Abstract
Recruitment to the Faroe Plateau cod (Gadus morhua) stock (62°N, 6.8°W) is highly variable between years, and availability of suitable prey during the early life stages may determine the year class strength. Here we first investigate interannual variability in recruitment (1983–2017) of cod based on survey data and data from stock assessments. Positive relationships between the spawning stock biomass and larval abundance and between juvenile abundance and recruitment at year 1 were detected. However, the relationship between larval abundance and juvenile abundance was not statistically significant, indicating variable survival during the larval/early juvenile stages. We then investigated if food availability at the larval and early juvenile stages could explain the variability in recruitment using an 8-year long time series of larval and juvenile gut content. Feeding at the early larval stage, present during the pre-bloom, was relatively stable between years. However, large interannual variability in feeding at the early juvenile stage was detected. When juvenile survival and mean length were high, juveniles clearly had less food in their guts. This contradiction is suggested to be due to intense grazing pressure on the zooplankton at high juvenile abundances. However, years with high larval survival coincided with years when the phytoplankton spring bloom was early and intense, suggesting that production of suitable prey during the larval stages determines larval growth and survival and that this production is dependent on the phytoplankton spring bloom characteristics.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atlantic cod (Gadus morhua) is a keystone fish species ecologically and commercially in most shelf ecosystems in the North Atlantic. Similar to other cod stocks, the Faroe Plateau cod stock shows large fluctuations in year class strength and recruitment (Brander 2007; Steingrund et al. 2010). The reproductive capacity of cod is related to the spawning stock biomass (SSB), although the relationship between recruitment and the SBB is weak for most cod stocks, including Faroe Plateau cod (ICES 2020). Understanding and predicting recruitment variability is a major unsolved challenge, but prey availability and feeding conditions during the larval and early juvenile stages are generally considered to be a major factor influencing survival of the offspring and driver of recruitment variability (Hjort 1914; Anderson 1988; Cushing 1990).
Cod reproduction has evolved to ensure that spawning and larval development take place when and where conditions are favorable for larval growth, survival and ultimately recruitment (Cushing 1990). Spawning of Faroe Plateau cod takes place over a 2–3 month period usually in February–April (i.e., during the pre-bloom) to the north and west of the islands at depths between 80 and 150 m (Ottosen et al. 2018). The eggs and larvae are dispersed in an anticyclonic direction around the islands, and are retained on the inner shelf by the tidal front situated at approximately 130 m bottom depth (Gaard and Steingrund 2001; Larsen et al. 2008). The majority of larvae start feeding in April.
Mortality during the early stages is very high. Mortality is expected to be inversely related to growth, since large larvae are less vulnerable to, e.g., predation (Anderson 1988). Growth is mainly dependent on the availability of food. For fish larvae and juveniles gape size is a major constraint during feeding (Munk 1997). As such, cod larvae and juveniles utilize a succession of zooplankton prey species and size ranges as they grow, and hence timing in the seasonal succession of zooplankton prey is a key to successful growth and endurance of the fish. In addition to mere food abundance, many studies highlight the importance of prey quality, e.g. species and size composition for survival (e.g., Beaugrand et al. 2003; Robert et al. 2011; Swalethorp et al. 2014; Jacobsen et al. 2020).
A synthesis of large-scale patterns in the planktonic prey of larval and juvenile Atlantic cod has shown that early cod larvae (see Fig. 1 for definition of cod developmental stages) in the northern part of the latitudinal range of cod prefer eggs and nauplii from the species Calanus finmarchicus, while early juveniles prefer late-stage C. finmarchicus copepodites (Heath and Lough 2007). Pseudocalanus sp. copepodites are often selected by late larvae (Kane 1984; Munk 1997; Rowlands et al. 2008; McLaren and Avendaño 2011; Robert et al. 2011; Swalethorp et al. 2014). The prey settings for fish larvae inhabiting the inner Faroe shelf during spring and summer is a mixture of neritic zooplankton species with variable influence from the oceanic environment (Gaard 1999; Jacobsen et al. 2018), and a recent study of the diet and prey preferences of larval and pelagic juvenile Faroe Plateau cod largely concurs with the synthesis of Heath and Lough (2007) (Jacobsen et al. 2020). That study showed how the diet changes through ontogenetic development and revealed positive selection towards certain species, i.e. copepod eggs and calanoid nauplii → Acartia sp. and Pseudocalanus sp. → late stage C. finmarchicus → decapod larvae and negative selection towards others, i.e. Oithona sp. and barnacle larvae, even when the prey were the same size. However, there appeared to be constraints in feeding at both the late larval stage and in juveniles as the mean size of prey in the guts was much smaller than the preferred size of prey. These constraints may ultimately lead to reduced food intake, survival and recruitment.
For Faroe Plateau cod, there is a positive relationship between the survey based 0-group (hereafter termed juvenile) index and subsequent recruitment, although it is stronger in some periods than others; e.g. strong in the 1980s and weak in 1990s (Jákupsstovu and Reinert 1994; Steingrund et al. 2010).The juvenile indices, i.e. length and abundance are highly correlated, suggesting that survival is growth dependent as described in the growth-mortality hypothesis (Anderson 1988). Furthermore, there is a positive relationship between cod juvenile indices and the local Primary Production Index (PPI) (Jacobsen et al. 2019) indicating that food availability to larvae and juveniles is critical for cod growth and survival. The causal mechanism is most likely a positive relationship between phytoplankton production and zooplankton reproduction affecting both growth and survival of larvae positively (Jacobsen et al. 2019). However, there is a negative relationship between the PPI and the zooplankton standing stock during summer (Gaard 2003; Hansen et al. 2005). Thus, the zooplankton standing stock does not necessarily reflect the productivity. Instead, it has recently been suggested that the negative relationship between the PPI and the zooplankton standing stock is generated by heavy grazing by juvenile fish on the summer zooplankton when the productivity is high (Jacobsen et al. 2019). In addition to fish juveniles, adult sand eel seems to be the only significant predator on the spring and summer zooplankton in the Faroe shelf ecosystem. However, due to large predation by, e.g., adult cod, haddock and many seabird species (Gaard et al. 2002), the biomass of adult sand eel is usually much smaller than the biomass of fish juveniles.
