Abstract
Devastating bleaching of coral communities at Amitori Bay, Iriomote Island, Japan, occurred in 2016 during the third global mass bleaching event in 2014–2017. The present study documented changes in coral communities in Amitori Bay from just before until after the 2016 bleaching event (2016–2020), by measuring coral cover and recruitment at nine sites (with two additional sites in 2018) in the bay. Spawning rates of acroporid corals were also monitored from 2017 to 2019 by visual observation and using bundle collectors to observe how long the effect of bleaching persisted. Reductions of 64.7 and 89.5% from 2016 to 2017 were observed in cover and recruitment of all coral families, respectively. Coral cover of all coral families recovered to pre-bleaching levels by 2020 and recruitment in 2020 was about two times greater than the pre-bleaching level. These results mirrored those of acroporids. Spawning rates of Acropora corals increased significantly from 40.6% in 2017 to 90.0% in 2019. Recovery of coral cover 4 years after the severe bleaching event was likely related to regrowth of remnants and of surviving juveniles of < 5 cm. The sudden increase in recruitment was likely driven by a combination of larval supply from other populations, increased numbers of reproductive adults, increases in spawning rates, and increased larval retention in the bay due to wind conditions in 2020. This study suggests that coral communities as in Amitori Bay will be critical for local-scale community persistence, serving as both source and sink populations.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tropical coral communities are subject to various natural and anthropogenic disturbances, but one of the most urgent global threats is sea-surface temperature (SST) warming, which can cause coral bleaching, often resulting in mass coral mortality (Wilkinson 1998, 2000; Hughes et al. 2017, 2018a). Severe bleaching events have become more frequent, having occurred once every 25 years in the 1980s to once every 5.9 years since 2010 (Hughes et al. 2018a). The most recent and severe global mass bleaching event occurred in 2014–2017 (Hughes et al. 2018a; Eakin et al. 2019). At that time, many coral communities across the Indo-Pacific Ocean were severely impacted (Hughes et al. 2018a, but see Hédouin et al. 2020). For example, at Jarvis Island, in the central Pacific, live coral cover declined by 93% in 2016 with nearly 100% mortality of non-massive genera (Barkley et al. 2018). In the Chagos Archipelago, in the central Indian Ocean, Acropora-dominated communities shifted to dominance of massive Porites, with an 86% decrease of Acropora from 2012 to 2016 (Head et al. 2019). A similar trend was also observed at Huvadhoo Atoll, in the southern Maldives (Perry and Morgan 2017). Moreover, in the Great Barrier Reef (GBR), only 8.9% of the reefs escaped bleaching (Hughes et al. 2017) and most reefs bleached severely, resulting in significant depletion of adult coral populations and subsequent suppression of larval recruitment in 2018 (Hughes et al. 2019). It was most severe among acroporids, and they were replaced by pocilloporids in the recruitment pool (Hughes et al. 2019), presumably affecting the recovery trajectory of those coral communities.
Post-bleaching trajectories of coral communities differed among reef locations and regions (Baker et al. 2008), with some reefs recovering to pre-bleaching coral coverage and composition (van Woesik et al. 2011; Gilmour et al. 2013), while others failed to recover and/or become dominated by algae or other benthic organisms (Stobart et al. 2005; Graham et al. 2015). In the past, many coral communities have taken a decade or more to recover after severe bleaching events (Adjeroud et al. 2009; van Woesik et al. 2011; Gilmour et al. 2013; McClanahan 2014; Graham et al. 2015), but for some, recovery was more rapid (e.g., 7 years in Palau, Golbuu et al. 2007; 3 years at lagoonal reefs in the Seychelles, Koester et al. 2020). In some severely devastated coral communities, regrowth of remnant colonies and new recruits supplied by neighboring reefs were the main drivers of recovery (Golbuu et al. 2007; van Woesik et al. 2011; Graham et al. 2015). In contrast, due to limited connectivity with other reefs, recovery of isolated coral communities in Western Australia was primarily driven by regrowth of coral remnants (Gilmour et al. 2013).
