Abstract
Rising sand temperatures resulting from climate warming may cause the ‘feminization’ of sea turtle populations, which have temperature-dependent sex determination. In July and October 2021, we conducted surveys using a drone (also referred to as an unmanned aerial vehicle or UAV) and shore-based observations to assess the operational sex ratio (OSR) (number of males and females in breeding condition) for green turtles (Chelonia mydas) in the southern Great Barrier Reef, Australia (23.44 °S, 151.92 °E). Using drone-imagery, the length:width (L:W) ratio of a turtle’s head was used to distinguish loggerhead turtles (Caretta caretta) from green turtles, and the L:W of the carapace was used to distinguish juveniles from adult-sized green turtles. The first breeding pair was observed in shore-based surveys on 14 September 2021 and the number of mating turtles then increased to a peak on 4 October, about 8 weeks before the peak in nesting. A total of 94 km of drone transects with associated video footage was analysed. In October, at the peak of the mating season, the ratio of adult turtles displaying breeding behaviours near the island was 0.51 female (95% CI ± 0.17), supporting previous conclusions that despite the female-biased (> 80%) hatchling sex ratio, this population likely has a fairly balanced OSR. These findings are likely explained by males breeding 2–3 times more frequently than females, which helps mitigate female-biased hatchling sex ratios. Assessing the OSR of populations with extreme female hatchling bias may help to inform whether intervention is needed to increase male hatchling production.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Climate warming research has burgeoned in recent years due to major concerns regarding how certain organisms will respond, particularly those already under threat from anthropogenic activities. Sea turtles are widely considered to be vulnerable to climate change (Poloczanska et al. 2009). One reason for concern is that all sea turtle species have temperature-dependent sex determination (TSD), whereby sex is determined by the nest temperature during the thermal sensitive period of development (reviewed in Wibbels 2003; Porter et al. 2021). Below the pivotal temperature (PT), a higher proportion of male hatchlings is produced and vice versa for females (Mrosovsky, 1994; Ackerman, 1997). There is a 2–4 °C transitional range of temperatures where hatchlings of both sexes are produced (Mrosovsky, 1994). Therefore, the 2–4 °C projected rise in atmospheric temperatures by 2100 (IPCC 2022) and the associated rise in sand temperatures may result in the ‘feminization’ of sea turtle populations globally (Poloczanska et al. 2009).
Operational sex ratios (OSR, the ratio of sexually active males to receptive females in a reproductive cohort) that are female-biased can increase competition between females to find a mate, reduce opportunities to breed, and decrease genetic diversity (Emlen and Oring 1977; Kvarnemo and Ahnesjo 1996). Furthermore, a localised extinction event could occur if the production of male offspring declines to a level at which it is functionally zero (Poloczanska et al. 2009; Mitchell and Janzen 2010). For example, the northern Great Barrier Reef (nGBR) green turtle (Chelonia mydas) genetic stock is under considerable threat from feminization induced by climate change (Jensen et al. 2018). On Raine Island, where the majority of this population nests (also the largest green turtle rookery in the world), sand temperatures since the mid-1970s are estimated to have been well over the PT, and as a result the clutches produced are > 99% female (Jensen et al. 2018; Blechschmidt et al. 2020; Booth et al. 2020). A major concern for rookery managers and conservationists is if female-biased cohorts recruit to the breeding population and as the older males die out there will not be enough breeding males to fertilise the clutches. Recent modelling by Jensen et al. (2022) has shown that under an extreme climate warming scenario, the nGBR green turtle population will require each adult male to mate with 15–30 different females per season just to prevent population decline.
Approximately 1500 km southeast of Raine Island is Heron Island, a green turtle nesting site located within the Capricorn Bunker Group (CBG) in the southern Great Barrier Reef (sGBR) of Australia. Around September and October, sGBR green turtles that are in breeding condition migrate from their foraging grounds to mate and nest in locations such as the CBG (Limpus 2008). Despite inter-annual variation in the number of nesting turtles (Limpus 2008), the sGBR green turtle population has increased in recent decades (Chaloupka and Limpus 2001). There is also a foraging population of green turtles that are year-round residents of Heron Reef (Forbes 1996; Fitzsimmons 1997). The resident population in the lagoons of Heron Reef are historically reported as ~ 50% female (Forbes 1996), whilst the primary hatchling sex ratio for Heron island is estimated to be 80–97% female (Booth and Astill 2001; Booth and Freeman 2006). Data from experimental studies on Heron Island also suggest that average nest temperatures have likely increased in recent years (Ischer et al. 2009; Booth and Evans 2011; Booth et al. 2013; Smith et al. 2021; SI Figs. 1, 2). Despite a female-biased primary sex ratio predicted for Heron Island, sand temperatures in the CBG are still expected to be highly variable due to beach orientation and high vegetation cover, inferring complete feminisation is still not likely to occur soon (Booth and Astill 2001; Limpus 2008).
Previously, OSRs for the sGBR population have been estimated using both ‘mark and recapture’ and drone surveys in the lagoons surrounding Heron Island, with the conclusion that the OSR is balanced or slightly male-biased in October (Fitzsimmons 1997; Yaney-Keller et al. 2021). However, the extent of inter-annual variability in the OSR is largely unknown for sGBR green turtles and many other populations. Here, we used drone surveys to assess whether a balanced OSR has been sustained since it was first measured in the 1990s. Previous work by Yaney-Keller et al. (2021) using drone and boat surveys indicated a balanced OSR on Heron Reef in the 2016 breeding season; however, the presence of non-breeding adults and subadult turtles in these surveys was not considered in the analyses. In this study, we suggest and adopt methods to minimise the potential for counting non-breeding turtles and other turtle species when using drone surveys to assess OSR. Lastly, we documented the phenology of breeding activity around the island and in this way ensure that drone surveys to estimate OSR were conducted around the peak of breeding activity.