Despite the potential importance of larval and juvenile feeding for subsequent recruitment, little is known about interannual differences in feeding and when critical periods relating to survival occur. The first feeding period has been heralded as the most important for successful recruitment (Hjort 1914), but also the timing of metamorphosis from larvae to juveniles has drawn attention as critical, since larvae at this time undergo extensive physiological and morphological changes and consequently have relatively high energy requirements (Fig. 1) (Thompson and Harrop 1991; Thorisson 1994). To improve our understanding of the recruitment variability in Faroe Plateau cod, the aim of this study is to examine relationships between prey species availability at the early life stages and interannual variability in recruitment. After examining the relationship between larval abundance, juvenile abundance and subsequent recruitment, we scrutinise a time series of larval and juvenile gut content for interannual differences in feeding and compare these with variability in the environmental prey availability.
Materials and methods
Cod stage data
The ICES North Western Working Group (NWWG) annually undertakes an assessment of the size of the Faroe Plateau cod stock (ICES 2020). The data regarding the spawning stock biomass and recruitment of 1-year olds shown in this study are given as perceived by an age disaggregated stock assessment performed by the stock assessment program SAM (Nielsen and Berg 2014). Even though these stock assessment results extend back to 1959, we only use data from 1983 to 2017. This choice is based on the fact that the 0-group juvenile surveys were commenced in 1983 and the most recent recruitment estimates (i.e. post-2017) are subject to high uncertainties.
Variations in stock structure and condition of the adults can affect the level of egg production and hence recruitment (Scott et al. 1999). Therefore, the egg production of Faroe Plateau cod was calculated from the numbers at age given by the ICES assessment of the size of the spawning stock and a wet-weight based fecundity relationship from the North Sea (Oosthuizen and Daan 1974).
Larval abundance 1997–2016 was assessed from a total of 326 stations sampled inside the 130 m bottom depth contour in late April. The larvae were collected in the upper 50 m with a Bongo net with a mesh size of 100 µm as described in Jacobsen et al. 2020. The net was equipped with a flow meter to measure the volume of the filtered water. Total larval abundance was estimated assuming a total size of the shelf ecosystem of 10,000 km2.
Juvenile total abundance and mean length was based on approximately 100 sampling stations covering the Faroe Plateau every year in the second half of June 1983–2017. Details on sampling procedures can be found in Jacobsen et al. 2020. Juvenile cod length and abundance are positively correlated (Jacobsen et al. 2019), but the abundance is more subject to uncertainties due to, e.g. patchiness during sampling. In addition, when the mean length is high (> 30 mm) a portion of the cod may already have settled potentially leading to lower catches.
Cod larva and juvenile gut content
The gut content data and zooplankton data used in this study are the same material as was used in Jacobsen et al. 2020. While the previous paper focused on the general diet and prey preferences of pelagic Faroe Plateau cod larvae and juveniles, this paper scrutinises the data for interannual differences in feeding.
In total, 4984 guts (3634 in April and 1350 in June) were examined from 200 stations (108 in April and 92 in June) (Table 1), of which approximately half have relating zooplankton data. All samples were collected inside the 130 m bottom depth contour, i.e. in the horizontally and vertically well-mixed central shelf (Larsen et al. 2008). Sampling was only done during the daytime between 6:30 and 18:30. In April, fish larvae and zooplankton were collected with a Bongo net (diameter 0.6 m) with a mesh size of 100 µm. In June, fish larvae/juveniles were collected with a pelagic trawl with 5 mm mesh size and a mouth opening of about 8 m (horizontally) times 4–5 m (vertically), while the zooplankton was collected with a 200 µm WP-2 ring net. For more details on data collection and sample handling please refer to Jacobsen et al. 2020.
Data analyses
Relationships between different cod life stages were examined in order to identify transitions where the correlation in the abundance of successive life stages brake down, which would indicate variable interannual survival. Correlations between life stages were calculated using Spearman Rank correlation tests.
After establishing a lack of correlation between the larval and the juvenile stage, and a positive relationship between juvenile abundance and subsequent recruitment, we investigated if larval and juvenile survival and growth were affected by the prey availability. Prey availability was measured indirectly as environmental zooplankton prey biomass, and directly as number, mass and composition of prey in larval and juvenile guts. An estimate of instantaneous mortality rate per day (z) of the offspring from the sampling in late April (early stage larvae) to the sampling in late June was calculated using the estimated abundances and the number of days between the mid-points of the two cruises:
z = (ln(abundance in April)-ln(abundance in June))/number of days between the cruises.
While the zooplankton samples contained many species, including meroplankton such as cirripedia larvae and cyclopoid and harpacticoid copepods, we focused our analysis on zooplankton species that are the main components in the cod larvae and juveniles diet (Jacobsen et al. 2020). Zooplankton data were log10(x + 1) transformed prior to any statistical analyses to meet assumptions of normality and homogeneity. Analysis of variance (ANOVA) was used to determine if there was variation in zooplankton densities among years and sampling times.
Log-transformed larval and juvenile mean lengths were examined for interannual differences using linear mixed models with Trawl stations as random factor. Significance of the Year fixed factor was tested by comparing models with and without Year included using likelihood-ratio tests.
