Abstract
Central place foragers such as pelagic seabirds often travel large distances to reach profitable foraging areas. King penguins (Aptenodytes patagonicus) are well known for their large-scale foraging movements to the productive Antarctic Polar Front, though their fine-scale travelling and foraging characteristics remain unclear. Here, we investigated the horizontal movements and foraging patterns of king penguins to understand their fine-scale movement decisions during distant foraging trips. We attached multi-channel data loggers that can record depth, speed, tri-axis acceleration, tri-axis magnetism, and environmental temperature of the penguins and obtained data (n = 8 birds) on their horizontal movement rates from reconstructed dive paths and their feeding attempts estimated from rapid changes in swim speed. During transit toward main foraging areas, penguins increased the time spent on shallow travelling dives (< 50 m) at night and around midday, and increased the time spent on deep foraging dives (≥ 50 m) during crepuscular hours. The horizontal movement rates during deep dives were negatively correlated with maximum dive depths, suggesting that foraging at greater depths is associated with a decreased horizontal travelling speed. Penguins concentrated their foraging efforts (more deep dives and higher rates of feeding attempts) at twilight during transit, when prey may be more accessible due to diel vertical migration, while they travelled rapidly at night and midday when prey may be difficult to detect and access. Such behavioural adjustments correspond to a movement strategy adopted by avian deep divers to travel long distances while feeding on prey exhibiting diel vertical migration.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Breeding seabirds are central place foragers dependent on patchily distributed marine resources (Ashmole 1963). Pelagic species often need to commute a large distance over the course of several days between their breeding sites and productive foraging areas. Many seabird species that breed in the Southern Ocean travel several hundred kilometers to mesoscale oceanic features (e.g. fronts and eddies) characterized by physical properties such as a large gradient in sea-surface temperature (SST) where productivity and prey availability increase (Weimerskirch 2007; Bost et al. 2009). During these trips, flying seabirds such as albatrosses and petrels can quickly travel a large distance (e.g. 300–500 km per day; Shaffer et al. 2003) at a low energetic cost (Weimerskirch 2007). Owing to their ability to travel quickly and efficiently, flying seabirds reduce the time spent travelling and the energetic cost of moving to and from productive foraging areas. In contrast, flightless seabirds such as penguins can travel only 20–120 km per day (e.g. Bost et al. 1997; Hull et al. 1997; Trathan et al. 2008) due to their slower mode of locomotion (i.e. swimming). Swimming is slower and incurs a higher energetic cost per unit travel distance (Davis et al. 1989; Green et al. 2002) than flying or gliding (Maina 2000). Therefore, how penguins must balance time spent travelling and foraging is an important constraint in enduring long-distance journeys to and from productive foraging areas. Larger penguin species can dive to depths of 100 m to > 500 m (Wilson 1995; Charrassin et al. 2002; Wienecke et al. 2007) during foraging dives in search of prey (Charrassin and Bost 2001; Bost et al. 2009). Therefore, penguins are expected to develop movement strategies for long-distance travel with behavioural adjustments that differ considerably from those of flying seabirds.
The king penguin (Aptenodytes patagonicus) is the second-largest, and second-most deep-diving penguin, after the emperor penguin (A. forsteri). King penguins can dive to depths of over 360 m for foraging (Charrassin et al. 2002; Pütz and Cherel 2005; Shiomi et al. 2016; Proud et al. 2021) and travel more than 400 km between their breeding sites and foraging zones during summer (Bost et al. 1997; Pütz 2002). During their long-distance travels, king penguins rapidly move toward their main foraging areas such as oceanic fronts and eddies (Cotté et al. 2007; Trathan et al. 2008; Scheffer et al. 2010; Bost et al. 2015). Although the large-scale foraging movements of king penguins according to mesoscale oceanic features are well known (Bost et al. 2009), the fine-scale movement characteristics of their travelling and foraging behaviours during long-distance trips remain unclear, due to the technical challenges of directly linking underwater movement paths of penguins and prey captures. Thus, it has been poorly documented how prey accessibility affects the local movement patterns of king penguins during their long-distance trips.
King penguins mainly forage for small mesopelagic fish, myctophids, in summer (Cherel and Ridoux 1992). Myctophid fish exhibit diel vertical migration, being distributed at deep depths (i.e. depth > 100 m) during the daytime, and at shallower depths during the nighttime when foraging on zooplankton (Zaselsky et al. 1985; Perissinotto and McQuaid 1992; Collins et al. 2008). As visual foragers, king penguins need sufficient light intensity to detect and pursue their prey underwater (Wilson et al. 1993; Pütz et al. 1998; Bost et al. 2002). The level of light intensity during the daytime, even at the great depths that king penguins forage, is greater than that at shallow depths during the nighttime (Bost et al. 2002). This supports the hypothesis of the ambient light level while foraging, explaining why king penguins primarily forage at depths > 100 m during the daytime, and less at shallower depths during the nighttime (Bost et al. 2002). Furthermore, foraging during twilight may be much more effective than during the daytime for penguins if there is sufficient light to detect and pursue their prey (Pütz and Cherel 2005). These crepuscular hours indeed correspond to the periods of myctophid vertical migration from the upper water column to greater depths during dawn, and vice versa during dusk; therefore, myctophids may be much more accessible in the shallower depths during the twilight than during the daytime.
This study aimed to understand the fine-scale movement decisions of king penguins during their foraging trips in relation to feeding opportunities. We investigated their 3D dive paths and the timing of feeding attempts using multi-channel data loggers, which allowed us to investigate the relationship between the availability of prey as indicated by feeding behaviour and finer-scale movement patterns. We hypothesized that during long-distance travel, king penguins continuously adjust their fine-scale travelling and foraging behaviours in response to the large-scale spatial distribution of their prey associated with sea temperature and diel changes in feeding opportunities. Specifically, we predicted that (i) penguins concentrate their foraging behaviours during twilight when feeding opportunities are expected to be higher, and (ii) focus on travelling behaviours during the middle of the day and nighttime when feeding opportunities are expected to be lower.
Methods
Fieldwork
This field study was performed at Possession Island (46°25′S, 51°45′E), Crozet Archipelago, South Indian Ocean between late January and early March 2011.
