Abstract
Social hierarchies within groups define the distribution of resources and provide benefits that support the collective group or favor dominant members. The progression of individuals through social hierarchies is a valuable characteristic for quantifying population dynamics. On coral reefs, some clownfish maintain size-based hierarchical communities where individuals queue through social ranks. The cost of waiting in a lower-ranked position is outweighed by the reduced risk of eviction and mortality. The orange clownfish, Amphiprion percula, maintains stable social groups with subordinate individuals queuing to be part of the dominant breeding pair. Strong association with their host anemone, complex social interactions, and relatively low predation rates make them ideal model organisms to assess changes in group dynamics through time in their natural environment. Here, we investigate the rank changes and isometric growth rates of A. percula from 247 naturally occurring social groups in Kimbe Island, Papua New Guinea (5° 12′ 13.54″ S, 150° 22′ 32.69″ E). We used DNA profiling to assign and track individuals over eight years between 2011 and 2019. Over half of the individuals survived alongside two or three members of their original social group, with twelve breeding pairs persisting over the study period. Half of the surviving individuals increased in rank and experienced double the growth rate of those that maintained their rank. Examining rank change in a wild fish population provides new insights into the complex social hierarchies of reef fishes and their role in social evolution.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Social hierarchies help group-living animals distribute resources, including food, shelter, and mating privileges (Koski et al. 2015). Individuals are ranked by their position in the hierarchy, which is driven by physical and competitive fitness (e.g., size, strength, coloration), and how they contribute to the functioning of the social group (Koski et al. 2015). Hierarchies are typically structured according to the individuals’ size, age, or sex, and moving up in social rank increases an individual’s access to food (Forrester 1991), mates (Buston 2003a), and habitat (Thompson et al. 2007). Social rank often corresponds to feeding success rates, which are correlated to growth rates, and allows dominant individuals to remain well-fed where food is limited (Gurney and Nisbet 1979). Reef fish are a popular vertebrate model to study hierarchy and dominance since they have a clear social structure and can be kept easily in laboratory settings; for example, the coral-dwelling goby Paragobiodon xanthosomus exhibits a size-based hierarchy in which members form a queue to inherit breeding status from the dominant pair. Cooperation between subordinates regulates growth and maintains a size ratio between ranks to avoid conflict, punishment, or eviction from the social group (Wong et al. 2007). While studies have examined the causes of rank change and dominance in reef fish by analyzing growth rates (Wong et al. 2007, 2016; Reed et al. 2019), they are conducted as short-term experiments (Buston 2003a, 2003b; Buston and Cant 2006; Rueger et al. 2018). No studies have examined rank change and growth rates in fish over one year in a natural setting. Long-term studies aid in the understanding of population dynamics by providing large datasets that are used to predict future population changes, but large datasets can be challenging to obtain in the marine environment (Reinke et al. 2019).
Mating behavior is essential in driving, establishing, enforcing, and maintaining the social hierarchy. Sexual selection within hierarchical societies elucidates patterns of conflict within as opposed to between sexes. Wong and colleagues determined that food limitation and paternal egg care lead to competition among female coral gobies for males meaning they are less likely to mate with subordinate males, leading to monogamy within social groups (Wong et al. 2008). Most clownfish live in groups comprising one dominant breeding pair and several non-breeding subordinates where the female is the dominant individual. The species Amphiprion bicinctus and Amphiprion akallopisos have been shown to exhibit monogamy based on the individual recognition (Fricke 1973) and pair-bonding (Fricke 1974). Fricke and Fricke also found that several pairs of A. bicinctus remained on the same anemone without changing partners for at least three years (Fricke and Fricke 1977), revealing that social structures are relatively stable for females and males.