To evaluate the importance of the food items we applied the index of relative importance (IRI) (Pinkas et al. 1971):
where N percentage abundance of prey, V percentage weight of prey and F percentage occurrence in the gut. The IRI was calculated for cod in length intervals of 5 mm and presented as yearly plots. To assess interannual differences in total stomach content, we used Kruskal–Wallis tests and Wilcoxon signed-rank tests.
Remaining interannual comparisons of gut content were done on early stage larvae, i.e., 5–8 mm in length in April (n = 1357) and early juveniles 20–30 mm in June (n = 878). Comparisons on late larvae (9–19 mm) and late juveniles (≥ 31 mm) were not applied, because of a low number of observations.
Heat maps with dendrograms of scaled gut content were produced to identify interannual disparity and similarity in the diet components using the heatmap.2 function in R (R Core Team 2020). All other statistical analyses were also done using the statistical program R (R Core Team 2020).
Results
The SSB of Faroe Plateau cod peaked at 125 kt in 1984, but then collapsed to below 25 kt in the early 1990s (Fig. 2a). The stock recovered rapidly in the mid 1990s and early 2000s, but then collapsed again around 2005 and has been historically low since. The recruitment, on the other hand, is characterized by peaks in 1983, 1987, 1993, 1999, 2008 and 2016 (Fig. 2e). The average recruitment/SSB ratio from 1983 to 2017 was ~ 0.4 (range 0.05–1.65).
Changes in a spawning stock biomass, b estimated egg production, c larval abundance in April, d juvenile abundance (filled circles) and length (open circles) in June and e recruitment at age 1 (advanced 1 year to align with the 0-group) of Faroe Plateau cod for the period 1983–2017. The shaded yellow area highlights the gut content study period
The number of eggs produced was significantly positively related to the SSB. Furthermore, larval abundance in late April was significantly positively related to the egg production as well as the SSB (Table 2). However, the relationship between the abundance of larvae in April and juveniles in June was not statistically significant (Fig. 2c, d, Table 2). Recruitment at age 1 was significantly positively correlated to juvenile abundance the previous year (Fig. 2d, e, Table 2), but was not significantly correlated to the SSB (Table 2).
Over the entire time series, the ratio of juvenile abundance (June survey) to larval abundance (April survey), which is an estimate of survival, varied by a factor 552. This translated into annual daily instantaneous mortality between late April and late June ranging from 0.098 to 0.203 (Fig. 3a). Moreover, there was a strong negative relationship between mean juvenile length in late June and daily instantaneous mortality (Fig. 3b).
a Daily instantaneous mortality of cod larvae and juveniles between the April and June cruises. b Relationship between cod juvenile daily instantaneous mortality and mean annual juvenile length in June [z = − 0.114*ln(L) + 0.5181], Pearson’s R = 0.79. Yellow dots are years covered by gut content analyses
In order to investigate if feeding conditions at the early life stages affect juvenile indices and subsequent recruitment, a detailed gut content study of larvae (sampled in April) and pelagic juveniles (sampled in June) during the years 1998–2005, i.e., a period covering both high and low larval survival (Fig. 3a) and recruitment (Fig. 2e), is presented.
The dry weight (hereafter termed biomass for the ease of the reader) of zooplankton species that are the main components in the cod larvae and juvenile diet is shown in Fig. 4. A one-way ANOVA indicated significant differences in mean zooplankton biomass between years in both April and June for most cod larva and juvenile prey groups (Table S1). An exception to this was the biomass of copepod eggs in April (Fig. 4a, Table S1). The mean biomass of calanoid nauplii and the medium-sized copepods Acartia sp., Pseudocalanus sp. and early stage C. finmarchicus in April was significantly different between years (Table S1) with exceptionally high values in year 2000 (Fig. 4b). The biomass of the largest zooplankton items T. longicornis, late stage C. finmarchicus and decapod larvae; the preferred prey of early and late cod juveniles, varied by a factor 2 in April and a factor 15 in June with very low values in 2000 and 2001 and high values in 2002–2005 (Fig. 4c).
Temporal development in the environmental biomass of 8 zooplankton prey groups on the inner Faroe shelf. a small prey: Copepod eggs and calanoid nauplii, b medium-sized prey: Early stage C. finmarchicus, Pseudocalanus sp. and Acartia sp., and c large prey: T. longicornis, late stage C. finmarchicus and decapod larvae. Zooplankton samples in June were collected with a 200 µm net, hence there is no data available for the smallest zooplankton prey groups, i.e. calanoid nauplii and copepod eggs in June. Additional data gaps are indicated by ×
Linear mixed models with Year (fixed) and Trawl station (random) were used to investigate interannual differences in the lengths of larvae and juveniles used for gut content analysis. A likelihood-ratio test indicated that the model including Year provided a better fit for the data than a model without Year for both the larval (χ2(6) = 81.3, P < 0.01) and juvenile (χ2(5) = 104.7, P < 0.01) model. The estimates of the year effect showed that juvenile mean length in June was larger in year 2000 and 2001 compared with 2002–2005 (Table 1, Fig. 2d). However, this characteristic high-low pattern in cod length was not evident in larvae in April (Table 1). In April, the mean length of cod larvae (sampled for gut analyses) was largest in 2003 and 2001 (Table 1).
Total prey biomass in the guts of early larvae (5–8 mm) in April fluctuated among years, but with no clear temporal pattern (Fig. 5a). In addition, the interannual variability in total gut content in early larvae did not seem to reflect the biomass of available prey (Fig. 4). Overall, the difference in total gut content between years in early larvae in April was significant (Kruskal–Wallis test, H6 = 76.71, P < 0.01), but no single year was clearly distinguishable from the other years (Wilcoxon signed-rank test, P > 0.05). In early juveniles (20–30 mm) in June, the total gut content appeared to mimic the environmental biomass of prey, with a very low value in 2000 and to a lesser extent also in 2001 and higher values in 2002–2005 (Fig. 5b). Interannual differences in total gut content in early juveniles in June were significant (Kruskal–Wallis test, H5 = 132.83, P < 0.01) with particularly low values in year 2000 and high values in 2004 compared to the other years (Wilcoxon signed-rank test, P < 0.01).
