the sample homogenate and 100 µl of NaOH (6 M). Absorbance was read at 450 nm after 10 min of incubation at room temperature against a blank where an equal volume of homogenization buffer substitutes the protein solution.
For this study, two esterases—acetylcholinesterase (AChE) and carboxylesterase (CbE) – were measured to evaluate the physiological condition of embryos. AChE activity was measured using a modification of the method of Ellman et al. (1961) adapted to a microplate reader (Rodríguez-Fuentes et al. 2008). Each well contained 10 µL of the enzyme supernatant and 180 µL of 5, 5-dithiobis (2 nitrobenzoic acids) (DTNB) 0.5 mM in 0.05 M Tris buffer pH 7.4. The reaction started by adding 10 µL of acetylthiocholine iodide (final concentration 1 mM). Absorbance rate of change at 405 nm was measured for 120 s; CbE activity was measured using ρ-nitrophenyl-α- arabinofuranoside (ρNPA) substrate, as indicated by Hosokawa and Satoh (2001) with some modifications. Each assay included 25 μL of the supernatant and 200 μL of ρNPA. The reaction was recorded for 5 min at 405 nm. SOD, AChE, and CbE activities were reported per mg protein in the sample (Bradford 1976).
Statistical analyses
The relationship between oxygen consumption and egg wet weight was linearized using a semi-log transformation (Ln wet weight). After that, an analysis of covariance (ANCOVA) was performed to identify statistical differences between the linear models obtained. Changes in each parameter measured (morphological, ANTIOX, and OXD) were also analyzed to show how temperature is affecting each one. We made an ANOVA analysis of each parameter (morphological, ANTIOX, or ODA) to show a more conventional analysis of the data (see supplementary data). To evaluate how the maternal thermal stress affected embryo performance (morphological changes, ANTIOX, and OXD) of the embryos exposed at different temperatures, a multivariate analysis, permutational MANOVA (PERMANOVA), and Principal Coordinate Analysis (PCO) were performed using Primer v 7.0 + PERMANOVA add on. Raw data of this study were stored in ZENODO repository (Domínguez-Castanedo et al. 2022). In the end, we used the PCO to interpret the results obtained because of the high integrative value that have this type of analysis.
Results
Routine oxygen consumption in vivo
When embryo routine metabolism was related to the embryo’s wet weight, was noted that the respiratory metabolism was affected by the maternal thermal condition but not by the embryo's thermal condition (Fig. 2). For that reason, MO2 data of embryos from each thermal female treatment (24 or 30 °C) were grouped (Fig. 2). The results obtained from the ANCOVA showed that no statistical differences were recorded between the slopes obtained from the Ln MO2–Ln embryo wet weight relationship of embryos from females conditioned at 24 or 30 °C (F = 2.9; DFn = 1; DFd = 242; p = 0.09). However, statistical differences were observed between intercepts (F = 28; DFn = 1, DFd = 243; p = 0.001) with lower values in embryos from females maintained at 24 °C (Ln MO2–24 °C = Ln 1.3x–Ln 0.98; p ˂ 0.0001; F = 491; DFd = 1.16) than those obtained of embryos from females maintained at 30 °C (Ln MO2–30 °C = Ln 1.1x—ln 1.6; p ˂ 0.0001; F = 75; DFd 1.81) (Fig. 2).
Transgenerational effect of Octopus maya female thermal condition (24 and 30 °C) on the respiratory metabolism of embryos exposed at 24 °C and 30 °C. Blue dots indicate the metabolism of embryos from females conditioned at 24 °C and incubated at 24 and 30 °C. Red squares indicate the metabolism of embryos from females conditioned at 30 °C and incubated at 24 and 30 °C. (Ln MO2(Fem 30 °C, n = 248) = Ln 1.1 ww–Ln 1.6; R2 = 0.48; p ˂ 0.0001; LnMO2(Fem 24 °C, n = 165) = Ln 1.3 ww–Ln 0.98; R2 = 0.75; ANCOVA Slope: F = 2.9; DFn 1; DFd = 242; p = 0.09; ANCOVAIntercept: F = 28; DFn = 1; DFd = 243; p < 0.0001)
Embryonic development
The PCO analysis indicated that the transgenerational effect of the maternal thermal condition affected the morphometric characteristics of the embryos of the next generation. Embryos from females maintained at 24 °C (incubated at 24 and 30 °C) showed larger yolks and eggs than those of embryos from females maintained at 30 °C (incubated at 24 and 30 °C; Fig. 3; Figure S-1A and B).
Principal coordinates PCO1 vs PCO2 of the transgenerational effect of Octopus maya female thermal condition (24: blue and green; and 30 °C: orange and red) on morphological characteristics of the embryos exposed at 24 °C and 30 °C. In the codes for the treatments, the first number identifies the thermal condition of females and the second one thermal condition of embryos
Embryos in the growth phase from females maintained at 24 °C (incubated at 24 °C and 30 °C) and from females maintained at 30 °C showed higher eye diameter and mantel length than embryos from females maintained at 30 °C and incubated at 24 °C (p ˂ 0.001; Figure S-1C and D). Also, was observed that at the end of embryo development (phase 4) embryos from females maintained at 24 °C and incubated at 24 °C had larger arms than the rest of the embryos, being the shorter arms observed in embryos from females maintained at 30 °C and incubated at 30 °C (p ˂ 0.001; Fig. 3; Fig S-1E). Exponential curves were obtained from the wet weight of embryos during embryo development (Fig S-1F). ANCOVA analysis showed that there were no statistical differences between slopes but differences between intercepts were detected with higher wet weight of embryos from females maintained at 24 (incubated at 24 and 30 °C) than embryos from females maintained at 30 °C (p ˂ 0.001; F = 23. DFn = 2, DFd = 244; Fig. S-1F). When PERMANOVA was applied to all morphological data was evident that from an integrative analysis, embryo from females maintained at 24 °C was larger and heavier than embryos from females maintained at 30 °C (Fig. 3).