In 2016, coral communities in Japan also suffered severe mass bleaching, especially at Iriomote Island in the Ryukyu Islands (Fig. 1). There, 94% of corals bleached, compared with 7 – 70% at other islands (Biodiversity Center of Japan 2017). In Amitori Bay, in northwestern Iriomote Island, more than 50% of corals were bleached at depths of 3–20 m in August 2016, with 32.5 and 3.9% of corals bleached at depths of 30 and 40 m, respectively (Murakami et al. 2017). In the bay, seawater temperatures exceeded 30 ℃ from 9 to 29 August 2016, 12 m below the surface, a temperature significantly higher than in non-bleaching years (Murakami et al. 2017). During this period, no typhoon came close to the region until 17 September, whereas in a normal year, more than one typhoon strikes this region during July and August. Murakami et al. (2017) suggested that a lack of typhoons in July and August contributed to the persistence of elevated seawater temperatures in the bay.
The objective of the present study was to document changes in coral communities in Amitori Bay from just before until after the 2016 mass bleaching event and to understand factors that promoted recovery of its coral communities. Acroporid coral communities, dominated by the genus Acropora, in the bay were hypothesized to be maintained by sexually produced recruits from within the bay and nearby areas (Nakamura et al. 2017) and adult acroporid cover decreases would therefore cause recruitment to decline in the area. To quantify the relationship between coral cover and recruitment, we recorded changes in both parameters. Because bleaching can cause substantial decreases in reproductive output of Acropora corals (Ward et al. 2000; Baird and Marshall 2002), we also observed spawning rates of remnant Acropora colonies. Moreover, impacts of bleaching on reproduction of corals can be long-lasting (Levitan et al. 2014; Johnston et al. 2020). We therefore documented the effects of bleaching of five Acropora coral species.
Materials and methods
Study sites
Amitori Bay is located in northwestern Iriomote Island (24°15’ – 25’N, 123°40’ – 55’E) in the Yaeyama District of the southwestern Ryukyu Archipelago, in southwestern Japan (Fig. 1). This bay is an inner bay with an entrance ~ 2.2 km wide and a length of ~ 3.5 km. The deepest water is about 75 m at the mouth of the bay and it is about 30 m at its innermost point. Two rivers enter the bay. The bay is uninhabited, and there is no access to it from populated areas of the island. It was designated as a natural conservation area in 2015 due to minimal anthropogenic impact. Before the 2016 bleaching event, coral communities in the bay comprised 317 coral species representing 65 genera (Yokochi et al. 2019). Coral composition, abundance, and cover differ along the two sides of the bay and also vary with the distance from its mouth (Murakami et al. 2012; Yokochi et al. 2019).
Therefore, in 2016 and 2017, we selected nine sites in the bay, five sites (A1–A5, Fig. 1) along the Amitori side and four sites (S1–S4, Fig. 1) along the Sabasaki side. Two more sites (A0 and S0, Fig. 1) were added in 2018 outside the entrance to the bay. Three subsites were established within each site.
Coral cover and recruitment
Coral cover was estimated using 10 × 2 m belt-transects at each site at a depth of 5 m. One to three belt-transects were set per subsite. Five 1 × 1 m quadrats were randomly situated along each belt-transect. Coral assemblages within each quadrat were photographed and the areas covered by corals were determined using Image J (Schneider et al. 2012), following which percent coral cover was calculated. When corals overlapped, only areas covered by the coral above were used for the area of the overlap. Corals were identified to the family level (Acroporidae, Poritidae, Pocilloporidae, and other families). Percentage cover of all coral families, referred to hereafter as “All-coral” cover, was the sum of the percentage cover of Acroporidae, Poritidae, Pocilloporidae, and other families. Surveys were conducted at A1–A5 and S1–S4 in 2016 and 2017, and at A0–A5 and S0–S4 in 2018, 2019 and 2020 (Fig. 1, Supplementary Table 1). Surveys were conducted at the three subsites (Sub1–Sub3) of all sites from 2016 to 2019, but only at Sub2 of all sites in 2020, because of limited manpower. The number of belt-transects and quadrats per site differed among years (Supplementary Table 1), with only five quadrats per site in 2020. Although low replication compromises the robustness of the data, we decided to show the data to supplement the outlined changes in coral cover of the bay in previous years.