The composition of prey in the guts of the cod larvae and juveniles (Fig. 6) reflected the environmental biomass of preferred prey (Fig. 4). While the relative fraction of copepod eggs to calanoid nauplii was approximately 50/50 in yolk-sac and early stage larvae in 1998–1999 and 2001–2005, larvae from year 2000 consumed a much higher proportion of calanoid nauplii. In the two years (2000 and 2001) with high juvenile survival (Fig. 3a) and length (Fig. 2d), early juveniles in late June consumed much less of late stage C. finmarchicus than the 2002–2005 cohorts (Fig. 6), most likely because they were almost absent from the shelf (Fig. 4c).
The relative importance of 11 of the most common prey groups expressed by the index of relative importance in pelagic cod sampled on the inner Faroe shelf 1998–2005. Numbers refer to number of observations in each cod length interval with prey in their guts. Vertical black lines indicate shifts in cod developmental stages. The detailed analysis in the remainder of the study concerns the early larva and early juvenile developmental stages, which across years were the most abundant life stages
Heat maps were produced to identify yearly patterns in species composition of the diet with potential links to survival. Heat map analyses of the gut content revealed two clusters of reoccurring yearly patterns in early larvae in April and early juveniles in June, respectively (Fig. 7). In April, year 1999 was separated from the other years. Year 1999 diet composition was characterized by elevated biomass of early stage C. finmarchicus copepodites and reduced biomass of the preferred calanoid nauplii (Fig. 7a). The mortality from larvae to juveniles in 1999 was the lowest observed in the times-series covered by the stomach content analysis (0.113 day−1, Fig. 3a). The second year partitioned in the cluster analyses was 2001 in which Acartia sp., Pseudocalanus sp and copepod eggs were important in the diet. The mortality in 2001 was intermediate at 0.130 day−1. The remaining years when mortality varied between 0.115 and 0.169 day−1 were not clearly separated, hence there were no clear indications of a relationship between diet composition in the larvae and survival.
Heat map and dendrogram showing yearly feeding patterns in a early larvae in April and b early juveniles in June. Scaled biomass of 6 of the most important prey items in the cod guts are in the columns. Low prey biomass is represented in blue and high biomass is in red. Rows are sampling years and font size is scaled to survival of larvae to the juvenile stage (refer to Fig. 3). Similarities (clustering) between prey groups and years are based on Euclidian distance. Black boxes highlight extreme values
In June, years 2000 and 2001 were clearly different from the other years with reduced gut biomass of C. finmarchicus and Pseudocalanus sp. (Fig. 7b). These were also the years when the cod larvae experienced the lowest mortality, 0.127 and 0.130 respectively, in the times series covered by the juvenile stomach content analyses. 2002 and 2003 grouped and in both years early stage C. finmarchicus copepodites were important. Similarly, 2004 and 2005 grouped and their diets had a high contribution of late stage C. finmarchicus copepodites. The years 2003–2005 were characterised by high mortality (0.145–0.169 day−1). No estimate of mortality was calculated for 2002 due to missing larval abundance data.
Discussion
When is interannual variation in recruitment generated?
The analysis of the time series of abundance of the different cod life stages showed that recruitment is not significantly related to the size of the SSB. Hence, variable survival occurs after spawning, that is not related to the size of the SSB. This is similar to many other cod stocks (ICES 2020), as well as other species of fish (Peterman et al. 1988; Nash and Dickey-Collas 2005). However, there was a significant positive relationship between the egg and larval abundance in April and the size of the SSB, underscoring the importance of having a healthy sized SSB. Despite of this, large variation in instantaneous mortality from late April to late June and hence a low correlation (R2 ~ 0.16) between larval abundance in April and juvenile abundance in June indicates a critical survival period at the larval and early juvenile stage. Highly variable survival during the larval stages has also been reported in, e.g. Arcto–Norwegian cod, Baltic cod, Icelandic cod and North Sea cod (Sundby et al. 1989; Köster et al. 2003; Beaugrand et al. 2003; Jonasson et al. 2009). In Arcto–Norwegian cod there is no correlation between egg abundance and recruitment. However, after the first 2–3 months of life, the correlation between larval/juvenile abundance and recruitment increases dramatically to R2 ~ 0.7 (Sundby et al. 1989). Similar results have been reported for Baltic cod (Köster et al. 2003) and in other fish species like walleye pollock in the western Gulf of Alaska, North Sea herring and Northern anchovy (Peterman et al. 1988; Bailey and Spring 1992; Nash and Dickey-Collas 2005).
Survival of Faroe Plateau cod larvae to the juvenile stage appears food and growth dependent, since mortality is negatively related to juvenile mean length. To what extent this variation is driven by subtle variations in mortality acting over the entire period between the two annual sampling times or is driven by more episodic mortality events is not clear from the present analysis (Houde 1989). It has previously been suggested that juvenile growth and survival is bottom-up regulated through food availability during spring, but that a high juvenile abundance may put top-down pressure on the zooplankton biomass during summer (Jacobsen et al. 2019). Our study of the cod larva and juvenile gut content has provided additional support for these hypotheses. However, from the gut content time series the critical timing related to survival does not appear to be during the first-feeding period in April, nor during the early juvenile period in June, but somewhere in between the two sampling times. This is discussed below.
Feeding conditions during the pre-bloom in April
The critical period in Faroe Plateau cod larval survival is most likely not during the initial phase, i.e. the first-feeding period in April as larval abundance reflected the SSB and the overall interannual variability in feeding was low at this stage. Furthermore, cod larvae in 1999 experienced poor feeding conditions in April, but still had very high recruitment at age 1.