Survival
Embryo survival was affected by maternal temperature (Fig. 4). Embryos from females maintained at 30 °C had significantly lower survival (mean value of 34.5% survival) than observed in embryos from females held at 24 °C (76.5% survival).
Antioxidant enzymes and oxidant damage
Pair-wise tests indicated statistical differences for pair levels of maternal temperature within levels of embryo temperature during the activation phase. The antioxidant defense mechanism of the embryos from the females maintained at 24 °C and incubated at 24 and 30 °C showed higher concentrations of PO and CAT activity (Fig. S-2A and D). High variability in GST and GSH values were observed in embryos from females maintained at 24 °C and incubated at 30 °C (Fig. S-2B and C). In contrast, the results of the embryos from females maintained at 30 °C showed low activities of CAT both in activation and growth phases (Fig. S-2A; Table S-1).
In the activation phase, PERMANOVA indicated that the variable maternal temperature and embryo incubation temperature have a significant effect on antioxidant defense mechanisms and oxidant damage (Fig. 5). Pair-wise tests indicated statistical differences for pair levels of maternal temperature within levels of embryo temperature during the activation phase. The antioxidant defense mechanism of the embryos from the females maintained at 24 °C and incubated at 24 and 30 °C showed higher concentrations of PO and CAT activity (Fig. S-2A and D). High variability in GST and GSH values were observed in embryos from females maintained at 24 °C and incubated at 30 °C (Fig. S-2B and C). In contrast, the results of the embryos from females maintained at 30 °C showed low activities of CAT both in activation and growth phases (Fig. S-2A; Table S-1).
Principal coordinates PCO1 vs PCO2 of the transgenerational effect of Octopus maya female thermal condition (24: blue; and 30 °C: red) on antioxidant defense mechanisms of the embryos at activation stage (A), and growth stage (B) and exposed at 24 °C and 30 °C. In the symbols, the first number identifies the thermal condition of females and the text the phase of embryo development. Centroids of each PCO at the right side of each one. CAT Catalase, GST Glutathione-s-Transferace, GSH Total Glutathione, PO Protein carbonilation
The results of the antioxidant defense mechanisms during the growth phase indicated no interaction of the variables maternal and incubation temperatures of embryos. However, a significant effect of maternal stress and thermal stress in embryos significantly affected the antioxidant defense mechanisms in embryos during the growth phase (Table S-2). Samples of embryos from females kept and incubated at 30 °C showed lower CAT activity, total GSH concentration and higher activities of GST. Samples from female embryos maintained at 24 °C and kept at 24 and 30 °C showed the highest PO levels (Fig. S-2; Table S-2).
Esterase
Esterase activities were not affected by the thermal origin of the embryos nor incubation temperature (p ˃ 0.05; Fig. 6). Differences in activities of CbE and AChE were only detected according to development stage, with low values at heart activation and circulatory system activation in comparison with activities measured during the growth phase (SS 1507; DF = 1; MS 1507; F = 44.9; p ˂ 0.0001; Fig. 6).
Effect of incubation temperature on Carboxylesterase (A; CbE) and acetylcholinesterase (B; AChE) activities of Octopus maya embryos (Stages XIV–XVI = activation; VII–XIX = growth) from females acclimated at 24 °C (blue circle) and 30 °C (red circle). The first number on X-axis indicates the female temperature acclimation. The second number indicates the embryo incubation temperature. Values ± SD. Different letters (a and b) mean statistical differences between phases at p < 0.0001
Discussion
The present study shows a transgenerational effect of temperature on cross-generation plasticity (CGP) in O. maya. Thermally stressed females produced embryos with high metabolic rates and less yolk than females maintained in optimal conditions. At the end of the embryo development, maternal thermal stress provoked the smallest hatchlings than females non-thermally stressed, indicating that parental thermal stress can have severe impacts, at least between two generations.
Several studies made in O. maya embryos, juveniles and adults have demonstrated that this species is relatively sensitive to high temperatures, of which the thermal limit for embryos and spawners is around 27 °C (Ángeles-González et al. 2021; Caamal-Monsreal et al. 2016; Domínguez-Estrada et al. 2022; Juárez et al. 2015, 2016, 2022; López-Galindo et al. 2018; Meza-Buendia et al. 2021; Noyola et al. 2013). In a previous study, Juarez et al. (2016) observed that juveniles of thermally stressed females had a growth rate of approximately half of that recorded in animals of non-stressed females, suggesting that a transgenerational high-temperature effect could be occurring in this octopus species (Juarez et al. 2016). Additionally, these authors observed that egg fecundity was affected by temperatures of 30 °C (Juárez et al. 2015). The existence of endocrine control mechanisms in optic (Domínguez-Estrada et al. 2022) and oviducal glands to inhibit spawning at elevated temperatures (Juárez et al. 2022) was recently demonstrated, suggesting that when the temperature is stressing, females avoid potential stress for embryos inhibiting spawning. These results support the hypothesis assuming that in warming scenarios, adults could migrate to deeper waters searching for lower temperatures (22–26 °C) where reproductive processes can occur successfully (Angeles-Gonzalez et al. 2021; Pimentel et al. 2012). Unfortunately, ecological adjustments as migrations require time (decades, hundreds of years?) due to deep and complex modifications in the ecosystem that is invaded and inevitably provokes overlapping niches (Ángeles-González et al. 2021; Eddy et al. 2020). Sadly, ocean warming is occurring faster than physiological adjustments in species and structure of the ecosystems, leaving adaptations linked to phenotypic plasticity of the population ability (or not) to respond to the rapid environmental changes (Tittensor et al. 2021). In this sense, it is highly probable that female octopuses that cannot migrate to cooler waters during ovarian maturation (i.e. due to niche overlap with O. americanus (Avendaño et al. 2020) would experience negative transgenerational consequences of high temperatures in their progeny.