Nine chick-rearing king penguins were gently captured using a hooked pole before their departure for foraging trips. To record their diving behaviours, each bird was equipped with a multi-channel data logger (W1000L-3MPD3GT, Little Leonardo Ltd., Tokyo, 166 mm in length, 26 mm in diameter, weighing 132 g in the air, i.e. 1.2% of the mean body mass of equipped king penguins), using waterproof tape (Tesa tape, 4651; Tesa), stainless steel cables (4.5 mm in width, STB-360S; Hellermann Tyton), and instant glue (Loctite, 401; Henkel, Germany). The multi-channel data logger recorded swim speed (m·s−1), depth (m), ambient temperature (°C), tri-axis magnetism (nT) at a rate of 1 Hz, and tri-axis acceleration (m·s−2) at a rate of 8 Hz for two birds (K6 and K9) and 16 Hz for all other birds. Two out of the nine penguins (K3 and K5) were equipped with GPS data loggers (CatTrack, recustomized with a 1500 mAh lithium-iron phosphate battery and a deep depth casting; final size was ca. 60 × 40 × 25 mm, 50 g in air), together with the multi-channel data loggers (GPS data not used in this study due to short battery lifetime and limited available data). One out of the nine penguins (K8) was equipped with an oesophageal temperature logger in addition to the multi-channel data logger. The temperature sensor of this logger was set in the oesophagus and the body of the logger was set in the stomach (see methods in Charrassin et al. 2001; Bost et al. 2007). The deployment procedure for each penguin was completed within an hour. Attaching data loggers can affect diving behaviours in king penguins (Ropert-Coudert et al. 2000a). In previous studies and this study, the foraging trip duration of king penguins with loggers was longer than that of penguins without loggers (e.g. Bost et al. 1997; Charrassin et al. 2001, 2002). Nevertheless, the range and mean of diving depths in our penguins (1–366 m, 52 ± 74 m) were similar to that reported in a previous study (3–343 m, 55 ± 16 m, mean ± standard deviation) that used smaller loggers (e.g. 98.5 × 20 × 10 mm, 30 g: Pütz and Cherel 2005). Thus, we expect the effect of our data loggers on diving capacity to be relatively limited. After their foraging trips, birds were recaptured upon their return to their colony, and the data loggers were retrieved.
Diving behaviour
Data analysis was performed using IGOR Pro 8 (WaveMetrics, USA) with the program package Ethographer (Sakamoto et al. 2009). Submersions with a depth ≥ 1 m that lasted ≥ 30 s were considered dives. Deep dives were defined as dives of ≥ 50 m depth (Charrassin et al. 1998, 2002; Pütz et al. 1998; Ropert-Coudert et al. 2000a; Hanuise et al. 2013) and shallow dives were defined as dives of < 50 m depth. It is well known that the foraging movements of the king penguins at Crozet are associated with the areas of relatively low SST (4–5 °C) in summer (Park et al. 1993, 1998; Bost et al. 1997). We, therefore, calculated the SST experienced by the birds as the mean ambient temperature recorded by the loggers during 1–10 m depths (Charrassin and Bost 2001). Diving data were analyzed with respect to the time of day (night, dawn, daylight, dusk). The times for sunset and sunrise were downloaded from the Hydrographic and Oceanographic Department of Japan Coast Guard website (www1.kaiho.mlit.go.jp/KOHO/automail/sun_form3.html), which are determined as the instant uppermost portion of the sun is at the horizon when viewed from the position of the breeding colony. During the experimental period (27 January–01 March 2011), sunrise and sunset times were 05:17–06:07 and 19:23–20:14, respectively, per the local time (GMT + 4 h). Dawn and dusk were defined as 1 h before sunrise and after sunset, respectively. The period between dawn and dusk was defined as “daytime,” while “nighttime” was the period between the end of dusk and the onset of dawn. “Twilight” refers to the combined periods of dawn and dusk. We used sunrise and sunset times at the breeding colony for the corresponding dates because we do not know the accurate locations of birds at sea. The sunrise and sunset times at the breeding colony could deviate from those at the foraging locations of the study birds, but the deviations would be less than 20 min within a typical latitudinal movement range (up to 5° south of the breeding colony). Therefore, we considered that the use of sunrise and sunset times at the breeding colony would have a minimal impact on the main results in this study.
Swim speeds were calculated from the rotations of a propeller (rev·s–1), which was located at the front part of the multi-channel logger. To convert the number of rotations into absolute speeds (m·s−1), we set a constant value for each bird using the calibration method described by Shiomi et al. (2008). First, using the number of propeller rotations per second and the angle of the logger’s longitudinal axis (relative to the horizontal plane), which was calculated from gravitational acceleration (Sato et al. 2003), the ‘simulated vertical movement rate’ (penguin’s depth change per second) was obtained. Then, the simulated vertical movement rate at each second was summed from the start to the end of each dive to reconstruct the simulated depth profiles. Then, the attachment angle of the logger relative to the penguin’s body axis was estimated so as to make the simulated depth at the end of the dive zero (see Fig. 3 in Sato et al. 2003). Finally, the simulated depth profiles were compared with the actual depth profiles measured by a pressure sensor. We then chose the optimal constant value for each dive so that the simulated depth profiles were consistent with the actual depth profiles (see Fig. 1 in Shiomi et al. 2008). The constant values obtained for all dives of each bird were averaged, and the average value was used to calculate actual swimming speeds for all dives.
After converting propeller rotations into swim speed, the mean swim speed within a dive was calculated to identify dives with speed recording failures. Speed data from some dives were compromised due to propeller failure (e.g. temporal intrusion of materials such as algae or feathers). These dives (3.2% of all dives) with a mean swim speed below a threshold of 1.0 m·s−1 were excluded from further analyses. We chose this threshold by visually checking a plot of average swim speed against stroke rate within a dive (Shiomi et al. 2016).