The abundance of recruits (in this study used as proxy for coral recruitment) was assessed on ten pairs of 10 × 10 cm panels made of fiber-reinforced cement, which were deployed at each subsite at ca. 5 m depth in the vicinities of the belt-transect surveys. Two panels were fastened together with a 2-cm gap, allowing coral larvae to settle between them, while excluding grazers. Panels were never reused, and every year, new settlement panel pairs were deployed. Panel pairs were randomly set on substrates, at least 1 m apart. The lower surface of each lower panel was attached to the substrate using underwater epoxy glue. Panel pairs were pre-conditioned in flow-through aquaria for 3–4 weeks and were deployed about 1 month before the full moon of May (Supplementary Table 2), the expected major coral spawning period (Murakami et al. 2015). Settlement panels were retrieved about 1 month after the observed spawning (Supplementary Table 2). Retrieved panel pairs were bleached in a chlorine solution for 1 week to eliminate organic matter and then dried for observation under a stereo microscope. Upper and lower surfaces of the upper panel and the upper surface of the lower panel (0.03 m2) were examined for each settlement panel pair. Coral recruits on panels were identified to the family level (Acroporidae, Poritidae, Pocilloporidae, and other families) based on skeletal morphology (Babcock et al. 2003). Numbers of recruits on panels were counted for each family. Recruitment of all corals (sum of acroporids, poritids, pocilloporids, and other families) is referred to hereafter as “All-coral” recruitment. Settlement panel pairs were deployed at sites A1–A5 and S1–S4 in 2016, 2017, and 2020, and at A0–A5 and S0–S4 in 2018 and 2019 (Fig. 1, Supplementary Table 3). In 2020, water conditions were very rough outside the entrance of the bay at the time of settlement panel deployment; thus, they could not be deployed at A0 and S0 in 2020. Moreover, settlement panel pairs were deployed at three subsites (Sub1–Sub3) in 2016 to 2018, but only at Sub 2 in 2019 and 2020 because of limited manpower.
Spawning rate of surviving Acropora colonies after the 2016 bleaching
Spawning rates were observed for Acropora corals that survived the 2016 bleaching event, among which the most abundant species of colonies > 30 cm in diameter were Acropora gemmifera, A. nasta, A. cf. selago, and A. tenuis from 2017 to 2019 and A. hyacinthus in 2018 and 2019. In 2020, observations were only conducted in April, due to restrictions imposed thereafter in response to the coronavirus pandemic. Spawning was assessed through (1) visual observation by night diving and (2) bundle collectors (funnel-shaped devices with plankton-net and plastic bottle for collection of bundles, which are capsules that contain eggs and sperm). Visual observation was conducted around A3 (Fig. 1) because of its proximity to the Amitori Center of the Okinawa Regional Research Center of Tokai University. Colonies > 30 cm in diameter of each acroporid species were observed spawning by night diving. Spawning of colonies (11 colonies in 2017 and 12 in 2018) was recorded once the release of egg-sperm bundles into the water column was observed. Bundle collectors were set on colonies > 30 cm in diameter in the late afternoon at various sites in the bay. These were checked the next morning to see if they contained bundles. There were 21 collectors in 2017, 28 in 2018, and 30 in 2019. In 2019, colonies observed visually were the same as those monitored with bundle collectors. These surveys were conducted from May to August in 2017, April to June in 2018, and April to June in 2019 (Supplementary Table 4). Observed colonies largely differed among years, because tagging of corals or substrata was discouraged in the bay. From these surveys, spawning rates of five Acropora species were estimated as the ratio of the number of coral colonies that spawned divided by the number of coral colonies observed visually by night diving and with bundle collectors.