Several potential mechanisms can explain the negative transgenerational effect of thermal stress in O. maya. First, embryos from thermally stressed females had morphological alterations, showing a smaller amount of yolk and smaller sizes than embryos of non-stressed females. Second, embryos from thermally stressed females had higher metabolic rates than those observed in embryos of non-stressed females. Third, embryos from thermally stressed females had lower GSH levels and lower CAT activities, suggesting stress in those embryos due to high temperatures (Olivares et al. 2019).
The negative transgenerational effects of thermal stress in O. maya can be associated with the use of yolk reserves and consequences on embryo morphology and physiology, which compose an energetic mechanism. In octopus embryos, as in many other aquatic organisms, yolk quantity and quality are key aspects of this phase of their life cycle (Caamal-Monsreal et al. 2016). In fish, embryonic and early larval development and metabolism are fueled entirely by maternally-derived nutritional resources (yolk and oil) before the onset of exogenous feeding, which is a good indicator of parental physiological condition (Hou and Fuiman 2022). Thus, the transgenerational effect of high temperature in O. maya could start with the inability of females to store enough yolk (or yolk with enough quality?) in the eggs. Previous experiments made at the UNAM-Sisal laboratory demonstrated that adult females exposed to 30 °C experienced a reduction in aerobic scope. This result suggests that in such thermal conditions, females had not enough energy to reach an adequately ovarian maturation (Meza-Buendia et al. 2021), even when in laboratory conditions, females were fed ad libitum with a paste that covered nutritional requirements for reproduction (Tercero-Iglesias et al. 2015). In this sense, hypothesizing that embryos from thermally stressed females were equipped with limited yolk quantities (quality?) by reducing the energy available for tissue and organ synthesis, the final size of the animals was affected (Fig. 6). In adults of the coral colonies of Pocillopora acuta exposed to thermal stress, offspring were also smaller than those obtained in adults without thermal stress, which suggests, as observed in octopus embryos, that smaller progeny can be a transgenerational effect in other ectotherm animals (McRae et al. 2021).
Although the reason why embryos of thermally stressed females have high metabolic rates is still not known, we may hypothesize that some epigenetic alterations could be involved (Eirin-Lopez and Putnam 2019; Zhao et al. 2018) (Fig. 7). Invertebrates have wide breathing strategies to facilitate oxygen extraction of the surrounding water (Bell and Syed 2012). In cephalopod embryos, in the absence of any circulatory mechanism to aid oxygen transport to the tissue, oxygen must pass by diffusion from the external environment through the egg capsule to the embryo (Pimentel et al. 2012). During embryo development, changes occur in surface area and reduction of the egg wall thickness when, at the same time, the metabolic demand rises due to cellular growth, organogenesis, and muscular activity (Caamal-Monsreal et al. 2016; Cronin and Seymour 2000; Sánchez-García et al. 2017; Uriarte et al. 2012). What kind of mechanisms could then be epigenetically altered because of parental thermal stress?
The flow diagram shows the hypothesis to explain the negative transgenerational effect of maternal thermal stress in Octopus. maya embryos (red circle) and embryos from non-stressed females (blue circle). Yellow arrows indicate that although thermally stressed females had a high routine metabolic rate, this stress prevents the use of enough energy for yolk synthesis. Also, epigenetic alterations could modify the energetic metabolism, provoking high metabolic rates in embryos. As a result, embryos from thermally stressed females with high metabolic rates have less yolk and, consequently, a smaller size than embryos laid by non-stressed females. RMR Routine metabolic rate, SMR Standard metabolic rate, AS Aerobic scope
According to Eirin-López and Putnam (2019), a wide range of epigenetic mechanisms might be modulated because of environmental stress, ending up with changes in the phenotypes of organisms. One of these stressors is the temperature, which is known to modify the use of nutrients that are channeled to yolk production (Caamal-Monsreal et al. 2016). It has been reported that levels of acetyl-CoA, a donor to acetylation reactions, are correlated with the extent of histone acetylation and, that this epigenetic mark favors gene expression (Gibney and Nolan 2010; Lu and Thompson 2012; Sidoli et al. 2019; Tsuchiya et al. 2014). If embryos from stressed females have less yolk content, it can be proposed that suboptimal available levels of acetyl-CoA might thus trigger a histone hypoacetylation event which in turn might favor the silencing of a set of genes (probably, metabolism-related genes) that under normal embryo development conditions are necessary for growth. In fact, a direct link between the acetyl-CoA levels and the subsequent acetylation of histones at growth-related genes has been observed (Cai et al. 2011). Another mechanism by which hypoacetylation might be occurring is because elevated temperatures could alter the degradation of amino acids (AA) in the female during yolk synthesis processes in the embryo. If temperature modifies how AA are used as a cellular energy source in the ovary, then NAD + can be synthesized de novo from AA as tryptophan. NAD + is an obligatory co-factor of sirtuin (SIRT1 and SIRT 6) activities, which deacetylate histones H3K9/14 and H3K9/56, respectively. The deacetylation of histone H3 by these SIRT could down-regulate the expression of metabolic genes in the embryo, thereby altering various metabolic pathways. The latter would cause the uncoupling of blocks 1 and 2, (the latter comprising mostly the tri-carboxylic acid cycle) with block 3 (electron transport system ETS); the consequent escape of protons, and an increase in ROS production (Etchegaray and Mostoslavsky 2016) provokes at the same time an increment in mitochondrial oxygen