Feeding behaviour
To estimate the timings of the feeding activities of penguins during dives, the following approach was used. First, the oesophageal temperature data obtained from one king penguin (K8) was used to measure feeding activities. A decrease in oesophageal temperature was considered a reliable index for ingestion events since king penguins feed on ectothermic prey (Charrassin et al. 2001; Hanuise et al. 2010). We defined a decrease of 0.06 °C·s−1 in oesophageal temperature as a feeding event as described by Charrassin et al. (2001). Next, a steep increase and decrease in swim speeds were considered to be related to feeding events such as the pursuit and capture of prey (Wilson et al. 2002; Shiomi et al. 2016; Brisson‑Curadeau et al. 2021). Steep increases in swim speeds (up to 4.0 m·s−1) that interrupt cruising speed (approximately 2.0 m·s−1) were often observed in the swim speed profiles and were termed “dashes” (Ropert-Coudert et al. 2000b). To quantify the dashes in swim speeds of other instrumented penguins, swim speed, U, was converted into accumulated values of acceleration following Ropert-Coudert et al. (2000b):
where U't is the function describing the increase of the swim speed as a function of time (t). The threshold value on acceleration peaks (dashes) was determined based on the number of dashes per dive to match the number of feeding events estimated from oesophageal temperature changes and a value of ≥ 0.68 m·s−2 (y = 0.93x + 0.06, R2 = 0.75, n = 1442 dives, Fig. S1) was defined as a “feeding attempt”. This value was applied to all the individuals and used for further analysis of feeding behaviour. We note that the number of oesophageal temperature drops may underestimate the actual number of prey captures, i.e. a single decrease in oesophageal temperature may reflect up to several prey captures when penguins catch small myctophid prey (less than 2 g) (see Hanuise et al. 2010). Feeding dives were defined as dives in which at least one feeding attempt occurred. The number of feeding attempts per minute submersed was calculated as a feeding attempt rate (n·min−1) for each feeding dive. The feeding attempt depth refers to the depth where each feeding attempt occurred.
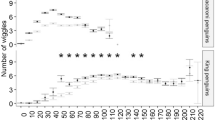
Assuming that breeding king penguins travel to reach distant foraging areas at the Polar Front (PF), we divided their foraging trips into two phases: a “low-feeding phase” representing the transit period and a “high-feeding phase” representing periods of intense feeding in main foraging areas based on the total number of feeding attempts per day. Based on rapid changes in swim speed, we estimated king penguins made 3–665 feeding attempts daily (Figs. S2, S3), with a higher number of feeding attempts in the middle of their foraging trips (Figs. 1, S3). The frequency distribution of the total number of feeding attempts per day was bimodal (Fig. S2). This enabled the separation between the two trip phases. We defined ‘high-feeding phases’ as the phases when the birds made more than 300 feeding attempts per day and the ‘low-feeding phases’ as the phases when the birds made less than 300 feeding attempts per day (Figs. 1, S2). However, one bird (K8) made 286 feeding attempts on day 8 and 400 and 665 feeding attempts on days 7 and 9, respectively (Fig. S3). We, therefore, considered day 8 of K8 as a high-feeding phase (Fig. S3). We did not analyze inward and outward low-feeding phases separately (Bost et al. 1997) because the logger data mostly covered outward but not inward low-feeding phases due to the limitation of battery and memory capacity (Fig. 1; Table 1).
Horizontal movement
The 3D dive paths were reconstructed from the following data: tri-axis magnetism, tri-axis acceleration, swim speed, and depths using the dead-reckoning method (Johnson and Tyack 2003; Shiomi et al. 2008). A tri-axis acceleration sensor in the logger recorded the following two components of acceleration: (i) dynamic acceleration related to propulsive activities, and (ii) static acceleration derived from gravity related to posture changes. First, we used a low-pass filter with a threshold value of 0.69 Hz to extract only static accelerations from tri-axis accelerations. The threshold value of the filter was determined by analyzing the power spectral density of the raw acceleration data. We then used the freeware macro available online to reconstruct 3D dive paths (Narazaki and Shiomi 2010; Shiomi et al. 2010). Using this macro, 3D dive paths were estimated by the following procedure (Johnson and Tyack 2003; Shiomi et al. 2008). Firstly, the pitch and roll angles of the penguins were calculated from tri-axis static accelerations (Johnson and Tyack 2003; Shiomi et al. 2008). Then, the heading was calculated from the pitch, roll, and tri-axis magnetism as angles between the vector of the horizontal component of the total geomagnetic intensity and that of the longitudinal axis of the animal. Headings relative to true north were obtained by adding the declination of earth’s magnetism at the breeding colony, − 49.6° (the International Geomagnetic Reference Field model; https://www.ngdc.noaa.gov/geomag/calculators/magcalc.shtml#declination). Finally, the 3D dive paths were reconstructed from heading, swim speed, and depth recorded every second with a dead-reckoning method. From the reconstructed 3D dive paths, the horizontal straight-line distance from the start to the endpoints of each dive was calculated. The horizontal straight-line distance was divided by dive duration to calculate the horizontal movement rate (m·s−1) for each dive, which was used as an index of travelling behaviour (Fig. S4). The horizontal movement rate indicates that the speed of horizontal movement per unit time. High values indicate that the penguins move rapidly in a horizontal dimension. We also calculated path straightness as an index of the tortuosity of dive paths. The horizontal straight-line distance was divided by the cumulative horizontal distance travelled from the start to the end points of each dive (Fig. S4). Straightness values range from 0 to 1, with higher values indicating that the path is more linear (Benhamou 2004).
Statistical analysis
Statistical analysis was performed using R software (R Core Team 2020). A Brunner-Munzel test was used to compare the median dive depths in deep dives (≥ 50 m), feeding attempt rates, feeding attempt depths, and horizontal movement rates between two trip phases (low-feeding phase and high-feeding phase) for each bird using the R package lawstat (Gastwirth et al. 2020). This statistical test was selected because the distributions of behavioural parameters were not normally distributed. A generalized linear mixed model (GLMM) with Poisson error distribution was fitted to determine the effect of the mean SST per day on the number of daily feeding attempts. We selected Poisson error distribution because the dependent variable (number of feeding attempts per day) was count data. We determined the effect of maximum dive depth in deep dives on the horizontal movement rate using a linear mixed model (LMM). In this LMM model, we included the trip phase (low-feeding or high-feeding phase) as a categorical fixed factor and ‘maximum dive depth × trip phase’ as an interaction term. We also determined the effect of the number of feeding attempts per dive on the path straightness using LMM. In this model, we included trip phase as a categorical fixed factor, and ‘number of feeding attempts per dive × trip phase’ as an interaction term. We included BirdID as a random factor in all GLMM and LMM models. We used the glmer and lmer function, for GLMM and LMM, respectively, from the R package lme4 (Bates et al. 2015). We obtained the p-value of GLMM and LMM models using the glht function from the R package multicomp (Hothorn et al. 2008). In addition, we calculated marginal R2 and conditional R2 using r.squaredGLMM function from the R package MuMIn (Barton 2020). The marginal R2 and conditional R2 components are interpreted as the variance explained by the fixed effects only and the entire model, respectively (Nakagawa and Schielzeth 2013). Values are shown as the mean ± the standard error of the mean.