Analysis
All analyses were performed in R, version 4.1.1 (R Core Team 2021). To reveal the relationship between coral cover and recruitment in coral communities in Amitori Bay, we used data from sites A1–A5 and S1–S4 (Fig. 1) for 2016–2020 because of the lack of settlement data for A0 and S0 in 2016, 2017, and 2020 and coral cover data in 2016 and 2017. Yearly variability of coral cover and recruitment were analyzed for All-coral, and for Acroporidae, Poritidae, and Pocilloporidae individually from 2016 to 2020 using Kruskal–Wallis tests. For cases in which significant differences were revealed, Steel–Dwass tests were conducted for multiple comparisons. Here, non-parametric tests were used, because the assumptions for parametric tests were rejected for lack of normality and homogeneity of variances using the Shapiro and Bartlett tests, respectively. Pearson’s correlations were conducted to determine if there were some indices of stock–recruitment relationships between coral cover and recruitment for All-coral, Acroporidae, Poritidae, and Pocilloporidae.
Using the RVAideMemoire package in R, spawning rates were compared among years using Fisher’s exact test with Holm’s method. Tests were conducted on the five pooled species, and on Acropora gemmifera and A. tenuis, because numbers of observed colonies of the other three Acropora species were fewer than six each year.
Results
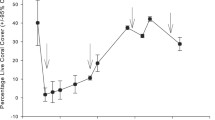
Mean coral cover varied significantly from 2016 to 2020 (Table 1) and annual variation of All-coral cover paralleled that of acroporid cover (Fig. 2a, b). All-coral cover decreased significantly from 20.25 ± 1.59% (mean ± SE) in 2016 to 7.15 ± 0.50% in 2017 (Fig. 2a), a relative reduction of 64.7%. In parallel, acroporid cover, which accounted for 66.4% of All-coral cover in 2016, showed a significant decrease from 13.44 ± 1.49% in 2016 to 2.94 ± 0.35% in 2017 (Fig. 2b), a relative reduction of 78.1%. Between 2017 and 2020, the cover of All-coral and acroporids gradually increased to 22.05 ± 2.91% and 12.34 ± 2.04%, respectively, reaching 108.9% (All-coral) and 91.8% (acroporid) of the pre-bleaching cover in 2016. By comparison, poritid and pocilloporid coral cover did not show a significant decrease from 2016 to 2017 (Fig. 2c, d). Poritid cover, which accounted for more than 20% of All-coral cover, except in 2016 (8.0%), gradually increased from 1.61 ± 0.36% in 2016 to 4.88 ± 1.70% in 2020. The cover of pocilloporids was less than 0.5% from 2016 to 2020 (except for 2019; 0.76 ± 0.20%) and accounted for less than 5% of All-coral cover in all years.