In this study, we focus on the orange clownfish, Amphiprion percula examining its rank change and growth over an eight-year time gap. The orange clownfish, A. percula (Lacepède, 1802), is one of 28 species of clownfish belonging to the subfamily Amphiprioninae within the family Pomacentridae. It is estimated to have a lifespan of 30 years (Buston and Garcia 2007) and is commonly associated with anemone hosts Heteractis magnifica and Stichodactyla gigantea (Fautin and Allen 1992). Anemone species may play an essential role in the growth and size of A. percula individuals. Previous studies found that, on average, females were 10% larger in H. magnifica compared to those living on S. gigantea, and individuals that settled on H. magnifica had a faster growth rate based on otolith increments (Salles et al. 2016). A recent study (Salis et al. 2021) concluded that juvenile A. percula delays the development of their white bars during metamorphosis depending on the anemone species they are hosted by, emphasizing the importance of anemone host species on a variety of biological factors. Additionally, anemone surface area significantly impacts the social structure, with larger anemones hosting larger social groups and individuals (Chausson et al. 2018), which may place additional pressure on resource sharing and individual growth.
Within social groups of A. percula, there is a strict size-based ratio between sequentially ranked individuals in which a dominant individual is approximately 1.26 times the size of its immediate subordinate (Buston 2003b). Similar to gobies, subordinates regulate their growth to maintain this ratio or are otherwise evicted or killed (Buston 2003b; Rueger et al. 2018). In theory, individuals who change rank grow faster due to the increase in the food they consume as they rise in status. This study aimed to examine rank changes and growth in naturally occurring social groups of A. percula clownfish over eight years on shallow coral reefs on Kimbe Island, Papua New Guinea. We hypothesize that individuals who change rank will grow faster than those who do not change rank, and those with higher rank will have better survivorship. During this time, individuals were exposed to natural levels of predation risk, food availability, and recruitment.
Materials and methods
Sample collection
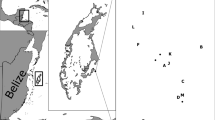
The Amphiprion percula population on Kimbe Island (5° 12′ 13.54″ S, 150° 22′ 32.69″ E), a remote island in Kimbe Bay, was sampled in March 2011 and April 2019. Each time, all individuals sized > 25 mm (n = 660 in 2011; n = 533 in 2019) were caught with hand nets, occasionally anesthetized with a dilute solution of clove oil (if necessary to facilitate capture), and measured to the nearest mm (total length, TL) (Fig. S1). Their host anemone species (S. gigantea and H. magnifica) were recorded and tagged with a cattle tag and buoy (Fig. 1). Due to the attrition of the tags on anemones, we could not confirm that all individual fish had remained on the exact anemone host between sampling times. For some fish, we can only confirm that they were found on the same species of anemone and general area based on GPS. To genotype all individuals, a tissue sample was taken from the caudal fin before they were released back onto their anemone host. The fin clip is regrown within approximately two weeks and has no adverse long-term effects on the fish. Samples were preserved in 96% ethanol. Rank was assigned based on the relative total lengths of individuals within each social group. Rank 1 was the largest individual and assumed to be the female; rank 2 was the second largest and assumed to be the male. Any additional individuals were classed as sub-adults.
A Visual of the study site, Kimbe Island (Papua New Guinea), and surrounding lagoon and reef habitats with tagged anemone locations (Heteractis magnifica in red and Stichodactyla gigantea in yellow). Kimbe Island = green, shallow (< 1 m) hard bottom substrate = gray, lagoons = light blue, and deeper ocean outside of the fringing reef habitat = dark blue. Host anemones Heteractis magnifica (B) and Stichodactyla gigantea (C) on the right. Aerial image credit to H.B.H., map courtesy U. Langner (RSRC), photographs credit to M.B.S
Sample processing and genotyping
Genomic DNA from A. percula fin clips ~ 2 mm2 was extracted using the Macherey–Nagel 96 tissue kits following the manufacturer’s protocols. Next, microsatellite loci were amplified using the QIAGEN Multiplex PCR kit following the protocol in Almany et al. (2017). Forward primers were labeled with fluorescent tags and pooled into four multiplex mixes indicated in Table S1. PCR products were analyzed on an ABI 3730 × 1 genetic analyzer (Applied Biosystems). Allele sizes for 20 microsatellite loci were successfully amplified and were manually scored in the fragment analysis software GENEMAPPER v4.0. Characteristics of the 20 microsatellite markers were analyzed in GenAlEx v6.5 (Peakall and Smouse 2012) and are presented in Table S2. Alleles were binned in R (R Core Team 2020) using the package MsatAllele (Alberto 2009).