Materials and methods
Study site
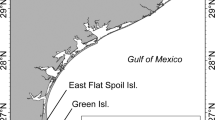
Heron Reef (23.44 °S, 151.92 °E) is ~ 80 km east of Gladstone, Queensland and within the Capricorn Bunker Group (CBG) of reefs in the Great Barrier Reef Marine Park (Fig. 1a). Heron Island is a sand cay and is an important nesting island for the sGBR green turtle genetic stock (Limpus 2008). Heron Reef is ideal for surveying the breeding behaviours of green turtles because individuals aggregate in the shallow lagoon from September to November to court and mate (Fitzsimmons 1997). The tidal range at Heron Reef is ~ 3 m, which means that on most low tides the reef rim of this platform reef is exposed, isolating the shallow lagoon, and potentially limiting access to sea turtles.
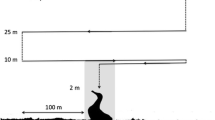
a Map of Queensland, Australia, and the geographic locations of focal green turtle nesting locations; Heron Island (green circle) in the southern Great Barrier Reef (GBR), and Raine Island (orange circle) in the northern GBR. This is an ‘equidistant cylindrical’ map that was adapted from a projection created in the ‘Maptool’ program from seaturtle.org. b Satellite image shows Heron Reef, where adult green turtles aggregate to breed annually. Drone surveys in July and October 2021 were conducted within the ‘nearshore’ (orange), ‘mid-lagoon’ (blue), ‘east-lagoon’ (pink) areas of Heron Reef. c Examples of two transect lines (yellow) flown by a drone in the ‘nearshore’ survey area. Paths have been adapted to exclude observational detours from the transect. Satellite images were generated from ArcGIS Software and adapted
Shore-based survey design
From 1 August 2021 to 17 January 2022, we conducted shore-based surveys around Heron Island to count adult green turtles close to shore. These surveys were used to estimate the start (when mating behaviour was first observed) and the peak (the observation periods when the frequency of mating behaviour was greatest) of the mating season. From August to November, these surveys were conducted weekly and opportunistically by the University of Queensland Research Station staff. During the final two months of the survey period, surveys were completed less regularly due to limited staff availability. However, courtship activity is known to substantially decrease by the beginning of December (Yaney-Keller et al. 2021), so this reduction in survey effort likely did not impact our findings. The observers walked around the island shore and counted the number of adult-sized green turtles and breeding pairs that were seen. Observers identified the mature turtles based on their relatively large size and speckled greyish-green carapace. Surveys took 30–45 min to complete and were conducted around high tide.
Survey equipment
A DJI Phantom™ 4 Pro drone (1375 g, SZ DJI Technology Co., Shenzhen, China) was used to conduct aerial surveys over several days in July and October. All flight paths were planned with the Litchi App™ on a tablet device (Apple Inc. iPad Mini 2™) and controlled by a licensed drone pilot. Each drone flight lasted 18–20 min depending on wind strength and direction. The drone carried a 4 K-quality video camera with a ND4 polarised filter added to reduce sun glare on the water’s surface. The camera was attached to a three-axis gimbal system that stabilised the video and camera angles were controlled remotely. Flights were carried out with 4 K video recording, in some instances, video resolution was reduced to 2.7 K to assist with backing up video data on cloud storage whilst on the island.
Drone survey design
Drone surveys were completed from 14 to 20 July and 8 to 12 October 2021. On each day that data were collected, 2–3 flights were conducted (wind and tide dependent), and one of the three following areas were surveyed; the ‘nearshore’, ‘mid-lagoon’, ‘east-lagoon’ areas of Heron Reef (Fig. 1b). All drone flights in October and most drone flights in July were launched and landed on a research vessel, with some land-based launches occurring in July 2021. No drone flights launched or landed on the island during the October survey period to avoid disturbance to the large numbers of black noddy terns (Anous minutus) nesting and breeding in the trees throughout October.
Surveys were conducted on a rising tide, between 08:00 and 17:00 on days where winds were forecast to be < 15 kts and with no rainfall. The vessel was positioned within 50–100 m of the first transect line of a survey route. The drone always remained within the line-of-sight of the pilot’s assistant (Civil Aviation Safety Authority regulations), so in some instances the drone was manually redirected to be within eyesight of the assistant. Transect lengths ranged from 500–700 m, were 50–100 m apart, with 2–6 lines per route and primarily in north–south oriented legs (Fig. 1c). Flight speed of each survey was 3–5 m/s and the altitude for the aerial surveys was 25–30 m (above the sea’s surface). However, altitude was reduced to 20 m in moderate wind conditions or to obtain closer observations of a turtle and assist with identification. The average flight altitude and camera angle were then used in the formulas for estimating the transect swathe (width), as provided in Burke et al. (2019). The total survey area of each transect was coarsely calculated by multiplying the horizontal distance travelled by the drone with the estimated swathe of each transect, restricting swathe width to 150 m. The total survey area covered was combined with the number of turtles observed during drone surveys to calculate standardised turtle density (turtles per km2) per survey period.
Data processing and analysis
After the surveys were completed, the drone videos were reviewed in the Behavioural Observation Research Interactive Software (BORIS, https://www.boris.unito.it/) created by Friard and Gamba (2016). All turtle observations, species, sex, size class (juvenile or adult) and behaviour (breeding/courting or foraging) were recorded and annotated within the video projects on BORIS. A still image was exported from the video file in BORIS for each turtle observation and labelled with a code representing species, size class, sex and behaviour.