Variability in larval cod length, which could be an indicator of the feeding conditions in April, was significantly different between years. However, there was up to a week’s difference in sampling time in April, which at this early stage could have a significant impact on the mean size of the larvae. In addition, the cruise in April is placed shortly after the peak spawning of cod. Therefore, the condition of the spawning females, i.e. size and condition factor probably has influence on the mean length of larvae in April as seen in, e.g. Iceland cod (Marteinsdóttir and Steinarsson 1998). Furthermore, the length of larvae in April is most likely closely related to the age of the larvae, i.e. the peak in time of spawning, which can vary by up to a month and is related to the ambient temperature (Ottosen et al. 2018) as well as female condition (Kjesbu 1994).
The environmental availability of copepod eggs and calanoid nauplii, which are preferred prey of early stage larvae abundant in April (Jacobsen et al. 2020), was lowest in 1999 and highest in 2000. Cluster analysis separated year 1999 from the other years. Larvae in 1999 contained more of large prey, i.e. early stage C. finmarchicus, which they usually select against, and less of the smaller prey, which they usually prefer (Jacobsen et al. 2020). The cohort from 1999 had the highest proportion of empty guts and had the lowest mean weight of prey in their guts compared with fish from the other years, indicating that larvae in April 1999 suffered from suboptimal feeding conditions. This is a conundrum, since survival to the juvenile stage and subsequent recruitment was very high in 1999.
On the other end of the spectrum, the larval cohort from year 2000 consumed a much higher proportion of calanoid nauplii compared with all the other cohorts, indicating favourable feeding conditions this particular year. It is known that cod larvae prefer late-stage nauplii to early stage nauplii (Swalethorp et al. 2014; Jacobsen et al. 2020), most likely because late-stage nauplii are more nutritious (Jung-Madsen et al. 2013). However, late-stage nauplii require food in order to survive (Hirche et al. 2001). An inspection of the seasonal progression in chlorophyll concentration on the Faroe shelf shows that out of the 8 years studied only in year 2000 had the spring bloom already begun at the time of sampling in April (Fig. 8). Therefore, we believe that the high consumption of nauplii this year is due to a higher proportion of late stage, nutritious nauplii this year compared with the other years.
Seasonal development in chlorophyll on the inner Faroe shelf 1998–2005 (from Debes et al. 2008a). Years with an early and intensive bloom are in green, while years with a late or low bloom are in grey. The shaded yellow areas show cod larvae and juvenile sampling times. The approximate timing of cod development stages is indicated below the x-axis. No chlorophyll sampling was done in early and mid April 2000
Although the data indicate poor feeding conditions in 1999 and good conditions in 2000, differences in feeding in April were generally small. This may be due to a low interannual variability in the environmental biomass of copepod eggs, one of the main prey for first feeding Faroe Plateau cod larvae. The abundance of copepod eggs in late April is mainly a result of pre-bloom egg production by the oceanic copepod C. finmarchicus, and this production is supported by their winter lipid reserves (Madsen et al. 2008), albeit primary production will also positively influence the egg production (Gaard 2000; Debes et al. 2008b). Previous studies have suggested that the production and abundance of C. finmarchicus eggs is relatively high in the Faroe area (Stenevik et al. 2007), indicating that newly spawned Faroe Plateau cod larvae in most cases have sufficient food resources during the first feeding period. However, phenological changes resulting in earlier emergence of C. finmarchicus have been observed in and around the Faroe area after the gut content study was conducted (Kristiansen et al. 2016; Jacobsen et al. 2018), and recent research has revealed that there has been a marked decline in C. finmarchicus pre-bloom egg production on the Faroe shelf after 2007 (Jacobsen et al. 2022). Thus the feeding conditions of first-feeding cod larvae might have changed in recent years.
Feeding conditions during the bloom in June
The environmental availability of late stage C. finmarchicus, which are preferred prey of early stage cod juveniles abundant in June, was very low in 2000–2001 and high in 2002–2005. That means that there was an inverse relationship between the environmental biomass of late stage C. finmarchicus in June and cod larval survival. When there was a high environmental availability of late stage C. finmarchicus as in 2002–2005, it was clearly reflected in the cod guts. However, in 2000 and 2001 late stage C. finmarchicus were virtually absent from the shelf, forcing the cod juveniles to turn to other prey species. In addition, the total gut content of cod juveniles was much lower in 2000 and 2001 compared with the other years, clearly suggesting poor feeding conditions.
Heath (2007) found that consumption by fish larvae during the first 3 months of life (from hatching to metamorphosis) can cause a significant impact on the zooplankton population in the North Sea. Furthermore, Cushing (1983) notes that abundant fish larvae are probably too dilute during the early larval phases to affect their food, but as the larvae grow this tendency is reversed and larvae progressively have more and more effect on their food. At the time of metamorphosis, larvae might produce a prey mortality severe enough to generate larval density-dependent growth (Cushing 1983).
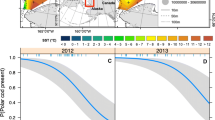
In our case, we believe that the contradiction between the apparently poor feeding conditions in June and increased cod juvenile length and abundance is best explained as follows: a temporal match between zooplankton and fish larval abundances during spring leads to increased growth and survival of fish larvae from spring spawning species including cod (Jacobsen et al. 2019). Indeed, the development in chlorophyll concentration (Fig. 8) indicates that there was a temporal match between the larvae and their prey in 2000 and 2001, and a comparison between the index of primary production on the Faroe shelf (Gaard 2003; Jacobsen et al. 2019) and the larval survival rates supports a bottom-up effect during spring, as there is a tight coupling between the two variables (Fig. 9). However, as the fish larvae grow, the increased biomass of larvae/juveniles eventually gives rise to intense top-down pressure on the summer (i.e., June) zooplankton. Since the juveniles are also larger than average in years with high abundance, possible competition for food has probably not been going on for long prior to the June cruise.