consumption as a compensatory reaction (Wallace and Fan 2010), which is consistent with what was observed in embryos of O. maya (Fig. 2). Concurrently, several questions can be addressed as follows: Is it possible that as a consequence of epigenetic alteration, octopus embryos from thermally stressed females have more mitochondria than those coming from non-stressed females? Are more enzymes participating in the energetic pathways of the embryos even if they have the same number of mitochondria? Do embryos have a lower chorion thickness that facilitates oxygen flow from water to the embryo? Moreover, other possibilities could be included in the epigenetic alterations due to parental thermal stress. Imperadore et al. (2019) showed that two pallial nerves innervate two stellate ganglia in O. vulgaris mantle, controlling mantle breathing movements. Are there any chances that epigenetic alterations affect the embryo’s nervous central system changing the control ability of the pallial nerves and provoking an uncontrolled muscle activity in embryos during activation and growth phases, enhancing energy requirements and causing higher oxygen consumption? AChE are β-esterases that degrade choline-based esters; their role in cephalopods, as in many other organisms, is to act as a neurotransmitter in the nervous system, controlling excitatory stimulus (Omedes et al. 2022). This study did not observe that female thermal stress provoked alterations in AChE activity levels, suggesting that if thermal stress provoked alterations in the nervous system, it was not through AChE inhibition. Esterases such as AChE and other esterases have other physiological roles, such as cell differentiation, apoptosis and arm regeneration, suggesting that other aspects of the embryo metabolism should be studied to find how the epigenome could be altering O. maya embryo metabolism.
Among the mechanisms involved in maintaining homeostasis in aquatic invertebrates, antioxidant defense mechanisms (ANTIOX) have a key role in neutralizing ROS and RNS overproduction (Abele et al. 2007; Rodríguez-Fuentes et al. 2017). Although ANTIOX mechanisms work all the time to neutralize ROS, in extreme temperatures, the rate of ROS production overwhelms ANTIOX capability and repair mechanisms, leading to cellular disparities (Rahaman and Rahaman 2021). These systems might sometimes fail to counteract the toxic effects of amplified oxygen and nitrogen radical formation, elevating lipid peroxidation levels and producing cellular oxidative stress (Regoli and Giuliani 2014). The magnitude of oxidative stress depends on temperature and exposure time besides the ability of organisms to upregulate the enzymes involved in antioxidative defenses (Matozzo et al. 2013; Parisi et al. 2021). It is interesting to note that carbonyl groups in oxidized proteins (PO) and CAT activity were higher in embryos from non-thermally stressed females than those from stressed females suggesting differences in the form in which ROS and ANTIOX acted in the two groups of embryos. In O. mimus embryos, a part of ROS and oxidized biomolecules are transferred by the female through the yolk even in optimal thermal conditions (Olivares et al. 2019). To neutralize the ROS inherited and the ones produced at respiration, embryos turn on the antioxidant mechanisms once the circulatory system is activated, allowing the enzymes to neutralize ROS after organogenesis when the heart and circulatory system start working (Sánchez-García et al. 2017). A similar sequence of events was observed in O. maya embryos of non-stressed females. High levels of PO at the time of activation may be closely related to inherited PO levels plus PO produced during respiration. In the case of embryos of thermally stressed females, other mechanisms could be operating. In those embryos, CAT activities were low evidencing that the antioxidant system is collapsing. CAT is an enzyme that catalyzes hydrogen peroxide to H2O + O2 and is linked to the superoxide dismutase (SOD) enzyme that alternately catalyzes the dismutation (or partitioning) of the superoxide radical into ordinary molecular oxygen and hydrogen peroxide. It means that being CAT and SOD are the first lines of defense of the antioxidant mechanisms of vertebrates and invertebrates, both enzymes are key in the neutralization of ROS processes (Regoli and Giuliani 2014). In this sense, it is possible to hypothesize that low activity of CAT in O. maya embryos should have hard consequences in juvenile stages, mainly if those hatchlings are exposed to thermally stressed environments.
Conclusion
The results in this research are consistent with the adverse parental carry-over of thermal effects, which may be associated with trade-offs between the female condition (less aerobic scope and energy for yolk synthesis at high temperatures) and the investment required (yolk quality/quantity?) to have a successful offspring. Embryos from thermally stressed females had smaller sizes, less yolk, and higher metabolic rates. Additionally, a collapse in the antioxidant defense system was observed in embryos from thermally stressed females, indicating they were unable to control the high load of ROS and oxidative damage, which was partially acquired by maternal inheritance.
To conclude, further research should (i) assess if, as a consequence of epigenetic alteration, octopus embryos -from thermally stressed females- have more mitochondria than those coming from non-stressed females; (ii) confirm, if more enzymes are participating in the embryo energetic pathways even if they have the same number of mitochondria; (iii) observe if the embryo chorion wall thickness depends on the female thermal condition. Confirm if global acetylations levels are minors in embryos coming from stressed females. Other possibilities could be included in epigenetic alterations due to parental thermal stress. Thus, future studies need be performed with the purpose of knowing if epigenetic alterations affect the organism nervous central system, changing the control ability of pallial nerves and provoking an uncontrolled muscle activity during activation and growth phases, enhancing energy requirements, and causing higher oxygen consumption.