Adult male green turtles were distinguished by their long tails (> 25 cm beyond carapace), whilst adult female green turtles were identifiable by short tails (< 5 cm beyond the carapace) that are often not visible from above (Fig. 2) (Limpus 1993; Fitzsimmons 1997). Juvenile versus adult-sized turtles were distinguished primarily by carapace colouration (juveniles = orange/brown, adults = dark grey/green and speckled) (Fig. 2c). Additionally, the length to width (L:W) ratio of the carapace was measured using the pixels of the still images in ImageJ Ops Software (https://imagej.net/Ops) to distinguish juveniles from adult-sized turtles. While we explored a similar approach to distinguish adults from subadult turtles, it was not possible from these measurements alone. This is primarily because the carapace length (therefore, the carapace shape) of the largest subadults is too close to the smallest of the adult turtles (i.e., subadults = 65–85 cm, adults ≥ 85 cm; Limpus 2008; Jensen et al. 2016; Hof et al. 2017). Tail length is not a reliable indicator of sex in subadult turtles, therefore, methods such as laparoscopic examination of the gonads are required to confidently identify sex and maturity (e.g. Fitzsimmons 1997). Additionally, subadults make up a significant proportion (~ 42%) of resident turtles in the lagoons of Heron Reef (Forbes 1996). Therefore, the results from the July and October surveys do not include a sex ratio estimate for the resident population because subadult turtles could be incorrectly counted as adult females.
Still images of green turtles captured from drone footage on Heron Reef in July and October 2021. a A breeding pair consisting of an adult male (tail not visible) mounted ontop of an adult female green turtle. b A courtship group consisting of a single adult female and two rival adult males (tails visible). c An adult breeding male (blue square) swimming away from a juvenile green turtle (white square) after attempting to mount. d A breeding pair (blue square) in close vicinity to two foraging turtles (white squares) in the ‘mid-lagoon’ area. Images have been cropped and adapted for this figure
Breeding and courtship behaviours observed in the drone surveys included mounting, chasing and/or avoiding other turtles (males and females), flipper slapping, escorting a mounted pair (termed ‘attendant’ male behaviour) and directional swimming in the water column (Fig. 2; Supplementary Information videos) (Schofield et al. 2017; Dickson et al. 2022). Observable foraging behaviours include individuals on the seafloor with the head down, presumably feeding on algae (e.g., Enteromorpha sp.) or patchy seagrass (Halophilia ovalis) (Forbes 1996), and/or crawling along the seafloor searching for food (Fig. 2d; Supplementary Information videos). Seafloor foraging may also be interspersed by the turtle surfacing for a breath before swimming straight back down to the seafloor. Both breeding and foraging turtles could be seen basking on the water’s surface, so this behaviour was excluded in our behavioural classification system. Tallies for adult male and female green turtle observations during the July and October periods were also converted into turtle density estimates based on turtles observed per km and km2 across the ‘nearshore’ survey area and all three survey areas; ‘nearshore’, ‘mid-Lagoon’ and ‘east-Lagoon’, combined.
Both adult green and loggerhead turtles (Caretta caretta) are year-round residents to the lagoon areas of Heron Reef, whereas resident hawksbill turtles (Eretmochelys imbricata) primarily remain on the reef crest and slope. Because green and loggerhead turtles occur in the same areas of the lagoons, they had to be distinguished from each other in the drone footage. Loggerhead turtles were identified by their ‘heart-shaped’ carapace, orange–brown scales, and a proportionally large head and smaller front and hind flippers in comparison to body size. Adult green turtles were identified by their oval-shaped carapace with a grey–green and black speckled pattern, and a proportionally small head and larger front and hind flippers in comparison to body size. To further distinguish green and loggerhead turtles, a protocol for identifying the species in the drone-imagery was developed. Firstly, a subsample of the still images of clearly identifiable green and loggerhead were imported into ImageJ Ops Software. If the head was clearly visible in the drone footage, then the head dimensions (length and width) were measured, to calculate the head L:W ratio of both species. Using this index, any ambiguous turtle images where the head was visible could be more confidently assigned.
Results
Shore-based surveys
No adult-sized turtles or courting pairs were observed in the shore-based surveys throughout August when the surveys started. The first pair of courting turtles was observed on 14 September 2021 (Fig. 3). The number of mating turtle observations then increased to a peak on 4 October and then decreased to 1–2 sightings per survey after 16 October (Fig. 3).
The number of adult-sized green turtles (light diamonds) and breeding pairs (dark circles) observed in the nearshore area from shore-based visual surveys on Heron Island conducted from 1 Aug 2021 to 17 January 2022. Adults are rarely observed in the nearshore areas outside of the breeding and nesting months (September–March) due to the lack of suitable food available close to the island
Size-class and species identification
The width and length of the carapace was measured for four juveniles and a subsample of 12 adult-sized green turtles (initial identification was based on carapace colouration), where the mean carapace L:W ratio was 1.09 (± 0.04 SE, range = 0.96–1.16) and 1.32 (± 0.03 SE, range = 1.14–1.47), respectively. Therefore, adult-sized turtles were identified as having a L:W carapace ratio > 1.15.
The L:W head ratio was calculated from measurements of 17 adult-sized turtles that could be confidently identified by the observer as a loggerhead or green turtle by their visible features (e.g. carapace shape, colouration, head, and limb proportions). The head L:W averaged 0.94 (± 0.06 SE, range = 0.83–1.09, n = 5) for loggerhead turtles and averaged 1.51 (± 0.06 SE, range = 1.2–1.82, n = 12) for green turtles (Fig. 4). Therefore, any individuals with a L:W ratio for head proportions (where the head is visible) > 1.2 were classified as green turtles.