Results
Data recovery
We recaptured the equipped birds and retrieved the loggers from 8 of the 9 birds after their foraging trips (trip duration: 15.7 ± 1.6 d, range 10.7–22.5 d). One bird (K7) did not leave the colony after deployment and hence foraging data could not be obtained. The logger data from the 7 birds covered 27–88% of the whole trip due to onboard memory and battery limitations of the loggers, while those from another bird (K6) covered a whole trip (Table 1). For two of the 8 birds (K2 and K8), tri-axis magnetism data were not available because of technical problems and thus 3D dive paths could not be calculated.
Diving behaviour
The median dive depths in deep dives (≥ 50 m) of king penguins did not differ clearly between the trip phases (Table 2). Among 5 birds, the median dive depths were significantly shallower in the high-feeding phase in 2 birds, while the opposite pattern (significantly shallower dives in low-feeding phases) was observed in 2 other birds, with no significant differences between trip phases in another bird (Table 2). As previously reported, diving depths showed a diurnal rhythm (Figs. 2, 3), with only shallow dives (< 50 m) occurring during the nighttime, and both deep and shallow dives occurring during the daytime. Dive depths gradually increased at dawn and decreased at dusk (Fig. 2).
Notably, the general diel pattern of deep and shallow dives differed between the two phases (Figs. 2, 3). In the low-feeding phase, deep dives occurred throughout the daytime but were often interspersed with shallow dives (Figs. 2a, 3a). Deep dives with some feeding attempts occurred intensively for several hours after sunrise and several hours before sunset (Figs. 2a, 3a) during this phase. In contrast, deep dives continuously occurred throughout the daytime in the high-feeding phase (Figs. 2b, 3b).
Feeding behaviour
The daily number of feeding attempts estimated from swim speed data was higher in colder water masses (Fig. 1) with a significant effect of SST on feeding activity (GLMM: y = exp (–0.48 x + 8.28), n = 57 days from 8 birds, p < 0.001, random effect = BirdID, Fig. S5). This indicated that penguins fed actively when they reached the low SST area (Fig. 1), presumably near or in the PF area. The median feeding attempt depths (depths of feeding attempts) did not differ clearly between the trip phases: the median feeding attempt depths of 2 of the 5 birds were shallower in the high-feeding phase than in the low-feeding phase, and deeper in the other birds (Table 2). In the high-feeding phase, the feeding attempt depths during all dives were concentrated at 100–140 m (Figs. 4a, S6). In contrast, in the low-feeding phase, the feeding attempt depths during deep dives showed more dispersed distributions (range of coefficient of variation: 30–45% in the low-feeding phase and 25–31% in the high-feeding phase, Figs. 4a, S6).
Comparison of the vertical distribution of feeding attempts between low-feeding and high-feeding phases of foraging trips. a Frequency distribution of the feeding attempt depths for a king penguin (K6) during the low-feeding (black) and high-feeding (red) phases. b Feeding attempt depth as a function of time of the day for a king penguin (K6) during the low-feeding (black coloured circle) and the high-feeding (red coloured circle) phases. Unshaded, light gray, and dark gray zones indicate daytime, twilight, and nighttime, respectively
Feeding attempts predominantly occurred during the daytime (80.2% ± 2.0%, n = 8 birds) and twilight (19.0% ± 2.1%, n = 8 birds), and during deep dives (≥ 50 m depth, 94.4% ± 0.9%, n = 8 birds, Fig. 4b). Feeding attempts occurred rarely during the nighttime (0.8% ± 0.2%, n = 8 birds, Fig. 4b), which occurred only at depths shallower than 50 m. The feeding attempt rates during feeding dives were relatively higher for several hours after sunrise and before sunset than during the nighttime and the daytime (Fig. 5). In the high-feeding phases, the feeding attempt rates during feeding dives were higher during the daytime than during the nighttime (Fig. 5b). In contrast, in the low-feeding phase, the feeding attempt rates during feeding dives were lower during the middle of the daytime than other daytime hours and during the nighttime (Fig. 5a).
Horizontal movement
The median horizontal movement rates during all dives were higher in the low-feeding phase than those in the high-feeding phase (Fig. 1; Table 2). Shallow dives showed a higher horizontal movement rate than that deep dives (1.62 ± 0.0002 m·s−1 in the shallow dives vs. 0.98 ± 0.01 m·s−1 in the deep dives, p < 0.001). During deep dives, the horizontal movement rates decreased with an increase in maximum dive depths (LMM: y = − 0.03x + 1.1, n = 3498 dives from six birds, marginal R2 = 0.35, conditional R2 = 0.46, p < 0.001, random effect = BirdID, Figs. 6a, S7). In addition, the horizontal movement rate in deep dives (≥ 50 m) for a given depth was higher in the low-feeding phase than in the high-feeding phase, and a significant ‘maximum dive depth × trip phase’ interaction effect was observed (p < 0.01, Fig. 6a). Path straightness in deep dives (≥ 50 m) decreased with an increase in the number of feeding attempts per dive (LMM: y = − 0.04x + 0.83, n = 2714 dives from three birds, marginal R2 = 0.48, conditional R2 = 0.5, p < 0.001, random effect = BirdID, Figs. 7, S8). In addition, the path straightness in deep dives (≥ 50 m) for a given number of feeding attempts was higher in the low-feeding phase than in the high-feeding phase, and a significant ‘number of feeding attempts × trip phase’ interaction effect was observed (p < 0.001, Fig. 7).