Relationship between daily instantaneous mortality from the cod larva to the cod juvenile stage and the local Primary Production Index (from Jacobsen et al. 2019) [z = − 0.058*ln(PPI) + 0.2696], Pearson’s R = 0.73. Yellow dots are years covered by gut content analyses
The grazing down of zooplankton by fish larvae could potentially lead to reduced growth or survival of cod juveniles later in life. However, that appears not to be the case as shown by the positive correlation between juvenile indices and subsequent recruitment. This is most likely because when the cod juveniles reach approximately 4 cm they settle to the bottom, stop feeding on zooplankton and target benthic prey instead.
When there was a shortcoming of late stage C. finmarchicus early juveniles turned to other prey species. In year 2000 late stage C. finmarchicus were replaced by a variety of smaller food items especially T. longicornis, while in 2001 they were replaced by larger food items, i.e. decapod larvae and small fish larvae. Although prey size and species has an effect on growth and survival of cod larvae (e.g., Beaugrand et al. 2003; Robert et al. 2011; Swalethorp et al. 2014), cod are opportunistic predators and prey abundance probably has more influence than prey species and size, especially in large larvae/juveniles (Seljeset et al. 2010).
In accordance with many with other studies our study shows, that year class failures can originate in the larval stage and that high survival during these stages is a necessary, but not always sufficient contribution to recruitment success. In addition to the highly variable survival during the pelagic larval and juvenile stage, recruitment to the Faroe Plateau cod stock is influenced by cannibalism from adult cod, shortly after settling, which may set the final recruitment level (Steingrund et al. 2010).
Conclusion
We here show that the spawning stock biomass of Faroe Plateau cod determines the initial abundance of larvae on the Faroe shelf. However, a critical period affecting survival and subsequent recruitment in Faroe Plateau cod occurs at the pelagic stage between the early larval stage and the early juvenile stage. Variable feeding conditions are most likely the cause of the variable survival as the survival appears growth dependent. Investigations of larval and juvenile gut content show, that the biomass of preferred prey in the environment is reflected in the guts of the larvae and juveniles. In most cases, first-feeding larvae experience pre-bloom conditions. Still, newly spawned larvae appear (for the most part) to have sufficient food, since the production of suitable prey (i.e. Calanus finmarchicus eggs) during the first-feeding period is, at least partially, independent of the phytoplankton spring bloom. Juvenile mean length and abundance are, however, clearly positively related to the timing and intensity of the local phytoplankton spring bloom. Nonetheless, there is a negative relationship between concurrent prey abundances and juvenile indices, indicating that large fish larvae/juveniles may have a significant top-down effect on their food during summer. In our time series, there is no relationship between the size of the SSB and recruitment, however, for the cod stocks south of 62oN, environmental variability may have an increased effect on recruitment, when the SSB is small (Brander 2005). This is of concern, since the SSB of the Faroe Plateau cod stock has been historically low since 2006 (ICES 2020). Hence, feeding conditions in the early life stages of Faroe Plateau cod may have been even more important for survival in the recent years than in the period reported in this paper.
Data availability
The datasets generated during and/or analysed during the current study are available from the corresponding author on reasonable request.
References
Anderson JT (1988) A review of size dependent survival during pre-recruit stages of fishes in relation to recruitment. J Northwest Atl Fish Sci 8:55–66. https://doi.org/10.2960/J.v8.a6
Bailey KM, Spring SM (1992) Comparison of larval, age-0 juvenile and age-2 recruit abundance indices of walleye pollock, Theragra chalcogramma, in the western gulf of Alaska. ICES J Mar Sci 49:297–304. https://doi.org/10.1093/icesjms/49.3.297
Beaugrand G, Brander KM, Lindley JA, Souissi S, Reid PC (2003) Plankton effect on cod recruitment in the North Sea. Nature 426:661–664. https://doi.org/10.1038/nature02164
Brander KM (2005) Cod recruitment is strongly affected by climate when stock biomass is low. ICES J Mar Sci 62:339–343. https://doi.org/10.1016/j.icesjms.2004.07.029
Brander KM (2007) The role of growth changes in the decline and recovery of North Atlantic cod stocks since 1970. ICES J Mar Sci 64:211–217. https://doi.org/10.1093/icesjms/fsl021
Cushing DH (1983) Are fish larvae too dilute to affect the density of their food organisms? J Plankton Res 5:847–854. https://doi.org/10.1093/plankt/5.6.847
Cushing DH (1990) Plankton production and year-class strength in fish populations: an update of the match/mismatch hypothesis. Adv Mar Biol 26:249–293
Debes HH, Gaard E, Hansen B (2008a) Primary production on the Faroe Shelf: temporal variability and environmental influences. J Mar Syst 74:686–697. https://doi.org/10.1016/j.jmarsys.2008.07.004