Frequency plot of head length to width (L:W) ratio of adult-sized green (green bars) and loggerhead (purple bars) turtles measured in ImageJ Software from a subsample of the turtles observed in footage from drone surveys on Heron Reef. This figure includes images of Caretta caretta (loggerhead) and Chelonia mydas (green turtle) as a reference to show key morphological differences seen in the two species at the adult life stage
Drone surveys
A total of 23 and 18 drone flights were conducted in July and October respectively, culminating in a combined survey distance of 94 km. Adult-sized green turtles were observed in July on 23 occasions (4 males and 19 females) and had an overall density of 3.8 turtles per km2 (Table 1). None of these individuals were observed courting, mounting, or chasing each other. Adult-sized turtles in July exhibited typically foraging behaviours on the seafloor of the lagoons or rising to the surface to breathe.
In the October survey period, 45 male and 68 female turtles were observed (n = 113). Overall density increased fivefold for adult-sized green turtles in October (21.2 turtles per km2) compared to July and resulted in density estimates of 8.4 males and 12.7 females per km2, respectively (Table 1). Whilst breeding behaviours were observed across all areas, foraging behaviours were only observed in the ‘mid-lagoon’ and ‘east lagoon’ areas. During the July surveys, no foraging behaviours or breeding aggregations of adult-sized turtles were observed in the ‘nearshore’ area. It is unlikely that an adult turtle would forage in the nearshore site because their preferred foods (seagrass or algal turf) are likely scarce immediately adjacent to the island. Therefore, to estimate OSR and exclude non-breeding individuals and subadults, we examined the sex ratio of adult-sized green turtles in the ‘nearshore area’ only. This resulted in an OSR of 1.0 M: 1.1 F, or 0.51 female (± 0.17 CI limit) (Table 2). Of the 113 observations of adult-sized turtles, 75 (66.4%) were engaged in courtship or other breeding behaviours. Of those 75 turtle observations, 38 were male and 36 were female. Breeding behaviours were seen in all survey areas during October, and the lowest observations of breeding activity occurred in the ‘east-lagoon’ area. On average, two breeding pairs were sighted per day (total = 10, SE ± 0.4) and there were two occasions where attendant males were sighted with a breeding pair. However, a female being courted by two or more males (not mounted) was only seen once during the survey period. The other 33.6% of turtle observations were of adult-sized green turtles that were either on the seafloor feeding, resting, or surfacing to breathe.
Discussion
Drone surveys of Heron Reef yielded an OSR estimate for the sGBR green turtle population of 1 M: 1.1 F during the peak of the courtship aggregation in October 2021. This corresponds with previous estimates of OSR for green turtles on Heron Reef made by Yaney-Keller et al. (2021) during drone surveys from 17 to 21 October 2016 (1.05 M: 1 F). Our estimate also corresponds to the balanced estimates from the 1993–1996 ‘mark and recapture’ surveys by Fitzsimmons (1997) (1.2 M: 1 F), and similarly found proportionally greater numbers of breeding males to non-breeding males present in the nearshore areas around the island (e.g. ‘shark bay’) during the courtship period. The consistency in the OSR across 25 + years suggests that the OSR may show little inter-annual variability in contrast to nesting numbers which can vary greatly between years (Limpus 2008). This may indicate there has been a stable recruitment of males into the adult cohort, which is also supported by sex ratios reported for juvenile, subadult and adult cohorts of the sGBR green turtle population at other GBR foraging sites from mark-recapture surveys (e.g. Jensen et al. 2018). This is likely due to the low sand temperatures within the CBG where this population primarily nests (Booth and Freeman 2006), and has likely buffered against climate change induced feminization. However, in recent years, there has been some evidence to suggest that rising sand temperatures on Heron Island may be causing reduced male production (SI Figs. 1, 2). Therefore, increased feminisation of the adult population may only become apparent in over 30 years-time, as those turtles reach maturity (Limpus 2008).
A balanced OSR despite a female-biased hatchling sex ratio
To estimate the OSR of sea turtle populations, researchers have typically used mark-recapture surveys (Fitzsimmons 1997; Hays et al. 2010), multiple paternity tests for clutches (Wright et al. 2012a; Stewart and Dutton 2014; Prakash et al. 2022) and more recently, drone surveys (Schofield et al. 2017; Yaney-Keller et al. 2021). Regardless of the method used or species surveyed, most estimates of OSR reported for sea turtle populations around the world to date do not reflect the female-skewed ratio typically seen in hatchling sex ratio estimates (Phillips et al. 2013; Wright et al. 2012a; Stewart and Dutton 2014; Schofield et al. 2017; Yaney-Keller et al. 2021; Prakash et al. 2022). Theoretical considerations of the energetics of breeding suggest that the interval between breeding seasons (termed the remigration interval) will generally be much shorter in males than females across sea turtle populations (Hays et al. 2017). This suggestion is also supported by empirical observations (Fitzsimmons 1997; Limpus 2008; Wright et al. 2012b; Hays et al. 2017; Papafitsoros et al. 2022). It is likely that the mating system of sea turtles also allows populations to persist even with female-biased hatchling sex ratios because of the male’s ability to breed with multiple females (Limpus 1993; Fitzsimmons 1997; Lee and Hays 2004; Papafitsoros et al. 2022). The differential survival between males and females is another mechanism by which OSRs could differ from hatchling sex ratios (Hays et al. 2022). Additionally, some freshwater turtle species are known to have high intra-population variation for pivotal temperature and the transitional range in temperatures, so males can be produced at warmer temperatures (Carter et al. 2019). However, such phenomena (including inter-clutch variation) are yet to be confirmed for most sea turtle populations (Mrosovsky 1988; Lockley et al. 2020; Porter et al. 2021). Regardless, our findings show optimistically that female-skewed hatchling sex ratios may still translate into more balanced OSRs.