Percentage cover and recruitment of All-coral (a, e), Acroporidae (b, f), Poritidae (c, g), and Pocilloporidae (d, h) between 2016 and 2020 in Amitori Bay. Boxplots show median and interquartile range; diamond marks show mean; significant differences among year are displayed as lines (*p < 0.05, **p < 0.001, ***p < 0.0001). Note the different y-axis scales. The number of replicates for percentage cover was 15 quadrats per site in 2016, 45 in 2017, 45 in 2018, 15 in 2019, and 5 in 2020. Those for recruitment were 30 settlement panel pairs per site in 2016–2018 and ten in 2019 and 2020
Mean recruitment varied significantly from 2016 to 2020 (Table 1, 2) and annual variation of All-coral recruitment showed a trend similar to that of acroporid recruitment (Fig. 2e, f). All-coral and acroporid recruitment decreased significantly between 2016 and 2017 (Fig. 2e, f), decreasing from 11.95 ± 0.83 (All-coral) and 7.60 ± 0.66 (acroporid) recruits per panel pair in 2016 to 1.26 ± 0.12 (All-coral) and 0.19 ± 0.03 (acroporid) recruits per panel pair in 2017, which represents relative decreases of 89.5% (All-coral) and 97.6% (acroporid). The number of All-coral and acroporid recruits gradually increased from 2017 to 2019 and a substantial increase was observed from 2019 to 2020 (Fig. 2e, f). All-coral recruitment in 2020 was 25.08 ± 2.44 recruits per panel pair, which was about seven times that in 2019 (3.18 ± 0.40 recruits per panel pair). Acroporid recruitment in 2020 was 20.19 ± 2.15 recruits per panel pair, which was 13 times that in 2019 (1.60 ± 0.21 recruits per panel pair). These recruitment values were about two-to-three times greater than in 2016 before the bleaching event. Compared to acroporids, poritid and pocilloporid recruitment was very low, < 2 recruits per panel pair. However, similar trends to recruitment of All-coral and acroporids were observed for poritids and pocilloporids (Fig. 2g, h). Poritid recruitment decreased significantly from 1.39 ± 0.24 recruits per panel pair in 2016 to 0.31 ± 0.06 recruits in 2017 (Fig. 2g). Pocilloporid recruitment also decreased significantly from 1.69 ± 0.28 recruits per panel pair in 2016 to 0.21 ± 0.03 recruits per panel pair in 2017 (Fig. 2h). By 2020, recruitment of poritid and pocilloporid recruitment had returned to 2016 levels.
There were no significant correlations between mean recruitment and mean cover for All-coral (Pearson’s R = 0.82, p = 0.088), Acroporidae (Pearson’s R = 0.79, p = 0.12), Poritidae (Pearson’s R = – 0.078, p = 0.90), or Pocilloporidae (Pearson’s R = – 0.14, p = 0.90) (Supplementary Fig. 1).
Spawning rates of Acropora increased from 40.6% in 2017 to 90.0% in 2019 (Table 3). The spawning rate in 2019 was significantly higher than in 2017 (p = 0.00015) and 2018 (p = 0.046). The spawning rate of A. tenuis in 2019 was also significantly higher than 2017 (p = 0.00034), but not than 2018 (p = 0.19). In 2017, only 1 of 13 observed colonies of A. tenuis spawned (spawning rate: 7.7%). In 2018, 5 of 11 colonies spawned (spawning rate: 45.5%). In 2019, nine of ten colonies spawned (spawning rate: 90.0%). For A. gemmifera, the spawning rate did not increase significantly from 2017 to 2019 (p = 0.91). Among nine A. gemmifera colonies, six spawned in 2017 (spawning rate: 66.7%). In 2018, 12 of 14 colonies spawned (spawning rate: 85.7%). Nine of ten colonies spawned in 2019 (spawning rate: 90.0%). Three other Acropora species also showed an increase in the spawning rate from 2017 to 2019 (Table 3).
Discussion
Following severe thermal stress and coral bleaching, reefs can undergo drastic changes in coral community composition (Graham et al. 2015; Hughes et al. 2017, 2018b), e.g., through the steady replacement of thermally sensitive corals by weedy and stress-tolerant corals (Darling et al. 2013; Edmunds et al. 2014; McClanahan et al. 2014, 2020; Palumbi et al. 2014). For instance, in many reefs, thermally sensitive Acropora species have declined in number and/or coverage following thermal stress events, while massive, stress-tolerant Porites were unaffected (Loya et al. 2001; Adjeroud et al. 2009; McClanahan 2014; Head et al. 2019). A similar trend was observed in this study. Acroporid cover in Amitori Bay declined significantly from 2016 to 2017, but poritid cover did not change.