Debes HH, Eliasen K, Gaard E (2008b) Seasonal variability in copepod ingestion and egg production on the Faroe Shelf. Hydrobiologia 600:247–265. https://doi.org/10.1007/s10750-007-9238-3
Gaard E (1999) The zooplankton community structure in relation to its biological and physical environment on the Faroe Shelf, 1989–1997. J Plankton Res 21:1133–1152. https://doi.org/10.1093/plankt/21.6.1133
Gaard E (2000) Seasonal abundance and development of Calanus finmarchicus in relation to phytoplankton and hydrography on the Faroe Shelf. ICES J Mar Sci 57:1605–1611. https://doi.org/10.1006/jmsc.2000.0963
Gaard E (2003) Plankton variability on the Faroe Shelf during the 1990s. ICES Mar Sci Symp 219:182–189
Gaard E, Steingrund P (2001) Reproduction of Faroe Plateau cod: spawning grounds, egg advection and larval feeding. Fróðskaparrit 48:87–103
Gaard E, Hansen B, Olsen B, Reinert J (2002) Ecological features and recent trends in the physical environment, plankton, fish stocks, and seabirds in the faroe shelf ecosystem. In: Sherman K, Skjoldal HR (eds) Large marine ecosystems in the North Atlantic. Elsevier, Amsterdam, pp 245–265. https://doi.org/10.1016/s1570-0461(02)80060-x
Hansen B, Eliasen SK, Gaard E, Larsen KMH (2005) Climatic effects on plankton and productivity on the Faroe Shelf. ICES J Mar Sci 62:1224–1232. https://doi.org/10.1016/j.icesjms.2005.04.014
Heath MR (2007) The consumption of zooplankton by early life stages of fish in the North Sea. ICES J Mar Sci 64:1650–1663
Heath MR, Lough RG (2007) A synthesis of large-scale patterns in the planktonic prey of larval and juvenile cod (Gadus morhua). Fish Oceanogr 16:169–185. https://doi.org/10.1111/j.1365-2419.2006.00423.x
Hirche HJ, Brey T, Niehoff B (2001) A high-frequency time series at ocean weather ship station M (Norwegian Sea): population dynamics of Calanus finmarchicus. Mar Ecol Prog Ser 219:205–219. https://doi.org/10.3354/meps219205
Hjort J (1914) Fluctuations in the great fisheries of northern Europe viewed in the light of biological research. Rapports Et ProcésVerbaux Des Réunions Du Conseil Permanent International Pour L’exploration De La Mer 20:228
Houde ED (1989) Subtleties and episodes in the early life of fishes. J Fish Biol 35:29–38. https://doi.org/10.1111/J.1095-8649.1989.TB03043.X
ICES (2020) Report of the North-Western working group (NWWG) 2020. ICES Sci Rep 2:52. https://doi.org/10.17895/ices.pub.6051
Jacobsen S, Gaard E, Larsen KMH, Eliasen SK, Hátún H (2018) Temporal and spatial variability of zooplankton on the Faroe Shelf in spring 1997–2016. J Mar Syst 177:28–38. https://doi.org/10.1016/j.jmarsys.2017.08.004
Jacobsen S, Gaard E, Hátún H, Steingrund P, Larsen KMH, Reinert J, Ólafsdóttir SR, Poulsen M, Vang HBM (2019) Environmentally driven ecological fluctuations on the Faroe Shelf revealed by fish juvenile surveys. Front Mar Sci 6:1–12. https://doi.org/10.3389/fmars.2019.00559
Jacobsen S, Nielsen KK, Kristiansen R, Grønkjær P, Gaard E, Steingrund P (2020) Diet and prey preferences of larval and pelagic juvenile Faroe Plateau cod (Gadus morhua). Mar Biol 167:122. https://doi.org/10.1007/s00227-020-03727-5
Jacobsen S, Gaard E, Hátún H (2022) Declining pre-bloom Calanus finmarchicus egg production adjacent two major overwintering regions in the Northeastern Atlantic. Front Mar Sci 9:1–11. https://doi.org/10.3389/fmars.2022.822978
Jákupsstovu SHÍ, Reinert J (1994) Fluctuations in the Faroe Plateau cod stock. ICES Mar Sci Symp 198:194–211
Jonasson JP, Gunnarsson B, Marteinsdóttir G (2009) Abundance and growth of larval and early juvenile cod (Gadus morhua) in relation to variable environmental conditions west of Iceland. Deep Sea Res Part II 56:1992–2000. https://doi.org/10.1016/j.dsr2.2008.11.010
Jung-Madsen S, Nielsen TG, Grønkjær P, Hansen BW, Møller EF (2013) Early development of Calanus hyperboreus nauplii: response to a changing ocean. Limnol Oceanogr 58:2109–2121. https://doi.org/10.4319/lo.2013.58.6.2109
Kane J (1984) The feeding habits of co-occurring cod and haddock larvae from Georges Bank. Mar Ecol Prog Ser 16:9–20. https://doi.org/10.3354/meps016009
Kjesbu OS (1994) Time of start of spawning in Atlantic cod (Gadus morhua) females in relation to vitellogenic oocyte diameter, temperature, fish length and condition. J Fish Biol 45:719–735. https://doi.org/10.1111/j.1095-8649.1994.tb00939.x
Köster FW, Hinrichsen H, Schnack D, John MAST, Mackenzie BR, Tomkiewicz J, Möllmann C, Kraus G, Plikshs M, Makarchouk A, Aro E (2003) Recruitment of Baltic cod and sprat stocks: identification of critical life stages and incorporation of environmental variability into stock-recruitment relationships. Sci Mar 67:129–154
Kristiansen I, Gaard E, Hátún H, Jónasdóttir SH, Ferreira SA (2016) Persistent shift of Calanus spp. in the southwestern Norwegian Sea since 2003, linked to ocean climate. ICES J Mar Sci 73:1319–1329. https://doi.org/10.1093/icesjms/fsv222
Larsen KMH, Hansen B, Svendsen H (2008) Faroe Shelf water. Cont Shelf Res 28:1754–1768. https://doi.org/10.1016/j.csr.2008.04.006
Madsen ML, Gaard E, Hansen BW (2008) Wax-ester mobilization by female Calanus finmarchicus (Gunnerus) during spring ascendance and advection to the Faroe Shelf. ICES J Mar Sci 65:1112–1121. https://doi.org/10.1093/icesjms/fsn097