Individuals that survived from 2011 to 2019 were identified in the R package AlleleMatch (Galpern et al. 2012). Only individuals classed as females, males, or sub-adults were included in this analysis (n = 660 in 2011 and n = 533 in 2019). A sensitivity analysis indicated that up to 8 genotypic mismatches could confidently identify identical genotypes within the sample.
Statistical analyses
We measured growth between 2011 and 2019 as the percent increase in body length between the two measurements. Variation in growth between anemone species, rank status, or change was assessed using generalized linear mixed models with a beta error distribution with a logit link in the R package glmmTMB (Friedman et al. 2010). The interactions between anemone species and rank were explored and found not to be important. Estimated marginal means and Tukey post hoc pairwise comparisons with 95% confidence intervals were calculated using the package emmeans (Lenth 2021) and plotted using the package ggplot2 (Wickham 2016). Model residuals were inspected in the DHARMa package (Hartig 2020) and checked for homogeneity of variance, dispersion, and outliers. Model selection was informed by AIC scores using the MuMIn package (Bartoń 2020). All analyses were performed in R version 4.0.2 (R Core Team 2020).
Results
In total, 134 of the 660 individuals (i.e., 20%) sampled in 2011 were recaptured in 2019, including 36 females, 52 males, and 46 sub-adults, and comprised 35 of the original 247 social groups from 2011. Of the 134 survivors, 72 individuals were confirmed to have been collected from the same anemone with other individuals from the same social group. We found no evidence of an individual changing anemone host over the eight years; the other 62 individuals were identified in anemones without a matching anemone ID tag between 2011 and 2019 but on the same species of anemone, at similar depths, in the same general location. We found that 60% of the 134 survivors were from anemone H. magnifica, and 40% were from S. gigantea.
Of the 134 survivors, 67 individuals (50%) maintained the same rank within their respective social groups, and 67 individuals (50%) changed social rank to become male or female (Fig. 2). Additionally, we identified 12 mating pairs that remained together since 2011, of which three remained with other individuals from their original social group. The host anemone, initial rank, and rank change strongly affected individuals’ growth between 2011 and 2019. Changes in rank had the strongest effect on growth resulting in a 26.8% (95% CI [24.5–29.1]) increase in growth for individuals that changed rank compared to 11.7% (95% CI [10.0–13.6]) in individuals that did not change rank, and the difference was significant between the two groups (p < 0.0001). The strongest effects were observed in sub-adults that became either male (27.0% growth, 95% CI [23.2–31.2]) or females (31.4% growth, 95% CI [26.4–36.9]) during the eight-year window (Table 1). Males that became females grew by 24.7% (95% CI [21.8–27.8]). Of the individuals that did not change rank, sub-adults grew fastest with 18.9% growth (95% CI [14.4–24.4]), faster than either females (10.8% growth, 95% CI [8.9–13.1]; p = 0.015) or males (10.0% growth, 95% CI [7.6–13.0]; p = 0.012). Anemone species also had a strong effect on growth; over eight years, individuals grew 15.7% on H. magnifica (95% CI [14.0–17.5]) compared to 23.1% on S. gigantea (95% CI [20.6–25.7]), and the difference was significant (p < 0.0001) (Fig. 3).
Discussion
Our study examined growth and rank change over eight years in a wild population of A. percula clownfish in Kimbe Island, Papua New Guinea. Tracking individuals in the marine environment over long periods can be extremely challenging. The close association of clownfish with their anemones provides a valuable model system to investigate the effects of rank change on growth, providing unique insights on survivorship, growth rates, rank changes, and the persistence of monogamous breeding pairs in coral reef fish. Our findings indicate strong survivorship of individuals over eight years, with 20% of individual resamples in 2019. Related studies of the same population but over ten years found that ~ 1% of the population had survived from the beginning to the end of the study (Salles et al. 2016). This suggests a complete population turnover after ten years which is short given the potential lifespan of 30 years for A. percula females (Buston and Garcia 2007).