Despite the 78.1% decrease in acroporid cover in Amitori Bay, post-bleaching recovery was rapid, compared to recovery times reported from reefs in Palau (Golbuu et al. 2007), Japan (van Woesik et al. 2011), Australia (Gilmour et al. 2013), Kenya (McClanahan 2014), and the Mexican Caribbean (Contreras-Silva et al. 2020), where recovery took a decade or more. Our study demonstrated that acroporid coral cover, dominated by Acropora, at Amitori Bay returned to pre-bleaching levels within 4 years after the severe bleaching event of 2016. As many acroporids follow a competitive life-history strategy, characterized by rapid growth (e.g., species of the genus Acropora; Darling et al. 2012), quick regrowth of remnant corals, as also observed at Scott Reef, Australia (Gilmour et al. 2013), Palau (Golbuu et al. 2007), and Sesoko Island, Japan (van Woesik et al. 2011), may therefore have promoted fast recovery at Amitori Bay. In addition, rapid regeneration of remnant branching Acropora may also have helped recovery of coral cover, as observed in the Keppel Islands in the southern GBR, where coral cover recovered to or exceeded pre-bleaching levels in less than a year (Diaz-Pulido et al. 2009). Moreover, survival of juvenile Acropora (< 5 cm) may also have contributed to the recovery as observed at Sesoko Island, Japan, where small Acropora colonies survived the 1998 bleaching event and rapidly grew afterward (Loya et al. 2001; van Woesik et al. 2011). Further studies also found higher resistance of small corals to thermal stress (Nakamura and van Woesik 2001; Papina et al. 2002; Bou-Abdallah et al. 2006; Alvarez-Noriega et al. 2018). In Amitori Bay, we frequently observed Acropora colonies of ca. 5 cm and smaller in April 2017, 8 months after the bleaching event (M. Nakamura, pers. obs.). As small Acropora may become more than double their sizes within 2–3 years at Iriomote Island (Muko et al. 2013), the small Acropora corals we observed may have helped the rapid recovery of acroporid corals in Amitori Bay. A note of caution regarding this study is that although coral cover recovered to the pre-bleaching level, the species composition likely changed due to different thermal tolerances and strategies of each species (Darling et al. 2012). In addition, sampling was reduced in 2020 because of limited manpower. Therefore, further analyses considering species composition with greater sample sizes would improve understanding of recovery states of coral communities in Amitori Bay.
All-coral recruitment decreased substantially in 2017 in Amitori Bay, reflecting a significant decrease in acroporid recruitment. The substantial reduction in recruitment after the severe bleaching event may have been caused by the reduction in local brood stock and brood stocks of connected reefs (Gilmour et al. 2013; Hughes et al. 2019). The decreased acroporid cover in the bay likely accounts for much of the recruitment failure of 2017, because acroporid coral communities in Amitori Bay have been estimated to be maintained mainly by new recruits from within the bay and nearby areas (Nakamura et al. 2017). However, as Amitori Bay likely also receives larvae from other populations (Murakami et al. 2020), the recruitment failure in 2017 could also have been caused by reduced brood stocks of connected reefs. For example, 70% mortality of acroporid corals was reported in 2016 in Sakiyama Bay, lying to the west of Amitori Bay (Biodiversity Center of Japan 2017), and assumed to be a potential source population for Amitori Bay (Nakamura et al. 2017). Another potential factor for the recruitment failure in 2017 could be low spawning rates. Spawning rates of Acropora species varied between 7.7 and 80.0% (40.6% of total, Table 3). Given that bleaching suppresses reproduction of corals (Ward et al. 2000; Baird and Marshall 2002; Levitan et al. 2014; Hagedorm et al. 2016; Johnston et al. 2020), low reproductive output likely reflected the 2016 bleaching event. As changes in fecundity of acroporid corals drive changes in recruitment (Hughes et al. 2000), the substantial reduction in acroporid recruitment in 2017 may have stemmed from both the reduction in brood stocks and suppression of reproductive capacity of remnant adult colonies.