The OSR can be estimated using multiple paternity tests of clutches (Wright et al. 2012a; Stewart and Dutton 2014; Prakash et al. 2022). However, there is evidence for relatively low rates of multiple paternity in clutches (n = 13) of the sGBR green turtle population when compared to other nesting populations around the world (Lee et al. 2018). The incidence of multiple paternity in clutches is likely driven by adult male–female encounter rates and so it is important to investigate the links between the OSR and the incidence of multiple paternity at nesting sites (Lee et al. 2018). It may be, for example, that a very high incidence of multiple paternity is linked to sites where the OSR is more heavily male-biased than that reported at Heron Island.
Breeding phenology
Shore-based observational surveys were used in this study to corroborate that mating activity peaked in early to mid-October, a finding consistent with previous estimates for the peak of mating activity for Heron Reef green turtles (Limpus 1993; Fitzsimmons 1997). Shore-based surveys of the incidence of mating pairs have been used elsewhere (Godley et al. 2002) and provide a simple, cheap and effective method of assessing mating seasonality and so complement drone-only surveys. The nesting season on Heron Island peaks from December to early January (Limpus 2008), approximately 8–10 weeks after the peak in mating. Our findings are also consistent with observations from around the world. For example, in green turtles nesting on Ascension Island and loggerhead turtles nesting in Greece, the mating season also peaks 8–10 weeks before the peak of the nesting season (Godley et al. 2002; Schofield et al. 2013). Throughout the mating season, the sex ratio of breeding sea turtles is also highly dynamic, with males arriving earlier to the breeding grounds and departing earlier than females (Schofield et al. 2013). Several studies have also noted that over the course of several weeks, the adult sex ratio shifts from predominately male, to balanced and then to predominately female (Schofield et al. 2017; Yaney-Keller et al. 2021). Our results suggest that 8–10 weeks before the peak in nesting activity is the optimal period during which to survey mating interactions and assess the relative abundance of breeding males.
Accounting for residents and other species in drone surveys
Measuring sex ratios of the adult cohort in a sea turtle population is made difficult because unlike females, males do not come ashore and so at-sea census surveys are often needed (Hays et al. 2010; Schofield et al. 2017). Surveying Heron Reef to determine the OSR is further complicated by the presence of (i) non-breeding, resident adults and (ii) subadult turtles (e.g. Limpus 1993, 2008; Forbes 1996; Fitzsimmons 1997), so it is not as straightforward to identify adult breeding turtles in drone footage. For example, the July drone surveys from our study do not allow an accurate estimate of the sex ratio of the foraging population because subadult individuals cannot be reliably distinguished from small adult female turtles in drone videos. We also acknowledge that discriminating visiting breeding turtles from resident foraging turtles by only their observed behaviours in the drone videos is not as rigorous as other methods. Potentially some of the adult turtles seen “foraging” on the sea floor may in fact be breeding adults resting or opportunistically feeding between bouts of courtship activity (Schofield et al. 2017). A study by Tucker and Read (2001) conducted on Raine Island (nGBR) during the nesting season, found that 50% of the female green turtles sampled (n = 48) had traces of vegetation in the upper digestive tract. However, the prevalence of opportunistic foraging by breeding turtles from the sGBR stock remains understudied (Forbes 1996). The short duration of observations in the present study and the occurrence of breeding activities in known foraging areas (e.g. ‘mid’ and ‘east-lagoon’) might make assignments of breeding status erroneous. Therefore, we identified breeding adults by both their behaviours and occurrence in the ‘nearshore’ area where no observations of adult-sized turtles foraging were ever documented. However, improved methods to distinguish breeding turtles from both non-breeding adults and subadults will certainly help improve OSR estimates at Heron Reef. In courtship areas where both non-breeding adults and subadults are rare, then it is easier to reliably estimate the OSR from drone surveys, such as for loggerhead turtles in Greece (Schofield et al. 2017). A further potential complication in some areas, may be that some females do not nest close to courtship and mating sites. In such cases, potentially high-resolution satellite tracking (e.g., Esteban et al. 2017; Shimada et al. 2019) may help identify the links between where females mate and where they nest, thereby revealing further key sites for drone surveys to assess OSRs.
In some locations, identifying the turtle species in drone footage is very simple, as only a single species occurs in the area (Schofield et al. 2017). However, around the world multiple turtle species are often found coexisting in the same general area on both foraging and breeding grounds. In such cases, objective criteria are needed to distinguish species. Sea turtles differ in several external characteristics, most notably their scute patterns and carapace colour and shape. Species also differ in the general shape of the head, for example, loggerheads having a relatively large and wide head consistent with powerful jaws needed to eat their hard prey (Tomas et al. 2001). We demonstrated how the L:W ratio of the head could be used to distinguish loggerhead from green turtles in drone surveys. This approach for distinguishing species could be extended to other species of sea turtles as drone surveys become more widely used with this group.