Furthermore, we found that individuals that changed rank (i.e., sub-adult to male, sub-adult to female, and male to female) grew faster than those that did not change rank. Half of the surviving individuals changed rank and grew significantly more than those that did not change rank. This is likely not a standard growth, but opportunistic growth that occurs when a female or male is removed from the group since A. percula has been known to regulate or suppress their growth to decrease the likelihood of being evicted (Buston 2003b). A recent review by Buston and Clutton-Brock examined strategic growth in social vertebrates and concluded that clownfish species Amphiprion perideraion and Amphiprion frenatus females acquire dominant positions by increasing in length relative to similarly-sized individuals and that the increase in size is limited by the size of the anemone (Buston and Clutton-Brock 2022).
The 12 male and female pairs that persisted between 2011 and 2019 strongly support that A. percula is monogamous. This is consistent with previous studies of mating behavior in reef fish that are site-attached, whereby breeding pairs remain monogamous until one mate is physically removed from the social hierarchy. A review of monogamy in marine fishes included 18 families, and four clownfish species (A. bicinctus, Amphiprion clarkii, A. perideraion, and A. frenatus) identified A. percula as monogamous from observations only (Whiteman and Cote 2004). We can confirm from genetic analyses that 12 pairs have remained in the same host anemone over eight years and are likely reproducing. Monogamy and breeding stability are often hard to confirm in the field over long-term studies without the adequate genetic analysis of all individuals present, making this challenging in many other field study systems.
Additionally, we found that species of anemone host significantly affected growth in A. percula. All individuals hosted by H. magnifica grew slower than those hosted by S. gigantea. However, previous research on early settlement in Kimbe Bay (Salles et al. 2016) found that individuals that settled on H. magnifica grew faster than those on S. gigantea. This may indicate a selective advantage on growth in H. magnifica for post-settlement recruits that are lost in subsequent social ranks. Since anemone surface area is positively correlated to the size of the individuals’ (Chausson et al. 2018), the faster growth rates are likely explained by the larger surface area provided by H. magnifica compared to S. gigantea as observed in (Salles et al. 2016). However, this effect may also be confounded by other environmental factors associated with the different habitats occupied by each anemone. Heteractis magnifica are found further offshore and in deeper habitats compared to S. gigantea anemones found in sheltered shallow habitats close to shore (Fig. 1). Further studies that control variables, such as temperature, flow, turbidity, nutrient/food availability, predation pressure, and intraspecific competition, may provide further insight into the mechanisms that regulate growth in different anemone species. However, the scale at which these variables need to be measured to understand their impacts on the physiology and growth of clownfish would be very difficult to achieve (Burgess et al. 2021).
Conclusion
Together, our findings support the notion that social and environmental characteristics can influence the outcome of group structure and growth (Robbins et al. 2005). This study provides the first long-term evaluation of the social structure and individual growth in a wild population of reef fish, demonstrating that growth in A. percula is socially mediated and strongly dependent on the host anemone. Further work should investigate the demographic consequence of faster growth and survivorship in clownfish and whether the loss of dominant and highly reproductive female fish (Saenz-Agudelo et al. 2015) can be mitigated by the rapid progression through social hierarchies and growth of lower-ranked groups.
Availability of data and material
All data generated or analyzed during this study are linked in this published article’s supplementary information files.