The sudden, significant increase in acroporid recruitment in 2020 may be explained by several factors: (1) larval supply from connected reefs, (2) re-acquisition of reproductive ability of acroporid coral communities, (3) increases in brood stock of the bay due to the recovery of coral cover, and (4) a higher retention rate of larvae produced in the bay due to wind conditions in 2020. Given that acroporid larvae take approximately 3–5 days to obtain settlement competency after fertilization (Harii et al. 2007), recruitment from sites outside the bay is likely (Murakami et al. 2020). Regarding reproductive capacity and brood stocks, the number of spawned acroporid corals increased year by year and the spawning rate reached nearly 100% for 2–3 years after bleaching events, although changes from 2017 to 2019 differed among acroporid species. Moreover, with growth of remnant corals, adult brood stocks likely increased year by year. Larger numbers of acroporid colonies likely spawned in 2020, resulting in increased recruitment. Moreover, winds blew from the southeast on the spawning day in 2020, and then shifted to the northeast for the following 3 days, according to data from the Japan Meteorological Agency (https://www.data.jma.go.jp/obd/stats/etrn/index.php, in Japanese). Hence, larval retention was estimated to be higher than when winds blow from the south (Murakami et al. 2020). These factors may partly explain the abrupt increase in acroporid recruitment in 2020.
The recovery of coral communities in Amitori Bay may be more rapid than intervals of recurrent bleaching events. Thermally sensitive corals such as acroporids are expected to be vulnerable and to have difficulty surviving in progressively warming sea-surface temperatures (van Woesik et al. 2011; Darling et al. 2013; Edmunds et al. 2014; McClanahan et al. 2014, 2020; Palumbi et al. 2014; Head et al. 2019; Hughes et al. 2019). However, our study demonstrated that acroporid coral cover reached pre-bleaching levels within 4 years. This rapid recovery was likely dependent on regrowth of remnants and of surviving juveniles (< 5 cm). In addition, recruitment largely surpassed the pre-bleaching level after 4 years, which will contribute to further persistence of communities (Gilmour et al. 2013; Graham et al. 2015). This could offer a glimmer of hope for the future of coral communities. However, it has recently been estimated that severe bleaching events will occur at increasingly shorter intervals (Hughes et al. 2018a). Our results therefore suggest that coral communities as in Amitori Bay will be critical for persistence of thermally sensitive acroporid species, serving as both source and sink populations when bleaching frequency increases. Appropriate management strategies, such as frequent monitoring, protection from human impacts, i.e., tourisms, fishing, implementation of wider protected areas, including source-sink populations for larval connectivity, will be necessary to ensure persistence of these local communities.
Data availability
Data and any available visuals, such as figures and tables, will be provided upon request to the corresponding author.
References
Adjeroud M, Michonneau F, Edmunds PJ, Chancerelle Y, Lison de Loma T, Penin L, Thibaut L, Vidal-Dupiol J, Salvat B, Galzin R (2009) Recurrent disturbances, recovery trajectories, and resilience of coral assemblages on a South Central Pacific reef. Coral Reefs 28:775–780
Alvarez-Noriega M, Baird AH, Bridge TCL, Dornelas M, Fontoura L, Pizarro O, Precoda K, Torres-Pulliza D, Woods RM, Zawada K, Madin JS (2018) Contrasting patterns of changes in abundance following a bleaching event between juvenile and adult scleractnian corals. Coral Reefs 37:527–532
Babcock RC, Baird AH, Piromvaragorn S, Thomson DP, Willis BL (2003) Identification of scleractinian coral recruits from Indo-Pacific reefs. Zool Stud 42:211–226
Baird AH, Marshall PS (2002) Mortality, growth and reproduction in scleractinian corals following bleaching on the Great Barrier Reef. Mar Ecol Prog Ser 237:133–141
Baker AC, Glynn PW, Riegl B (2008) Climate change and coral bleaching: an ecological assessment of long-term impacts, recovery trends and future outlook. Estuar Coast Shelt Sci 80:435–471
Barkley HC, Cohen AL, Mollica NR, Brainard RE, Rivera HE, DeCarlo TM, Lohmann GP, Drenkard EJ, Alpert AE, Young CW, Vargas-Angel B, Lino KC, Oliver TA, Pietro KR, Luu VH (2018) Repeat bleaching of a central Pacific coral reef over the past six decades (1960–2016). Commun Biol 1:177
Biodiversity Center of Japan (2017) Report on monitoring 1000 coral reef survey in fiscal year 2016. The Ministry of the Environment, Japan.