Conclusion
Assessing OSR has been identified as a key factor to advance our knowledge and to assist with the conservation effort of sea turtle populations (Rees et al. 2016). There may be many high-risk populations; however, the current literature demonstrates that balanced OSRs can persist even with more female-biased primary sex ratios (Wright et al. 2012b). The findings from our drone surveys further emphasise this point, and it is likely that the more frequent breeding by male turtles helps to mitigate female-biased hatchling sex ratio skews (Hays et al. 2014, 2022). However, climate warming has raised concerns for the viability of populations with extreme female-bias in hatchling sex ratios (Poloczanska et al. 2009; Hays et al. 2017). Hence, estimating OSR should be prioritised for populations such as in the nGBR green turtle genetic stock, where the primary sex ratio is already 99% female (Jensen et al. 2018; Booth et al. 2020). It is hoped that the techniques we have described can be used to estimate OSR for more populations and to better understand turtle mating systems under a changing climate. More broadly, OSR estimates may help to inform wildlife managers whether direct intervention by nest cooling (e.g., Esteban et al. 2018; Smith et al. 2021) is required to increase male hatchling production (Santidrián Tomillo et al. 2021).
Data availability
Data used in this study is available via The University of Queensland’s (UQ eSpace) database with mediated access (by request) https://doi.org/10.48610/aa1b050.
References
Ackerman RA (1997) The nest environment and the embryonic development of sea turtles. In: Lutz PL, Musick JA (eds) The biology of sea turtles. CRC Press, Boca Raton, pp 83–106. https://doi.org/10.1201/9780203737088
Blechschmidt J, Wittmann MJ, Blüml C (2020) Climate change and green sea turtle sex ratio—preventing possible extinction. Genes 11:588. https://doi.org/10.3390/genes11050588
Booth DT, Astill K (2001) Temperature variation within and between nests of the green sea turtle, Chelonia mydas (Chelonia: Cheloniidae) on Heron Island Great Barrier Reef. Aust J Zool 49:71. https://doi.org/10.1071/zo00059
Booth DT, Freeman C (2006) Sand and nest temperatures and an estimate of hatchling sex ratio from the Heron Island green turtle (Chelonia mydas) rookery, southern Great Barrier Reef. Coral Reefs 25:629–633. https://doi.org/10.1007/s00338-006-0135-4
Booth DT, Feeney R, Shibata Y (2013) Nest and maternal origin can influence morphology and locomotor performance of hatchling green turtles (Chelonia mydas) incubated in field nests. Mar Biol 160:127–137. https://doi.org/10.1007/s00227-012-2070-y
Booth D, Dunstan A, Bell I, Reina R, Tedeschi J (2020) Low male production at the world’s largest green turtle rookery. Mar Ecol Prog Ser 653:181–190. https://doi.org/10.3354/meps13500
Booth DT, Evans A (2011) Warm water and cool nests are best How global warming might influence hatchling green turtle swimming performance. PLoS ONE 6:e23162. https://doi.org/10.1371/journal.pone.0023162
Burke C, Rashman M, Wich S, Symons A, Theron C, Longmore S (2019) Optimizing observing strategies for monitoring animals using drone-mounted thermal infrared cameras. Int J Remote Sens 40:439–467. https://doi.org/10.1080/01431161.2018.1558372
Carter AL, Bodensteiner BL, Iverson JB, Milne-Zelman CL, Mitchell TS, Refsnider JM, Warner DA, Janzen FJ (2019) Breadth of the thermal response captures individual and geographic variation in temperature-dependent sex determination. Funct Ecol 33:1928–1939. https://doi.org/10.1111/1365-2435.13410
Chaloupka M, Limpus C (2001) Trends in the abundance of sea turtles resident in southern Great Barrier Reef waters. Biol Conserv 102:235–249. https://doi.org/10.1016/s0006-3207(01)00106-9
Dickson LC, Tugwell H, Katselidis K, Schofield G (2022) Aerial drones reveal the dynamic structuring of sea turtle breeding aggregations and minimum survey effort required to capture climatic and sex-specific effects. Front Mar Sci 9:685. https://doi.org/10.3389/fmars.2022.864694
Emlen ST, Oring LW (1977) Ecology, sexual selection, and the evolution of mating systems. Science 197:215–223
Esteban N, Mortimer JA, Hays GC (2017) How numbers of nesting sea turtles can be over-estimated by nearly a factor of two. Proc Roy Soc B 284:20162581. https://doi.org/10.1098/rspb.2016.2581
Esteban N, Laloë J-O, Kiggen FSPL, Ubels SM, Becking LE, Meesters EH, Berkel J, Hays GC, Christianen MJA (2018) Optimism for mitigation of climate warming impacts for sea turtles through nest shading and relocation. Sci Rep 8:17625. https://doi.org/10.1038/s41598-018-35821-6
Fitzsimmons N (1997) Male marine turtles: gene flow, philopatry and mating systems of the green turtle Chelonia mydas. PhD Thesis, School of Biological Sciences, The University of Queensland, St. Lucia. https://www.espace.library.uq.edu.au/view/UQ:365841. Accessed 1 July 2020
Forbes GA (1996) The diet and feeding ecology of the green sea turtle (Chelonia mydas) in an algal-based coral reef community. PhD Thesis, James Cook University of North Queensland, Townsville. https://www.researchonline.jcu.edu.au/27399/. Accessed 23 Feb 2022
Friard O, Gamba M (2016) BORIS: a free, versatile open-source event-logging software for video/audio coding and live observations. Methods Ecol Evol 7:1325–1330. https://doi.org/10.1111/2041-210x.12584