Data availability
The datasets generated during and/or analyzed during the current study are available in the Zenodo repository: Dominguez-Castanedo, O; Palomino-Cruz, D; Mascaró, M.; Rodríguez-Fuentes, G; Juárez, O.E.; Galindo-Sánchez, C.E.; Caamal-Monsreal, C.; Galindo-Torres, P.; Díaz, F.; Rosas, C. 2022. Data of transgenerational effects of thermal stress in embryos of O. maya. Zenodo https://doi.org/10.5281/zenodo.6533870
References
Abele E, Philip E, Gonzalez PM, Puntarulo S (2007) Marine invertebrate mitochondria and oxidative stress. Front Biosci 12:933–946
Ángeles-González LE, Lima FD, Caamal-Monsreal C, Díaz F, Rosas C (2020) Exploring the effects of warming seas by using the optimal and pejus temperatures of the embryo of three Octopoda species in the Gulf of Mexico. J Therm Biol 94:102753. https://doi.org/10.1016/j.jtherbio.102753
Ángeles-González LE, Martínez-Meyer E, Rosas C, Guarneros-Narváez V, López-Rocha J, Escamilla-Ake A, Osorio-Olvera L, Yáñez-Arenas C (2021) Long term environmental data explain better the abundance of the red octopus (Octopus maya) when testing the niche centroid hypothesis. J Exp Mar Biol Ecol 544:151609
Avendaño O, Roura A, Cedillo-Robles CE, Gonzalez A, Rodrıguez-Canul R, Velazquez-Abunader I, Guerra A (2020) Octopus americanus: a cryptic species of the O. vulgaris species complex redescribed from the Caribbean. Aquat Ecol 54:909–925. https://doi.org/10.1007/s10452-10020-09778-10456
Baker MA, Cerniglia G, Zaman A (1990) Microtiter Plate Assay for the measurement of glutathione and glutathione disulfide in large numbers of biological samples. Anal Biochem 190:360–365
Bell HJ, Syed NI (2012) Control of breathing in invertebrate model system. Compr Physiol 2:1745–1766
Blount DJ, Vitikaine KIE, Stott I, Cant AM (2016) Oxidative shielding and the cost of reproduction. Biol Rev 91:483–497. https://doi.org/10.1111/brv.12179
Bradford MM (1976) A refined and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem 72:248
Byrne M, Foo SA, Ross PM, Putnam HM (2019) Limitations of cross- and multigenerational plasticity for marine invertebrates faced with global climate change. Glob Change Biol 26:80–102. https://doi.org/10.1111/gcb.14882
Caamal-Monsreal C, Uriarte I, Farias A, Díaz F, Sánchez A, Re AD, Rosas C (2016) Effects of temperature on embryo development and metabolism of O. maya. Aquaculture 451:156–162
Cadenas E (1989) Biochemistry of oxygen toxicity. Annu Rev Biochem 58:79–110
Cai L, Sutter BM, Li B, Tu BP (2011) Acetyl-CoA induces cell growth and proliferation by promoting the acetylation of histones at growth genes. Mol Cell 42(4):426–437
Cronin ER, Seymour RS (2000) Respiration of the eggs of the giant cuttlefish Sepia apama. Mar Biol 136:863–870
Dominguez-Castanedo O, Palomino-Cruz D, Mascaró M, Rodríguez-Fuentes G, Juárez OE, Galindo-Sánchez CE, Caamal-Monsreal C, Galindo-Torres P, Díaz F, Rosas C (2022) Data of transgenerational effects of thermal stress in embryos of O. maya. Zenodo. https://doi.org/10.5281/zenodo.6533870
Domínguez-Estrada A, Galindo-Sánchez C, Ventura-López C, Rosas C, Juárez O (2022) Optic gland pathways in response to thermal stress through the reproductive phase of Octopus maya females. J Molluscan Stud. 88:eyac018. https://doi.org/10.1093/mollus/eyac018
Eddy TD, Bernhardt JR, Blanchard JL, Cheung WL, Colléter M, du Pontavice H, Fulton EA, Gascuel D, Kearney KA, Petrik C, M., Roy, T., Rykaczewski, R.R., Selden, R., Stock, C.A., Wabnitz, C.C., Watson, R.A., (2020) Energy Flow Through Marine Ecosystems: Confronting Transfer Efficiency. Trends Ecol Evol 36:76–86. https://doi.org/10.1016/j.tree.2020.1009.1006
Eirin-Lopez JM, Putnam HM (2019) Marine environmental epigenetics. Annu Rev Mar Sci 11:335–368. https://doi.org/10.1146/annurev-marine-010318-095114
Ellman G, Courtney K, Andres J, Featherstone R (1961) A new and rapid colorimetric determination of acetylcholinesterase activity. Biochem Pharmacol 7:88–95
Etchegaray JP, Mostoslavsky R (2016) Interplay between metabolism and epigenetics: a nuclear adaptation to environmental changes. Mol Cell 62:695–711
Feidantsis K, Georgoulis J, Giantsis I, Michaelidis, (2021) Treatment with ascorbic acid normalizes the aerobic capacity, antioxidant defence, and cell death pathways in thermally stressed Mytilus galloprovincialis. Comp Biochem Physiol Part B 255:110611
Fellous A, Favrel P, Riviere G (2015) Temperature influences histone methylation and mRNA expression of the Jmj-C histone-demethylase orthologues during the early development of the oyster Crassostrea gigas. Mar Genomics 19:23–30. https://doi.org/10.1016/j.margen.2014.1009.1002
Fellous A, Wegner KM, John U, Mark FC (2021) Windows of opportunity: Ocean warming shapes temperature-sensitive epigenetic reprogramming and gene expression across gametogenesis and embryogenesis in marine stickleback. Glob Change Biol 28:54–71. https://doi.org/10.1111/gcb.15942