Marteinsdottir G, Steinarsson A (1998) Maternal influence on the size and viability of Iceland cod Gadus morhua eggs and larvae. J Fish Biol 52:1241–1258. https://doi.org/10.1006/jfbi.1998.0670
McLaren IA, Avendaño P (2011) Prey field and diet of larval cod on Western bank, Scotian Shelf. Can J Fish Aquat Sci 52:448–463. https://doi.org/10.1139/f95-046a
Munk P (1997) Prey size spectra and prey availability of larval and small juvenile cod. J Fish Biol 51:340–351. https://doi.org/10.1111/j.1095-8649.1997.tb06107.x
Nash RDM, Dickey-Collas M (2005) The influence of life history dynamics and environment on the determination of year class strength in North Sea herring (Clupea harengus L.). Fish Oceanogr 14:279–291. https://doi.org/10.1111/j.1365-2419.2005.00336.x
Nielsen A, Berg CW (2014) Estimation of time-varying selectivity in stock assessments using state-space models. Fish Res 158:96–101. https://doi.org/10.1016/J.FISHRES.2014.01.014
Oosthuizen E, Daan N (1974) Egg fecundity and maturity of North Sea cod, Gadus morhua. Neth J Sea Res 8:378–397
Ottosen KM, Steingrund P, Magnussen E, Payne MR (2018) Distribution and timing of spawning Faroe Plateau cod in relation to warming spring temperatures. Fish Res 198:14–23
Peterman RM, Bradford M, Nancy C, Lo H, Methot RD (1988) Contribution of early life stages to interannual variability in recruitment. Can J Fish Aquat Sci 45:8–16
Pinkas L, Oliphante MS, Iverson ILK (1971) Food habits of albacore, bluefin tuna and bonito in California waters. Calif Fish Game 152:1–105
R Core Team (2020) R: a language and environment for statistical computing. R foundation for statistical computing, Vienna. R version 4.0.3. http://www.R-project.org/. Accessed 16 Oct 2020
Robert D, Levesque K, Gagne JA, Fortier L, Gagné JA, Fortier L (2011) Change in prey selectivity during the larval life of Atlantic cod in the southern Gulf of St Lawrence. J Plankton Res 33:195–200. https://doi.org/10.1093/plankt/fbq095
Rowlands WL, Dickey-Collas M, Geffen AJ, Nash RDM (2008) Diet overlap and prey selection through metamorphosis in Irish Sea cod (Gadus morhua), haddock (Melanogrammus aeglefinus), and whiting (Merlangius merlangus). Can J Fish Aquat Sci 65:1297–1306. https://doi.org/10.1139/F08-041
Scott B, Marteinsdottir G, Wright P (1999) Potential effects of maternal factors on spawning stock–recruitment relationships under varying fishing pressure. Can J Fish Aquat Sci 56:1882–1890. https://doi.org/10.1139/f99-125
Seljeset O, Vollset KW, Folkvord A, Geffen AJ (2010) The role of prey concentration and size range in the growth and survival of larval cod. Mar Biol Res 6:251–262. https://doi.org/10.1080/17451000903150355
Steingrund P, Mouritsen R, Reinert J, Gaard E, Hátún H (2010) Total stock size and cannibalism regulate recruitment in cod (Gadus morhua) on the Faroe Plateau. ICES J Mar Sci 67:111–124. https://doi.org/10.1093/icesjms/fsp240
Stenevik EK, Melle W, Gaard E, Gislason A, Broms CT, Prokopchuk I, Ellertsen B (2007) Egg production of Calanus finmarchicus—a basin-scale study. Deep Sea Res Part II 54:2672–2685. https://doi.org/10.1016/j.dsr2.2007.08.027
Sundby S, Bjørke H, Soldal AV, Olsen S (1989) Mortality rates during the early life stages and year-class strength of northeast Arctic cod (Gadus morhua L.). Rapports Et ProcésVerbaux Des Réunions Du Conseil Permanent International Pour L’exploration De La Mer 191:351–358
Swalethorp R, Kjellerup S, Malanski E, Munk P, Nielsen TG (2014) Feeding opportunities of larval and juvenile cod (Gadus morhua) in a Greenlandic fjord: temporal and spatial linkages between cod and their preferred prey. Mar Biol 161:2831–2846. https://doi.org/10.1007/s00227-014-2549-9
Thompson AB, Harrop RT (1991) Feeding dynamics of fish larvae on Copepoda in the western Irish Sea, with particular reference to cod Gadus morhua. Mar Ecol Prog Ser 68:213–223
Thorisson K (1994) Is metamorphosis a critical interval in the early life of marine fishes? Environ Biol Fishes 40:23–36. https://doi.org/10.1007/BF00002180
Acknowledgements
We thank Hjálmar Hátún and Anni Djurhuus for constructive discussion during the writing process, and we thank Dagunn H. J. Clementsen for providing the illustrations shown in Fig. 1 We are also grateful for the constructive comments made by the independent reviewers.
Funding
The making of this paper has been funded by STATOIL Faroes and the Faroese Research Council (Grant number 0443).
Author information
Authors and Affiliations
Contributions
This study was designed by KKN, RK, PG, EG and PS, the field work was carried out by EG, the data analysis was conducted by SJ, the story was composed by SJ with contribution from KKN, RK, PG, EG and PS, and SJ led the writing process.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
The authors declare that all applicable national and institutional guidelines for sampling, care and experimental use of organisms for the study have been followed and all necessary approvals were obtained.
Additional information
Responsible Editor: X. Irigoien.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Jacobsen, S., Nielsen, K.K., Kristiansen, R. et al. Influence of larval prey availability on year class strength in Faroe Plateau cod (Gadus morhua). Mar Biol 169, 153 (2022). https://doi.org/10.1007/s00227-022-04140-w
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04140-w