Godley B, Broderick A, Frauenstein R, Glen F, Hays G (2002) Reproductive seasonality and sexual dimorphism in green turtles. Mar Ecol Prog Ser 226:125–133. https://doi.org/10.3354/meps226125
Hays GC, Fossette S, Katselidis KA, Schofield G, Gravenor MB (2010) Breeding periodicity for male sea turtles, operational sex ratios, and implications in the face of climate change. Conserv Biol 24:1636–1643. https://doi.org/10.1111/j.1523-1739.2010.01531.x
Hays GC, Mazaris AD, Schofield G, Laloë J-O (2017) Population viability at extreme sex-ratio skews produced by temperature-dependent sex determination. Proc R Soc B-Biol Sci 284:20162576. https://doi.org/10.1098/rspb.2016.2576
Hays GC, Shimada T, Schofield G (2022) A review of how the biology of male sea turtles may help mitigate female-biased hatchling sex ratio skews in a warming climate. Mar Biol 169:89. https://doi.org/10.1007/s00227-022-04074-3
Hays GC, Mazaris AD, Schofield G (2014) Different male vs female breeding periodicity helps mitigate offspring sex ratio skews in sea turtles. Front Mar Sci 1:43. https://doi.org/10.3389/fmars.2014.00043
Hof C, Smallwood E, Meager J, Bell I (2017) First citizen-science population abundance and growth rate estimates for green sea turtles Chelonia mydas foraging in the northern Great Barrier Reef, Australia. Mar Ecol Prog Ser 574:181–191. https://doi.org/10.3354/meps12173
IPCC (2022) Climate change 2022: impacts, adaptation, and vulnerability. contribution of working group II to the sixth assessment report of the intergovernmental panel on climate change. Cambridge University Press. In Press
Ischer T, Ireland K, Booth DT (2009) Locomotion performance of green turtle hatchlings from the Heron Island rookery, Great Barrier Reef. Mar Biol 156:1399–1409. https://doi.org/10.1007/s00227-009-1180-7
Jensen M, Bell I, Limpus C, Hamann M, Ambar S, Whap T, David C, Fitzsimmons N (2016) Spatial and temporal genetic variation among size classes of green turtles (Chelonia mydas) provides information on oceanic dispersal and population dynamics. Mar Ecol Prog Ser 543:241–256. https://doi.org/10.3354/meps11521
Jensen MP, Allen CD, Eguchi T, Bell IP, Lacasella EL, Hilton WA, Hof CAM, Dutton PH (2018) Environmental warming and feminization of one of the largest sea turtle populations in the world. Curr Biol 28:154–159. https://doi.org/10.1016/j.cub.2017.11.057
Jensen MP, Eguchi T, Fitzsimmons NN, McCarthy MA, Fuentes MMPB, Hamann M, Limpus CJ, Bell IP, Read MA (2022) Integrating climate change and management scenarios in population models to guide the conservation of marine turtles. Bull Mar Sci 98:131–154. https://doi.org/10.5343/bms.2021.0033
Kvarnemo C, Ahnesjo I (1996) The dynamics of operational sex ratios and competition for mates. Trends Ecol Evol 11:404–408. https://doi.org/10.1016/0169-5347(96)10056-2
Lee PL, Hays GC (2004) Polyandry in a marine turtle: females make the best of a bad job. PNAS 101:6530–6535. https://doi.org/10.1073/pnas.0307982101
Lee PLM, Schofield G, Haughey RI, Mazaris AD, Hays GC (2018) A review of patterns of multiple paternity across sea turtle rookeries. Adv Mar Biol 79:1–31. https://doi.org/10.1016/bs.amb.2017.09.004
Limpus CJ (1993) The green turtle, Chelonia mydas, in Queensland: breeding males in the southern Great Barrier Reef. Wildl Res 20:513. https://doi.org/10.1071/wr9930513
Limpus CJ (2008) A biological review of Australian marine turtles. 2. Green turtle Chelonia mydas (Linnaeus). Queensland Environmental Protection Agency Brisbane, QLD. https://www.environment.des.qld.gov.au/wildlife/animals/discovering-wildlife/turtle-watching/publications. Accessed 1 July 2020
Lockley EC, Reischig T, Eizaguirre C (2020) Maternally derived sex steroid hormones impact sex ratios of loggerhead sea turtles. BioRxiv Preprint. https://doi.org/10.1101/2020.01.10.901520
Mitchell NJ, Janzen FJ (2010) Temperature-dependent sex determination and contemporary climate change. Sex Dev 4:129–140. https://doi.org/10.1159/000282494
Mrosovsky N (1988) Pivotal temperatures for loggerhead turtles (Caretta caretta) from northern and southern nesting beaches. Can J Zool 66:661–669. https://doi.org/10.1139/z88-098
Mrosovsky N (1994) Sex ratios of sea turtles. J Exp Zool 270:16–27. https://doi.org/10.1002/jez.1402700104
Papafitsoros K, Dimitriadis C, Mazaris AD, Schofield G (2022) Photo-identification confirms polyandry in loggerhead sea turtles. Mar Ecol 43:e12696. https://doi.org/10.1111/maec.12696
Phillips KP, Jorgensen TH, Jolliffe KG, Jolliffe S-M, Henwood J, Richardson DS (2013) Reconstructing paternal genotypes to infer patterns of sperm storage and sexual selection in the hawksbill turtle. Mol Ecol 22:2301–2312. https://doi.org/10.1111/mec.12235
Poloczanska ES, Limpus CJ, Hays GC (2009) Vulnerability of marine turtles to climate change. Adv Mar Biol 56:151–211. https://doi.org/10.1016/s0065-2881(09)56002-6
Porter E, Booth DT, Limpus CJ, Staines MN, Smith CE (2021) Influence of short-term temperature drops on sex-determination in sea turtles. J Exp Zool A Ecol Integr Physiol 335:649–658. https://doi.org/10.1002/jez.2509
Prakash SS, Lal MM, Dutton PH, Rico C, Piovano S (2022) Kinship genomics approach to study mating systems in a depleted sea turtle rookery. Reg Stud Mar Sci 51:102174. https://doi.org/10.1016/j.rsma.2022.102174
Rees A, Alfaro-Shigueto J, Barata P et al (2016) Are we working towards global research priorities for management and conservation of sea turtles? Endanger Species Res 31:337–382. https://doi.org/10.3354/esr00801