Fridovich I (1986) Biological effects of the superoxide radical. Arch Biochem Biophys 247:1–11. https://doi.org/10.1016/0003-9861(1086)90526-90526
Gibney ER, Nolan CM (2010) Epigenetics and gene expression. Heredity 105:4–13
Gillooly JF, Brown JH, West GB, Savage VM, Charnov EL (2001) Effects of size and temperature on metabolic rate. Science 293:2248–2251
Góth L (1991) A simple method for determination of serum catalase activity and revision of reference range. Clin Chim Acta 196:143–152. https://doi.org/10.1016/0009-8981(91)90067-M
Habig WH, Jakoby WB (1981) Glutathione S-transferases (rat and human). Methods Enzymol 77:218–231. https://doi.org/10.1016/S0076-6879(81)77029-0
Hadwan MH, Abed HN (2016) Data supporting the spectrophotometric method for the estimation of catalase activity. Data Brief 6:194–199. https://doi.org/10.1016/j.dib.2015.12.012
Halliwell B (2013) The antioxidant paradox: less paradoxical now? Br J Clin Pharmacol 75:637–644. https://doi.org/10.1111/j.1365-2125.2012.04272.x
Hayward LS, Wingfield JC (2004) Maternal corticosterone is transferred to avian yolk and may alter offspring growth and adult phenotype. Gen Comp Endocrinol 135:365–371
Hosokawa M, Satoh T (2001) Measurement of carboxylesterase (CES) activities. Curr Protoc Toxicol 10:4.7.1–4.7.14. https://doi.org/10.1002/0471140856.tx0407s10
Hou Z, Fuiman LA (2022) Incorporation of dietary lipids and fatty acids into red drum Sciaenops ocellatus eggs. Com Biochem Physiol B 258:110694. https://doi.org/10.1016/j.cbpb.2021.110694
Hulbert AJ, Pmplon R, Buffenstein R, Buttemer WA (2007) Life and death: metabolic rate, membrane composition, and life span of animals. Physiol Rev 87:1175–1213. https://doi.org/10.1152/physrev.00047.02006
Imperadore P, Parazzoli D, Oldani A, Duebbert M, Büschges A, Fiorito G (2019) From injury to full repair: nerve regeneration and functional recovery in the common octopus, Octopus vulgaris. J Exp Biol. https://doi.org/10.1242/jeb.209965
Juárez O, Galindo CE, Díaz F, Re AD, Sanchez-García AM, Caamal-Monsreal C, Rosas C (2015) Is temperature conditioning Octopus maya fitness? J Exp Mar Biol Ecol 467:71–76
Juárez O, Arreola-Meráz L, Sánchez-Castrejón E, Avila-Poveda OH, López-Galindo L, Rosas C, Galindo-Sánchez C (2022) Oviducal gland transcriptomics of Octopus maya through physiological stages and the negative effects of temperature on fertilization. PeerJ 10:e12895. https://doi.org/10.17717/peerj.12895
Juarez O, Hau V, Caamal-Monsreal C, Galindo CE, Díaz F, Re AD, Rosas C (2016) Effect of maternal temperature stress before before spawning over the energetic balance of Octopus maya juveniles exposed to a gradual temperature changes. J Exp Mar Biol Ecol 474:39–45
Klosing A, Casas E, Hidalgo-Carcedo C, Vavouri T, Lehner B (2019) Transgenerational transmission of environmental information in C. elegans. Science 356:320–323. https://doi.org/10.1126/science.aah6412
Lefevre S (2016) Are global warming and ocean acidification conspiring against marine ectotherms? A meta analysis of the respiratory effects of elevated temperature, high CO2 and their interaction. Conserv Biol. https://doi.org/10.1093/conphys/cow1009
Lin D, Han F, Xuan S, Chen X (2019) Fatty acid composition and the evidence for mixed-income–capital breeding in female Argentinean short-fin squid Illex argentinus. Mar Biol 166:90. https://doi.org/10.1007/s00227-00019-03534-00220
López-Galindo L, Galindo-Sánchez C, Olivares A, Avila-Poveda OH, Díaz F, Juárez OE, Lafarga F, Pantoja-Péreza J, Caamal-Monsreal C, Rosas C (2018) Reproductive performance of Octopus maya males conditioned by thermal stress. Ecol Ind 96:437–447. https://doi.org/10.1016/j.ecolind.2018.1009.1036
Lu C, Thompson CB (2012) Metabolic regulation nof epigenetics. Cell Metab 16(1):9–17
Madeira D, Costa PR, Vinagre C, Diniz M (2016) When warming hits harder: survival, cellular stress and thermal limits of Sparus aurata larvae under global change. Mar Biol 163:91–105. https://doi.org/10.1007/s00227-016-2856-4
Matozzo V, Chinellato A, Munari M, Bressan M, Marin MG (2013) Can the combination of decreased pH and increased temperature values induce oxidative stress in the clam Chamelea gallina and the mussel Mytilus galloprovincialis? Mar Pollut Bull 72:34–40. https://doi.org/10.1016/j.marpolbul.2013.1005.1004
McRae C, Huang W-B, Fan T-Y, Cóté IM (2021) Effects of thermal conditioning on the performance of Pocillopora acuta adult coral and their offspring. Coral Reefs 40:1491–1503. https://doi.org/10.1007/s00338-00021-02123-00339
Mesquita CS, Oliveira R, Bento F, Geraldo D, Rodrigues JV, Marcos JC (2014) Simplified 2,4-dinitrophenylhydrazine spectrophotometric assay for quantification of carbonyls in oxidized proteins. Anal Biochem 458:69–71. https://doi.org/10.1016/j.ab.2014.04.034