References
Alberto F (2009) MsatAllele_1.0: An R package to visualize the binning of microsatellite alleles. J Hered 100:394–397. https://doi.org/10.1093/jhered/esn110
Almany GR, Planes S, Thorrold SR, Berumen ML, Bode M, Saenz-Agudelo P, Bonin MC, Frisch AJ, Harrison HB, Messmer V, Nanninga GB, Priest MA, Srinivasan M, Sinclair-Taylor T, Williamson DH, Jones GP (2017) Larval fish dispersal in a coral-reef seascape. Nat Ecol Evol. https://doi.org/10.1038/s41559-017-0148
Bartoń K (2020) MuMIn: multi-model inference. R package version 1.43.17. https://CRAN.R-project.org/package=MuMIn
Burgess SC, Johnston EC, Wyatt AS, Leichter JJ, Edmunds PJ (2021) Response diversity in corals: hidden differences in bleaching mortality among cryptic Pocillopora species. Ecology. https://doi.org/10.1002/ecy.3324
Buston PM (2003a) Mortality is associated with social rank in the clown anemonefish (Amphiprion percula). Mar Biol 143:811–815. https://doi.org/10.1007/s00227-003-1106-8
Buston PM (2003b) Social hierarchies: size and growth modification in clownfish. Nature 424:145–146
Buston PM, Cant MA (2006) A new perspective on size hierarchies in nature: patterns, causes, and consequences. Oecologia 149:362–372. https://doi.org/10.1007/s00442-006-0442-z
Buston PM, Clutton-Brock T (2022) Strategic growth in social vertebrates. Trends Ecol Evo. https://doi.org/10.1016/j.tree.2022.03.010
Buston PM, Garcia MB (2007) An extraordinary life span estimate for the clown anemonefish Amphiprion percula. J Fish Biol 70:1710–1719. https://doi.org/10.1111/j.1095-8649.2007.01445.x
Chausson J, Srinivasan M, Jones GP (2018) Host anemone size as a determinant of social group size and structure in the orange clownfish (Amphiprion percula). PeerJ 6:e5841. https://doi.org/10.7717/peerj.5841
Fautin DG, Allen GR (1992) Field guide to anemone fishes and their host sea anemones. Western Australian Museum, Perth
Forrester GE (1991) Social rank, individual size and group composition as determinants of food consumption by humbug damselfish, Dascyllus aruanus. Anim Behav 42:701–711. https://doi.org/10.1016/S0003-3472(05)80116-2
Fricke HW (1973) Individual partner recognition in fish: field studies on Amphiprion bicinctus. Naturwissenschaften 60:204–205. https://doi.org/10.1007/BF00599441
Fricke HW (1974) Eco-ethology of the monogamous anemone fish Amphiprion bicinctus (field studies in the Red Sea). Z Tierpsychol 36:429–512
Fricke HW, Fricke S (1977) Monogamy and sex change by aggressive dominance in coral reef fish. Nature 266:830–832. https://doi.org/10.1038/266830a0
Friedman J, Hastie T, Tibshirani R (2010) Regularization paths for generalized linear models via coordinate descent. J Stat Softw 33:1–22
Galpern P, Manseau M, Smith K, Wilson P (2012) Allelematch: an R package for identifying unique multilocus genotypes where genotyping error and missing data may be present. Mol Ecol Resour 12:771–778. https://doi.org/10.1111/j.1755-0998.2012.03137.x
Gurney WSC, Nisbet RM (1979) Ecological stability and social hierarchy. Theor Popul Biol 16:48–80
Hartig F (2020) DHARMa: residual diagnostics for hierarchical (multi-level/mixed) regression models. R package version 0.3.3.0. https://CRAN.R-project.org/package=DHARMa
Koski J, Xie H, Olson I (2015) Understanding social hierarchies: the neural and psychological foundations of status perception. Soc Neurosci 10:527–550. https://doi.org/10.1080/17470919.2015.1013223
Lenth RV (2021) emmeans: estimated marginal means, aka least-squares means. R package version 1.5.4. https://CRAN.R-project.org/package=emmeans
Peakall R, Smouse PE (2012) GenAlEx 6.5: genetic analysis in Excel. Population genetic software for teaching and research—an update. J Bioinform 28:2537–2539. https://doi.org/10.1093/bioinformatics/bts460
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna
Reed C, Branconi R, Majoris J, Johnson C, Buston PM (2019) Competitive growth in a social fish. Biol Lett 15:1–4. https://doi.org/10.1098/rsbl.2018.0737
Reinke B, Miller DAW, Janzen FJ (2019) What have long-term field studies taught us about population dynamics? Annu Rev Ecol Evol Syst 50:261–278. https://doi.org/10.1146/annurev-ecolsys-110218-024717