Comparison of horizontal movements between low-feeding and high-feeding phases for a king penguin (K6). a Relationship between horizontal movement rates and maximum dive depth during deep dives (≥ 50 m) for a king penguin (K6). Deep dives during low-feeding (black coloured circle) and high-feeding (red coloured circle) phases are shown separately, with linear regression lines (low-feeding phase: y = –0.006x + 1.5, high-feeding phase: y = –0.003x + 1.6). Statistical analysis was conducted with a linear mixed effect model (see ‘Result’). Gray circles indicate shallow dives. b–e Examples of horizontal movement paths (b, c) and depth profiles (d, e) for dives during low-feeding (b, d) and high-feeding (c, e) phases. Closed and open circles represent the start and end of the dives, respectively. Arrows indicate travelling direction. Orange circles indicate the occurrence of feeding attempts
Relationship between path straightness and number of feeding attempts per dive for a king penguin (K6). Dives during low-feeding (black coloured circle) and high-feeding (red coloured circle) phases are shown separately, with linear regression lines (low-feeding phase: y = – 0.04x + 0.88, high-feeding phase: y = – 0.02x + 0.66). Statistical analysis was conducted with a general linear mixed effect model (see ‘Result’)
Discussion
This study examined the fine-scale patterns in the travelling and foraging behaviours of a deep-diving avian predator based on the rapid changes in swim speed and horizontal movements estimated by 3D dive path reconstruction. This novel approach shows that deep avian divers such as king penguins adjust fine-scale travelling and feeding patterns in relation to the distribution of their prey at different spatial and temporal scales. In this section, we discuss how king penguins adjust their local travelling and foraging behaviours in relation to (1) large-scale distribution of their prey associated with sea temperature changes, and (2) diel changes in feeding opportunities.
Low-feeding phase vs. high-feeding phase of trips
King penguins are well known to rely on prey resources present in distant but predictable frontal areas such as the PF area, which are 300–500 km away from their colonies (Bost et al. 2015). They feed mainly on myctophids, which are abundant in the PF area and distributed near the water surface at nighttime but in deeper depths during the daytime (Sabourenkov 1991; Pakhomov et al. 1994). The prey ingestion rate of king penguins, based on oesophageal temperature and dive profile records (i.e. ‘wiggles’) is higher in the PF area (Bost et al. 2015). Our results confirmed a higher feeding activity in the middle of the trips, where the penguins experienced low SST, corresponding to the PF area (Table 2; Fig. 1). King penguins dive typically to and below thermocline depths where myctophids are thought to be distributed during the daytime, and their dives become shallower as they travel toward the PF area as a response to the relatively shallower thermocline (Charrassin and Bost 2001). Feeding attempt depths are considered to reflect the depth distribution of available prey for a given dive (Fig. 4b). A higher concentration of feeding attempt depths to particular depth zones (100–140 m depths) in high-feeding phase than in the low-feeding phase (Figs. 4, S6) suggests that myctophids might be more aggregated at these depths. Our new results reinforce the hypothesis that prey is more accessible and predictable for king penguins in the PF area (Bost et al. 2009).
The travelling behaviour of king penguins appears to reflect the high prey availability in the PF area. Higher horizontal movement rates in the low-feeding phase than in the high-feeding phase (Table 2; Fig. 1) suggest that penguins swam horizontally which favor a rapid transit rapidly to the PF area. A pattern of decreasing path straightness with an increasing number of feeding attempts per dive (Figs. 7, S8) suggests that penguins performed area-restricted search, with increased search effort associated with prey encounters (Kareiva and Odell 1987). Higher path straightness adjusted for the number of feeding attempts per dive (Figs. 7, S8) and higher horizontal movement rates during deep dives (≥ 50 m) for a given depth (Figs. 6a, S7) in the low-feeding phase than in high-feeding phase suggest that deep dives in the low-feeding phase may be intended for both travelling and opportunistic foraging (Fig. 6b–e). In the high-feeding phase, relatively low horizontal movement rates and path straightness reflected the increased foraging activity in the profitable PF area. Lower horizontal movement rates are likely to be the result of steeper diving body angles and lower straightness of dive paths of penguins (Fig. 6b–e). These results are in accordance with the previous findings that penguins use steeper diving body angles during the descent and ascent phases of their dives when they experience relatively high-feeding success, even for comparable maximum dive depths (Sato et al. 2004; Hanuise et al. 2013). Thus, our results suggest that king penguins may shift their prey search behaviour in relation to the high prey availability in the more distant but profitable PF area.
Diel changes in foraging and travelling behaviour
Myctophids perform diel vertical migration (Zaselsky et al. 1985; Perissinotto and McQuaid 1992; Collins et al. 2008), which results in diel changes in the feeding opportunities for visual foragers such as king penguins (Wilson et al. 1993; Bost et al. 2002). Higher feeding attempt rates around dawn and/or dusk than during the nighttime and around midday (Fig. 5) suggest that feeding opportunities are high for king penguins during twilight when myctophids transit between deep depths and the near-surface water column (Pütz and Cherel 2005; Scheffer et al. 2010). During the nighttime, penguins made few feeding attempts, as has been previously reported (Pütz and Bost 1994; Pütz et al. 1998; Shiomi et al. 2016). This has been attributed to the low light intensity available during nighttime for foraging penguins (Wilson et al. 1993; Pütz et al. 1998; Bost et al. 2002). During the daytime, penguins made more feeding attempts at deep depths where myctophid are thought to be distributed (Pütz and Bost 1994; Bost et al. 2002). Lower feeding attempt rates during daytime in the low-feeding phase than in the high-feeding phase (Fig. 5) probably reflect that thermocline depths where myctophids are distributed may be deeper and more difficult to reach by penguins in the low-feeding phases, presumably before penguins reach the PF area (Charrassin and Bost 2001). Thus, feeding opportunities are likely to be higher during dawn and dusk because prey are accessible at shallower depths with sufficient light intensity for visual detection of prey (Piersma et al. 1988; Zimmer et al. 2008; Regular et al. 2010). Furthermore, during these periods, penguins spend more time on relatively deep dives (Figs. 2, 3), suggesting that king penguins concentrate their foraging efforts when feeding opportunities are high, such as around dawn and dusk.