Meza-Buendia AK, Trejo-Escamilla I, Piu M, Caamal-Monsreal C, Rodríguez-Fuentes G, Díaz F, Re AD, Galindo-Sánchez CE, Rosas C (2021) Why high temperatures limit reproduction in cephalopods? The case of Octopus maya. Aquacult Res. https://doi.org/10.1111/are.15387
Moreira DS, Venancio LPR, Sabino MACT, Hermes-Lima M (2016) How widespread is preparation for oxidative stress in the animal kingdom? Comp Biochem Physiol A: Mol Integr Physiol 200:64–78. https://doi.org/10.1016/j.cbpa.2016.01.023
Naef A (1928) Die Cephalopoden (Embryologie). Fauna e Flora Del Golfo Di Napoli 35:1–375
Noyola J, Caamal-Monsreal C, Díaz F, Re AD, Sánchez A, Rosas C (2013) Thermal preference, tolerance and metabolic rate of early juveniles of Octopus maya exposed to different acclimation temperatures. J Therm Biol 38:14–19
Olivares A, Rodríguez-Fuentes G, Mascaró M, Sánchez A, Ortega K, Caamal-Monsreal C, Tremblay N, Rosas C (2019) Maturation trade-offs in octopus females and their progeny: energy, digestion and defence indicators. PeerJ 7:e6618. https://doi.org/10.7717/peerj.6618
Omedes S, Andrade M, Escolar O, Villanueva R, Freitas R, Solé M (2022) B-esterases characterisation in the digestive tract of the common octopus and the European cuttlefish and their in vitro responses to contaminants of environmental concern. Environ Res 210:112961. https://doi.org/10.1016/j.envres.2022.112961
Parisi MG, Giacoletti C, Mandaglio M, Sará G (2021) The entangled multi-level responses of Mytilus galloprovincialis (Lamarck, 1819) to environmental stressors as detected by an integrated approach. Marine Environ Res 168:105292
Pimentel MS, Trübenbach K, Faleiro F, Boavida-Portugal J, Repolho T, Rosa R (2012) Impact of ocean warming on the early ontogeny of cephalopods: a metabolic approach. Mar Biol 159:2051–2059. https://doi.org/10.1007/s00227-00012-01991-00229
Pörtner HO (2010) Oxygen- and capacity-limitation of thermal tolerance: a matrix for integrating climate-related stressor effects in marine ecosystems. J Exp Biol 213:881–893
Pörtner HO, Bock C, Mark FC (2017) Oxygen- and capacity-limited thermal tolerance: bridging ecology and physiology. J Exp Biol 220:2685–2696. https://doi.org/10.1242/jeb.134585
Pörtner HO, Roberts DC, Poloczanska ES, Mintenbeck K, Tignor M, Alegría A, Craig M, Langsdorf S, Löschke S, Möller V (2022) Climate Change 2022: impacts, adaptation, and vulnerability. In: Pörtner HO, Roberts DC, Tignor M, Poloczanska ES, Mintenbeck K, Alegría A, Craig M, Langsdorf S, Löschke S, Möller VAO, Rama A (eds) Contribution of Working Group to the Sixth Assessment Report of the intergovernmental panel on climate change, IPCC 2022 summary for policymakers. Cambridge University Press, Cambridge
Rahaman MS, Rahaman MS (2021) Effects of elevated temperature on prooxidant-antioxidant homeostasis and redox status in the American oyster: signaling pathways of cellular apoptosis during heat stress. Environ Res. https://doi.org/10.1016/j.envres.2020.110428
Regoli F, Giuliani ME (2014) Oxidative pathways of chemical toxicity and oxidative stress biomarkers in marine organisms. Mar Environ Res 93:106–117
Repolho T, Baptista M, Pimentel MS, Dionısio G, Trübenbach K, Lopes VM, Lopes AR, Calado R, Diniz M, Rosa R (2014) Developmental and physiological challenges of octopus (Octopus vulgaris) early life stages under ocean warming. J Comp Physiol B 184:55–64
Rodríguez-Fuentes G, Armstrong J, Schlenk D (2008) Characterization of muscle cholinesterases from two demersal flatfish collected near a municipal wastewater outfall in Southern California. Ecotoxicol Environ Saf 69:466–471
Rodríguez-Fuentes G, Murúa-Castillo M, Díaz F, Rosas C, Caamal-Monsreal C, Sánchez A, Paschke K, Pascual C (2017) Ecophysiological biomarkers defining the thermal biology of the Caribbean lobster Panulirus argus. Ecol Indic Press. https://doi.org/10.1016/j.ecolind.2017.03.011
Ross PM, Parker LP, Byrne M (2016) Transgenerational responses of molluscs and echinoderms to changing ocean conditions. ICES J Mar Sci 73:537–549. https://doi.org/10.1093/icesjms/fsv1254
Roumbedakis K, Mascaró M, Martins ML, Gallardo P, Rosas C, Pascual C (2017) Health status of post-spawning Octopus maya (Cephalopoda: Octopodidae) females from Yucatan Peninsula, Mexico. Hydrobiologia. https://doi.org/10.1007/s10750-017-3340-y
Salinas S, Brown SC, Mangel M, Munch SB (2013) Non-genetic inheritance and changing environments. Non-Genetic Inherit 1:38–50
Sánchez-García A, Rodríguez-Fuentes G, Díaz F, Galindo-Sánchez C, Ortega K, Mascaró M, López E, Caamal-Monsreal C, Juárez O, Noreña-Barroso E, Re D, Rosas C (2017) Thermal sensitivity of O. maya embryos as a tool for monitoring the effects of environmental warming in the Southern of Gulf of Mexico. Ecol Ind 72:574–585
Secretaría de Agricultura, Ganadería, Desarrollo Rural, Pesca y Alimentación (2014) Fishery Management Plan for octopus (O. Maya and O. Vulgaris) in the Gulf of Mexico and the Caribbean Sea. Plan-de-Manejo-Pesquero-de-Pulpo.pdf (inapesca.gob.mx).