Santidrián Tomillo P, Wallace BP, Paladino FV, Spotila JR, Genovart M (2021) Short-term gain, long-term loss: How a widely-used conservation tool could further threaten sea turtles. Biol Conserv 261:109260. https://doi.org/10.1016/j.biocon.2021.109260
Schofield G, Scott R, Dimadi A, Fossette S, Katselidis KA, Koutsoubas D, Lilley MKS, Pantis JD, Karagouni AD, Hays GC (2013) Evidence-based marine protected area planning for a highly mobile endangered marine vertebrate. Biol Conserv 161:101–109. https://doi.org/10.1016/j.biocon.2013.03.004
Schofield G, Katselidis KA, Lilley MKS, Reina RD, Hays GC (2017) Detecting elusive aspects of wildlife ecology using drones: new insights on the mating dynamics and operational sex ratios of sea turtles. Funct Ecol 31:2310–2319. https://doi.org/10.1111/1365-2435.12930
Shimada T, Limpus CJ, Hamann M, Bell I, Esteban N, Groom R, Hays GC (2019) Fidelity to foraging sites after long migrations. J Anim Ecol 89:1008–1016. https://doi.org/10.1111/1365-2656.13157
Smith C, Booth D, Crosby A, Miller J, Staines M, Versace H, Madden-Hof C (2021) Trialling seawater irrigation to combat the high nest temperature feminisation of green turtle Chelonia mydas hatchlings. Mar Ecol Prog Ser 667:177–190. https://doi.org/10.3354/meps13721
Stewart KR, Dutton PH (2014) Breeding sex ratios in adult leatherback turtles (Dermochelys coriacea) may compensate for female-biased hatchling sex ratios. PLoS ONE 9:e88138. https://doi.org/10.1371/journal.pone.0088138
Tomas J, Aznar FJ, Raga JA (2001) Feeding ecology of the loggerhead turtle Caretta caretta in the western Mediterranean. J Zool 255:525–532. https://doi.org/10.1017/s0952836901001613
Tucker AD, Read MA (2001) Frequency of foraging by gravid green turtles (Chelonia mydas) at Raine Island. Great Barrier Reef J Herpetol 35:500. https://doi.org/10.2307/1565970
Wibbels T (2003) Critical approaches to sex determination in sea turtles. In: Wibbels T, Lutz P, Musick J, Wyneken J (eds) The biology of sea turtles, vol 2. CRC Press, Boca Raton, FL, pp 103–134
Wright LI, Fuller WJ, Godley BJ, McGowan A, Tregenza T, Broderick AC (2012) Reconstruction of paternal genotypes over multiple breeding seasons reveals male green turtles do not breed annually. Mol Ecol 21:3625–3635. https://doi.org/10.1111/j.1365-294x.2012.05616.x
Wright LI, Stokes KL, Fuller WJ, Godley BJ, McGowan A, Snape R, Tregenza T, Broderick AC (2012) Turtle mating patterns buffer against disruptive effects of climate change. Proc R Soc B-Biol Sci 279:2122–2127. https://doi.org/10.1098/rspb.2011.2285
Yaney-Keller A, San Martin R, Reina RD (2021) Comparison of UAV and boat surveys for detecting changes in breeding population dynamics of sea turtles. Remote Sens 13:2857. https://doi.org/10.3390/rs13152857
Acknowledgements
We would like to acknowledge and pay our respects to the Gooreng Gooreng, Gurang, Bailai and Taribelang Bunda Peoples, who are the Traditional Custodians of the Sea Country around Heron Island where these surveys were conducted. We acknowledge the assistance of the UQ Heron Island Research Station staff in the shore-based surveys and planning of fieldwork. We also thank WWF-Australia and furniture company Koala for their continued financial and logistical support for this study, as part of WWF’s collaborative ‘Turtle Cooling’ Project. We also thank Dr Colin Limpus for useful discussions about this work and the reviewers of this manuscript for their constructive feedback and support.
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. This study was in collaboration with the World Wide Fund for Nature-Australia (WWF-Aus), the University of Queensland, and Deakin University. The project funds were entirely supported by WWF-Aus through their partnership with Koala.com.
Author information
Authors and Affiliations
Contributions
MNS, CES, CAM-H, DTB and GCH conceived the study and designed the methodology. MNS and CES planned fieldwork logistics and collected the field data. MNS processed the drone footage, classified, and measured individuals, and conducted the analyses for the study. MNS and GCH led the writing of the manuscript. All authors contributed critically to the drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no conflicts of interest to declare.
Ethical approval
Surveys were conducted within the permitted areas on Heron Reef under GBRMPA permit G20/44440.1 and Limited Impact Accreditation No. UQ001/2021. Survey methods were approved by the University of Queensland’s Animal Ethics Committee under Certificate No. SBS/258/20/DEAKIN/WWF and ANRFA No. 2021/AE000605. At the time of surveying, the drone used in the study was registered under the pilot’s name (M.N. Staines) with the Australian Civil Aviation Safety Authority (CASA) under ARN: 1095200.
Additional information
Responsible Editor: L. Avens.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Supplementary file2 (MP4 30805 KB)
Supplementary file3 (MP4 29184 KB)
Supplementary file4 (MP4 28881 KB)
Supplementary file5 (MP4 51998 KB)
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Staines, M.N., Smith, C.E., Madden Hof, C.A. et al. Operational sex ratio estimated from drone surveys for a species threatened by climate warming. Mar Biol 169, 152 (2022). https://doi.org/10.1007/s00227-022-04141-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04141-9