Overall, the travelling behaviour of king penguins appears to reflect the diel changes in feeding opportunities. At nighttime, penguins increased the time spent on shallow dives per hour (Fig. 3), suggesting that penguins may concentrate their travelling behaviour at nighttime when feeding opportunities are relatively low due to low light intensity for detecting their prey. During the daytime, penguins spent less time on shallow dives in the high-feeding phases (Fig. 3b), suggesting that they performed less travelling in the PF area (Cotté et al. 2007). In contrast, in the low-feeding phase, the time spent for shallow dives per hour tended to be high after dawn and during the middle of the daytime (Fig. 3a). This suggests that penguins may increase their travelling time when feeding opportunities are low (Fig. 5a). During deep dives (≥ 50 m), the horizontal movement rate decreased with increased dive depth (Figs. 6a, S7), indicating that foraging at greater depths is associated with a decreased horizontal travelling speed when penguins only have a limited time to prospect at a greater depth. These results showed that king penguins travelling to and from the PF area made behavioural adjustments by increasing their travelling behaviour during periods of low-feeding opportunities such as during the nighttime and the middle of the daytime due to the possible difficulty in detecting and accessing prey.
Conclusions
This study provides new insights into the fine-scale patterns of travelling and foraging of an avian deep diver, the king penguin, during foraging trips using multiple types of bio-logging measurements. At the scale of a foraging trip, king penguins appear to modify their travelling and foraging behaviours in relation to the large-scale spatial distribution of their prey. Thus, king penguins maximise horizontal travel and opportunistic foraging to main foraging areas and then fed intensively near or in the PF area throughout the daytime. At the diel scale, king penguins travelling to and from the PF appear to adjust travelling and foraging in relation to the diel changes in feeding opportunities associated with the diel vertical migration of prey. King penguins concentrate their foraging efforts during dawn and dusk when feeding opportunities are likely to be high because prey is much more accessible at shallow depths. The behavioural adjustments reported here might be an important movement strategy adopted by king penguins to travel long distances while foraging on prey exhibiting diel vertical migration. Diving predators can search for their prey in the three dimensions of the water column when travelling toward profitable foraging areas (Bost et al. 2009). Therefore, diving seabirds could take advantage of spatiotemporal changes in feeding opportunities underwater more easily than flying seabirds which are surface feeders. Such abilities might compensate for the higher energy costs of swimming and have developed a characteristic movement strategy in diving seabirds. Concurrent measurements of feeding activities and fine-scale movements are a promising tool to better understand the dynamics movement decisions of long-ranging diving predators.
Data availability
The datasets analyzed during the current study will be available on the Arctic Data archive System (ADS) of the National Institute of Polar Research (https://ads.nipr.ac.jp/dataset/A20220624-003).
References
Ashmole NP (1963) The regulation of numbers of tropical oceanic birds. Ibis 103(3):458–473. https://doi.org/10.1111/j.1474-919X.1963.tb06766.x
Bartoń K (2020) MuMIn: Multi-Model Inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn. Accessed 25 Aug 2021
Bates D, Mächler M, Bolker BM, Walker SC (2015) Fitting linear mixed-effects models using lme4. J Stat Softw 67(1):1–48. https://doi.org/10.18637/jss.v067.i01
Benhamou S (2004) How to reliably estimate the tortuosity of an animal’s path: straightness, sinuosity, or fractal dimension? J Theor Biol 229:209–220. https://doi.org/10.1016/j.jtbi.2004.03.016
Bost CA, Georges JY, Guinet C, Cherel Y, Pütz K, Charrassin JB, Handrich Y, Zorn T, Lage J, Le Maho Y (1997) Foraging habitat and food intake of satellite-tracked king penguins during the austral summer at Crozet Archipelago. Mar Ecol Prog Ser 150:21–33. https://doi.org/10.3354/meps150021
Bost CA, Zorn T, Le Maho Y, Duhamel G (2002) Feeding of diving predators and diel vertical migration of prey: King penguins’ diet versus trawl sampling at Kerguelen Islands. Mar Ecol Prog Ser 227:1–61. https://doi.org/10.3354/meps227051
Bost CA, Handrich Y, Butler PJ, Fahlman A, Halsey LG, Woakes AJ, Ropert-Coudert Y (2007) Changes in dive profiles as an indicator of feeding success in king and Adélie penguins. Deep Sea Res Part II Top Stud Oceanogr 54(3–4):248–255. https://doi.org/10.1016/j.dsr2.2006.11.007
Bost CA, Cotté C, Bailleul F, Cherel Y, Charrassin JB, Guinet C, Ainley DG, Weimerskirch H (2009) The importance of oceanographic fronts to marine birds and mammals of the southern oceans. J Mar Syst 78(3):363–376. https://doi.org/10.1016/j.jmarsys.2008.11.022
Bost CA, Cotté C, Terray P, Barbraud C, Bon C, Delord K et al (2015) Large-scale climatic anomalies affect marine predator foraging behaviour and demography. Nat Commun 6(1):1–9. https://doi.org/10.1038/ncomms9220
Brisson-Curadeau É, Handrich Y, Elliott KH, Bost CA (2021) Accelerometry predicts prey-capture rates in the deep-diving king penguin Aptenodytes patagonicus. Mar Biol 168(10):1–10. https://doi.org/10.1007/s00227-021-03968-y
Charrassin JB, Bost CA (2001) Utilisation of the oceanic habitat by king penguins over the annual cycle. Mar Ecol Prog Ser 221:285–298. https://doi.org/10.3354/meps221285
Charrassin JB, Bost CA, Pütz K, Lage J, Dahier T, Zorn T, Le Maho Y (1998) Foraging strategies of incubating and brooding king penguins Aptenodytes patagonicus. Oecologia 114(2):194–201. https://doi.org/10.1007/s004420050436