Robbins MM, Robbins AM, Gerald-Steklis N, Steklis HD (2005) Long-term dominance relationships in female mountain gorillas: strength, stability and determinants of rank. Behaviour 142:779–809. https://doi.org/10.1163/1568539054729123
Rueger T, Barbasch TA, Wong MYL, Jones GP, Buston PM (2018) Reproductive control via the threat of eviction in the clown anemonefish. Proc R Soc B 285:1–6. https://doi.org/10.1098/rspb.2018.1295
Saenz-Agudelo P, Jones GP, Thorrold SR, Planes S (2015) Mothers matter: contribution to local replenishment is linked to female size, mate replacement and fecundity in a fish metapopulation. Mar Biol 162:3–14. https://doi.org/10.1007/s00227-014-2556-x
Salis P, Roux N, Huang D, Marcionetti A, Mouginot P, Reynaud M, Salles OC, Salamin N, Pujol B, Parichy D, Planes S, Laudet V (2021) Thyroid horomones regulate the formation and environmental plasticity of white bars in clownfishes. PNAS. https://doi.org/10.1073/pnas.2101634118
Salles OC, Saenz-Agudelo P, Almany GR, Berumen ML, Thorrold SR, Jones GP, Planes S (2016) Genetic tools link long-term demographic and life-history traits of anemonefish to their anemone hosts. Coral Reefs 35:1127–1138. https://doi.org/10.1007/s00338-016-1485-1
Thompson VJ, Munday PL, Jones GP (2007) Habitat patch size and mating system as determinants of social group size in coral-dwelling fishes. Coral Reefs 26:165–174. https://doi.org/10.1007/s00338-006-0181-y
Whiteman EA, Cote IM (2004) Monogamy in marine fishes. Biol Rev 79:351–375. https://doi.org/10.1017/S1464793103006304
Wickham H (2016) ggplot2: Elegant Graphics for Data Analysis. Springer-Verlag, New York, USA
Wong MYL, Buston PM, Munday PL, Jones GP (2007) The threat of punishment enforces peaceful cooperation and stabilizes queues in a coral-reef fish. Proc R Soc B 274:1093–1099. https://doi.org/10.1098/rspb.2006.0284
Wong MYL, Munday PL, Buston PM, Jones GP (2008) Monogamy when there is potential for polygyny: tests of multiple hypotheses in a group-living fish. Behav Ecol 19:353–361. https://doi.org/10.1093/beheco/arm141
Wong MYL, Uppaluri C, Medina A, Seymour J, Buston PM (2016) The four elements of within-group conflict in animal societies: an experimental test using the clown anemonefish, Amphiprion percula. Behav Ecol Sociobiol 70:1467–1475. https://doi.org/10.1007/s00265-016-2155-6
Acknowledgements
We would like to thank everyone who assisted us in the field, including B. Cresswell and G. Galbraith. Additionally, we acknowledge the Mahonia Na Dari Research Station and the crew of the M. V. Febrina for providing logistical support. We thank U. Langner (KAUST), who assisted with the maps. Lastly, we also thank the anonymous reviewers for their help and feedback on this paper.
Funding
This research was supported by King Abdullah University of Science and Technology (baseline funds to MLB), the ARC Centre of Excellence for Coral Reef Studies (DP190103056 to GPJ), and the Australian Research Council. HBH was supported by an ARC DECRA Fellowship (DE160101141).
Author information
Authors and Affiliations
Contributions
LMF, HBH, and MLB: contributed to the study conception and design. Data collection was performed by LMF, HBH, PS-A, MS, JEM, LBE, BP, MBS, SRT, SP, GPJ, and MLB. Data analysis was performed by LMF, HBH, DJC, and PS-A. The first draft of the manuscript was written by LMF and all authors commented on previous versions. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
We declare no conflicts or competing interests.
Ethical approval
The authors declare that the sampling of specimens was performed in the respect of the ethical rules and Papua New Guinea laws. All samplings were authorized by King Abdullah University of Science and Technology (KAUST—Field Research Plan 20190227).
Additional information
Responsible Editor: D. Goulet.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Fitzgerald, L.M., Harrison, H.B., Coker, D.J. et al. Rank change and growth within social hierarchies of the orange clownfish, Amphiprion percula. Mar Biol 169, 128 (2022). https://doi.org/10.1007/s00227-022-04117-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04117-9