Sidoli S, Trefely S, Garcia BA, Carrer A (2019) Integrated analysis of Acetyl-CoA and histone modification via mass spectrometry to investigate metabolically driven acetylation. Methods Mol Biol 1928:125–147
Sillero-Ríos J, Sureda A, Capó X, Oliver-Codorniu M, Arechavala-López P (2018) Biomarkers of physiological responses of octopus vulgaris to different coastal environments in the western Mediterranean Sea. Mar Pollut Bull 128:240–247. https://doi.org/10.1016/j.marpolbul.2018.1001.1032
Storey KB, Storey JM (1978) Energy metabolism in the mantle muscle of the squid Loligo pealeii. J Comp Physiol 123:169–175
Tercero-Iglesias JF, Rosas C, Mascaró M, Poot-López GR, Domingues P, Noreña E, Caamal-Monsreal C, Pascual C, Estefanell J, Gallardo P (2015) Effects of parental diets supplemented with different lipid sources on Octopus maya embryo and hatching quality. Aquaculture 448:234–242
Tittensor DP, Novaglio C, Harrison CS, Heneghan RF, Barrier N, Bianchi D, Bopp L, Bryndum-Buchholz A, Britten GL, Büchner M, Cheung WWL, Christensen V, Coll M, Dunne JP, Eddy TD, Everett JD, Fernandes-Salvador JA, Fulton EA, Galbraith ED, Gascuel D, Guiet J, John JG, Link JS, Lotze HK, Maury O, Ortega-Cisneros K, Palacios-Abrantes J, Petrik CM, du Pontavice H, Rault J, Richardson AJ, Shannon L, Shin Y-J, Steenbeek J, Stock CA, Blanchard JL (2021) Next-generation ensemble projections reveal higher climate risks for marine ecosystems. Nat Clim Change 11:973–981. https://doi.org/10.1038/s41558-021-01173-9
Tsuchiya Y, Pham U, Hu W, Ohnuma SI, Gout I (2014) Changes in acetyl levels during the early embryonic development of Xenopus laevis. PLoS ONE 9(5):e97693. https://doi.org/10.1371/journal.pone.0097693
Uriarte I, Espinoza V, Herrera M, Zuñiga O, Olivares A, Carbonell P, Pino S, Farias A, Rosas C (2012) Effect of temperature on embryonic development of Octopus mimus under controlled conditions. J Exp Mar Biol Ecol 416–417:168–175
Vagnerová K, Vacková Z, Klusonová P, Staud F, Kopecky M, Ergang P, Milkcik I, Pácha J (2008) Reciprocal changes in maternal and fetal metabolism of corticosterone in rat during gestation. Reprod Sci 15:921–931
Ventura-López C, López-Galindo L, Rosas C, Sánchez-Castrejón E, Galido-Torres P, Pascual C, Rodríguez-Fuentes G, Juárez O, Galindo-Sánchez C (2022) Sex-specific role of the optical gland in Octopus maya: a transcritomic analysis. Gen Comp Endocrinol 320:114000. https://doi.org/10.1016/jygcen.2022.114000
Waite HR, Sorte CJB (2022) Negative carry-over effects on larval thermal tolerances across a natural thermal gradient. Ecology 103:e03565. https://doi.org/10.01002/ecy.03565
Wallace DC, Fan W (2010) Energetics, epigenetics, mitochondrial genetics. Mitocondrion 10:12–31. https://doi.org/10.1016/j.mito.2009.1009.1006
Zhao L, Yang F, Milano S, Han T, Walliser EO, Schöne BR (2018) Transgenerational acclimation to seawater acidification in the Manila clam Ruditapes philippinarum: Preferential uptake of metabolic carbon. Sci Total Environ 627:95–103. https://doi.org/10.1016/j.scitotenv.2018.1001.1225
Funding
This work was supported by Consejo Nacional de Ciencia y Tecnología for funding the project CONACYT 61503 and PAPIIT IN203022 to CR; D. Fischer provided English Edition. ODC had a postdoctoral position at Faculty of Sciences at UNAM thanks to CONACYT postdoctoral fellow. The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
DPC, ODC, and CCM conducted the experiments and make the preliminary analysis of data and the write the draft of the manuscript. MM and GRF make the analysis of the data and wrote the manuscript CCM, OJ, CGD, and PGT provided the octopus embryos, and pictures for the elaboration of the figure, and contributed to the writing of the manuscript FD and CR contributed to the design of experiments, the writing of the final version of the manuscript, and to administrate the project.
Corresponding author
Ethics declarations
Conflict of interest
No Competing interest declared in consequence, the authors have no relevant financial or non-financial interests to disclose.
Ethical approval
This study was performed following the European directive related to the use of cephalopods as experimental organisms (DIRECTIVA 2010/63/UE) and approved by the Bioethical Commission at UNAM (Universidad Nacional Autónoma de México) Faculty of Sciences (CEARC/Bioética/25102021).
Additional information
Responsible Editor: R. Rosa.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Domínguez-Castanedo, O., Palomino-Cruz, D., Mascaró, M. et al. Trans-generational physiological condition of embryos is conditioned by maternal thermal stress in Octopus maya. Mar Biol 170, 41 (2023). https://doi.org/10.1007/s00227-023-04183-7
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-023-04183-7