Charrassin JB, Kato A, Handrich Y, Sato K, Naito Y, Ancel A et al (2001) Feeding behaviour of free-ranging penguins (Aptenodytes patagonicus) determined by oesophageal temperature. Proc R Soc B 268:151–157. https://doi.org/10.1098/rspb.2000.1343
Charrassin JB, Le Maho Y, Bost CA (2002) Seasonal changes in the diving parameters of king penguins (Aptenodytes patagonicus). Mar Biol 141(3):581–589. https://doi.org/10.1007/s00227-002-0843-4
Cherel Y, Ridoux V (1992) Prey species and nutritive value of food fed during summer to king penguin Aptenodytes patagonicus chicks at possession Island. Crozet Archipelago Ibis 134(2):118–127. https://doi.org/10.1111/j.1474-919X.1992.tb08388.x
Collins MA, Xavier JC, Johnston NM, North AW, Enderlein P, Tarling GA et al (2008) Patterns in the distribution of myctophid fish in the northern Scotia Sea ecosystem. Polar Biol 31(7):837–851. https://doi.org/10.1007/s00300-008-0423-2
Cotté C, Park YH, Guinet C, Bost CA (2007) Movements of foraging king penguins through marine mesoscale eddies. Proc R Soc B 274(1624):2385–2391. https://doi.org/10.1098/rspb.2007.0775
Davis RW, Croxall JP, O’Connell MJ (1989) The reproductive energetics of gentoo (Pygoscelis papua) and macaroni (Eudyptes chrysolophus) penguins at South Georgia. J Anim Ecol 58:59–74. https://doi.org/10.2307/4986
Gastwirth JL, Gel YR, Wallace Hui WL, Lyubchich V, Miao W, Noguchi K (2020) Package ‘lawstat’. R package version 3.4. https://CRAN.R-project.org/package=lawstat. Accessed 25 Aug 2021
Green JA, Butler PJ, Woakes AJ, Boyd IL (2002) Energy requirements of female Macaroni Penguins breeding at South Georgia. Funct Ecol 16:671–681. https://doi.org/10.1046/j.1365-2435.2002.00670.x
Hanuise N, Bost CA, Huin W, Auber A, Halsey LG, Handrich Y (2010) Measuring foraging activity in a deep-diving bird: comparing wiggles, oesophageal temperatures and beak-opening angles as proxies of feeding. J Exp Biol 213(22):3874–3880. https://doi.org/10.1242/jeb.044057
Hanuise N, Bost CA, Handrich Y (2013) Optimization of transit strategies while diving in foraging king penguins. J Zool 290(3):181–191. https://doi.org/10.1111/jzo.12026
Hothorn T, Bretz F, Westfall P (2008) Simultaneous inference in general parametric models. Biometrical J 50:346–363. https://doi.org/10.1002/bimj.200810425
Hull CL, Hindell MA, Michael K (1997) Foraging zones of royal penguins during the breeding season, and their association with oceanographic features. Mar Ecol Prog Ser 153:217–228. https://doi.org/10.3354/meps153217
Johnson MP, Tyack PL (2003) A digital acoustic recording tag for measuring the response of wild marine mammals to sound. IEEE J Ocean Eng 28(1):3–12. https://doi.org/10.1109/JOE.2002.808212
Kareiva P, Odell G (1987) Swarms of predators exhibit “preytaxis” if individual predators use area-restricted search. Am Nat 130:233–270. https://doi.org/10.1086/284707
Maina JN (2000) What it takes to fly: the structural and functional respiratory refinements in birds and bats. J Exp Biol 203(20):3045–3064. https://doi.org/10.1242/jeb.203.20.3045
Nakagawa S, Schielzeth H (2013) A general and simple method for obtaining R2 from generalized linear mixed-effects models. Methods Ecol Evol 4:133–142. https://doi.org/10.1111/j.2041-210x.2012.00261.x
Narazaki T, Shiomi K (2010) Reconstruction of 3-D path (ThreeD_path). http://japan-biologgingsci.org/home/macro/threed_path/. Accessed 25 Aug 2021
Pakhomov EA, Perissinotto R, McQuaid CD (1994) Comparative structure of the macrozooplankton/micronekton communities of the Subtropical and Antarctic Polar Fronts. Mar Ecol Prog Ser 111(1):155–169
Park YH, Gambéroni L, Charriaud E (1993) Frontal structure, water masses, and circulation in the Crozet Basin. J Geophys Res 98:12361–12385. https://doi.org/10.1029/93JC00938
Park YH, Charriaud E, Fieux M (1998) Thermohaline structure of the antarctic surface water/winter water in the Indian sector of the Southern Ocean. J Mar Syst 17:5–23. https://doi.org/10.1016/S0924-7963(98)00026-8
Perissinotto R, McQuaid CD (1992) Land-based predator impact on vertically migrating zooplankton and micronekton advected to a Southern Ocean Archipelago. Mar Ecol Prog Ser 80(1):15–27. https://doi.org/10.3354/meps080015
Piersma T, Lindeboom R, Van Eerden MR (1988) Foraging rhythm of great crested grebes Podiceps cristatus adjusted to diel variations in the vertical distribution of their prey Osmerus eperlanus in a shallow eutrophic lake in The Netherlands. Oecologia 76(4):481–486. https://doi.org/10.1007/BF00397858
Proud R, Le Guen C, Sherley RB, Kato A, Ropert-Coudert Y, Ratcliffe N et al (2021) Using predicted patterns of 3D prey distribution to map king penguin foraging habitat. Front Mar Sci 8:745200. https://doi.org/10.3389/fmars.2021.745200
Pütz K (2002) Spatial and temporal variability in the foraging areas of breeding king penguins. Condor 104(3):528–538. https://doi.org/10.1093/condor/104.3.528
Pütz K, Bost CA (1994) Feeding behaviour of free-ranging King Penguins (Aptenodytes patagonicus). Ecology 75(2):489–497
Pütz K, Cherel Y (2005) The diving behaviour of brooding king penguins (Aptenodytes patagonicus) from the Falkland Islands: variation in dive profiles and synchronous underwater swimming provide new insights into their foraging strategies. Mar Biol 147(2):281–290. https://doi.org/10.1007/s00227-005-1577-x
Pütz K, Wilson RP, Charrassin JB, Raclot T, Lage J, Le Maho Y et al (1998) Foraging strategy of king penguins (Aptenodytes patagonicus) during summer at the Crozet Islands. Ecology 79(6):1905–1921. https://doi.org/10.1890/0012-9658(1998)079%5B1905:FSOKPA%5D2.0.CO;2
R Core Team (2020) R: A language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria. https://www.R-project.org/. Accessed 25 Aug 2021
Regular PM, Davoren GK, Hedd A, Montevecchi WA (2010) Crepuscular foraging by a pursuit-diving seabird: tactics of common murres in response to the diel vertical migration of capelin. Mar Ecol Prog Ser 415:295–304. https://doi.org/10.3354/meps08752
Ropert-Coudert Y, Bost CA, Handrich Y, Bevan RM, Butler PJ, Woakes AJ, Le Maho Y (2000a) Impact of externally attached loggers on the diving behaviour of the king penguin. Physiol Biochem Zool 73(4):438–444. https://doi.org/10.1086/317743
Ropert-Coudert Y, Sato K, Kato A, Charrassin JB, Bost CA, Le Maho Y, Naito Y (2000b) Preliminary investigations of prey pursuit and capture by king penguins at sea. Polar Biosci 13:101–112
Sabourenkov EN (1991) Mesopelagic fish of the southern ocean-summary results of recent Soviet studies. CCAMLR Selected Scientific Papers, pp 433–457
Sakamoto KQ, Sato K, Ishizuka M, Watanuki Y, Takahashi A, Daunt F, Wanless S (2009) Can ethograms be automatically generated using body acceleration data from free-ranging birds? PLoS One 4(4):e5379. https://doi.org/10.1371/journal.pone.0005379
Sato K, Mitani Y, Cameron MF, Siniff DB, Naito Y (2003) Factors affecting stroking patterns and body angle in diving Weddell seals under natural conditions. J Exp Biol 206:1461–1470. https://doi.org/10.1242/jeb.00265
Sato K, Charrassin JB, Bost CA, Naito Y (2004) Why do macaroni penguins choose shallow body angles that result in longer descent and ascent durations? J Exp Biol 207(23):4057–4065. https://doi.org/10.1242/jeb.01265
Scheffer A, Trathan PN, Collins M (2010) Foraging behaviour of king penguins (Aptenodytes patagonicus) in relation to predictable mesoscale oceanographic features in the Polar Front Zone to the north of South Georgia. Prog Oceanogr 86(1–2):232–245. https://doi.org/10.1016/j.pocean.2010.04.008
Shaffer S, Costa D, Weimerskirch H (2003) Foraging effort in relation to the constraints of reproduction in free-ranging albatrosses. Funct Ecol 17(1):66–74. https://doi.org/10.1046/j.1365-2435.2003.00705.x
Shiomi K, Sato K, Mitamura H, Arai N, Naito Y, Ponganis PJ (2008) Effect of ocean current on the dead-reckoning estimation of 3-D dive paths of emperor penguins. Aquat Biol 3(3):265–270. https://doi.org/10.3354/ab00087
Shiomi K, Narazaki T, Sato K, Shimatani K, Arai N, Ponganis PJ, Miyazaki N (2010) Data-processing artefacts in three-dimensional dive path reconstruction from geomagnetic and acceleration data. Aquat Biol 8(3):299–304. https://doi.org/10.3354/ab00239
Shiomi K, Sato K, Handrich Y, Bost CA (2016) Diel shift of king penguin swim speeds in relation to light intensity changes. Mar Ecol Prog Ser 561:233–243. https://doi.org/10.3354/meps11930
Trathan PN, Bishop C, Maclean G, Brown P, Fleming A, Collins MA (2008) Linear tracks and restricted temperature ranges characterise penguin foraging pathways. Mar Ecol Prog Ser 370:285–294. https://doi.org/10.3354/meps07638
Weimerskirch H (2007) Are seabirds foraging for unpredictable resources? Deep Sea Res Part II Top Stud Oceanogr 54(3–4):211–223. https://doi.org/10.1016/j.dsr2.2006.11.013
Wienecke B, Robertson G, Kirkwood R, Lawton K (2007) Extreme dives by free-ranging emperor penguins. Polar Biol 30(2):133–142. https://doi.org/10.1007/s00300-006-0168-8
Wilson RP (1995) Foraging ecology. In: Williams TD (ed) The penguins. Oxford University Press, Oxford, pp 81–106
Wilson RP, Pütz K, Bost CA, Culik BM, Bannasch R, Reins T, Adelung D (1993) Diel dive depth in penguins in relation to diel vertical migration of prey: whose dinner by candlelight? Mar Ecol Prog Ser 94:101–104. https://doi.org/10.3354/meps094101
Wilson RP, Ropert-Coudert Y, Kato A (2002) Rush and grab strategies in foraging marine endotherms: the case for haste in penguins. Anim Behav 63(1):85–95. https://doi.org/10.1006/anbe.2001.1883
Zaselsky VS, Kudrin BD, Poletayev VA, Chechenin SC (1985) Some features of the biology of Electrona carlsbergi (Tanning) (Myctophidae) in the Atlantic sector of the Antarctic. J Ichthyol 25:163–166
Zimmer I, Wilson RP, Beaulieu M, Ancel A, Plötz J (2008) Seeing the light: depth and time restrictions in the foraging capacity of emperor penguins at Pointe Géologie. Antarctica Aquat Biol 3(3):217–226. https://doi.org/10.3354/ab00082
Acknowledgements
We are especially indebted to the Institut Polaire Français Paul-Emile Victor (IPEV) for financial and logistical support to Programme N°394 (P.I. C.A. Bost). Special thanks to the participants of the 47th and 48th mission on Crozet and especially Marguerite Netchaïeff, Astrid Willener and Antoine Joris. We are grateful to two anonymous reviewers and the Associate Editor for their constructive comments on the manuscript and Editage (www.editage.com) for English language editing.
Funding
This study was funded by the Institut Polaire Français Paul-Emile Victor (IPEV, programme “Oiseaux Plongeurs,” no. 394, Leadership CAB), the Terres Australes et Antarctiques Françaises administration, Bio-Logging Science at the University of Tokyo (UTBLS), and Grant-in-Aid for JSPS Research Fellow (Grant number 20J22632, HW).
Author information
Authors and Affiliations
Contributions
HW: analyzed the data and drafted the initial manuscript. KSh: conducted the fieldwork and assisted with the initial data processing. AT and KSh: supervised the data analysis and the writing of the manuscript draft. KSa and CAB: administrated the project about field study. YH: participated the field work and helped collect the data. All authors contributed largely to the writing of the final manuscript. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Ethics approval
All experimental procedures were conducted under the permission of the ethics committee of the Terres Australes et Antarctic Françaises (CEP) and the French Environment Ministry, and the Office for Life Science Research Ethics and Safety, University of Tokyo (no. P10-26).
Additional information
Responsible Editor: T. Clay.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Watanabe, H., Shiomi, K., Sato, K. et al. King penguins adjust their fine-scale travelling and foraging behaviours to spatial and diel changes in feeding opportunities. Mar Biol 170, 29 (2023). https://doi.org/10.1007/s00227-022-04170-4
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04170-4