Bou-Abdallah F, Chasteen ND, Lesser MP (2006) Quenching of superoxide radicals by green fluorescent protein. Biochim Biophys Acta 1760:1690–1695
Contreras-Silva AI, Tilstra A, MiganiV TA, Pérez-Cervantes E, Estrada-Saldívar N, Elias-Ilosvay X, Mott C, Alvarez-Filip L, Wild C (2020) A meta-analysis to assess long-term spatiotemporal changes of benthic coral and macro algae cover in the Mexican Caribbean. Sci Rep 10:8897
Darling ES, Alvarez-Filip L, Oliver TA, McClanahan TR, Côté IM (2012) Evaluating life-history strategies of reef corals from species traits. Ecol Lett 15:1378–1386
Darling ES, McClanahan TR, Côté IM (2013) Life histories predict coral community disassembly under multiple stressors. Glob Change Biol 19:1930–1940
Diaz-Pulido G, McCook LJ, Dove S, Berkelmans R, Roff G, Kline DI, Weeks S, Evans RD, Williamson DH, Hoegh-Guldberg O (2009) Doom and boom on a resilient reef: climate change, algal overgrowth and coral recovery. PLoS ONE 4(4):e5239
Eakin CM, Sweatman HPA, Brainard RE (2019) The 2014–2017 global-scale coral bleaching event: insights and impacts. Coral Reefs 38:539–545
Edmunds PJ, Adjeroud M, Baskett ML, Baums IB, Budd AF, Carpenter RC, Fabina NS, Fan TY, Franklin EC, Gross K, Han X, Jacobson L, Klaus JS, McClanahan TR, O’Leary JK, van Oppen MJH, Pochon X, Putnam HM, Smith TB, Stat M, Sweatman H, van Woesik R, Gates RD (2014) Persistence and change in community composition of reef corals through present, past, and future climates. PLoS ONE 9(10):e107525
Gilmour JP, Smith LD, Heyward AJ, Baird AH, Pratchett MS (2013) Recovery of an isolated coral reef system following severe disturbance. Science 340:69–71
Golbuu Y, Victor S, Penland L, Idip D Jr, Emaurois C, Okaji K, Yukihira H, Iwase A, van Woesik R (2007) Palau’s coral reefs show differential habitat recovery following the 1998-bleaching event. Coral Reefs 26:319–332
Graham NAJ, Jennings S, MacNeil MA, Mouillot D, Wilson SK (2015) Predicting climate-driven regime shifts versus rebound potential in coral reefs. Nature 518:94–97
Hagedorm M, Carter VL, Lager C, Ciani JFC, Dygert AN, Schleiger RD, Henley EM (2016) Potential bleaching effects on coral reproduction. Reprod Fertil Dev 28:1061–1071
Harii S, Nadaoka K, Yamamoto M, Iwao K (2007) Temporal changes in settlement, lipid content and lipid composition of larvae of the spawning hermatypic coral Acropora tenuis. Mar Ecol Prog Ser 346:89–96
Head CEI, Bayley DTI, Rowlands G, Roche RC, Tickler DM, Rogers AD, Koldewey H, Turner JR, Andradi-Brown DA (2019) Coral bleaching impacts from back-to-back 2015–2016 thermal anomalies in the remote central Indian Ocean. Coral Reefs 38:605–618
Hédouin L, Rouzé H, Berthe C, Perez-Rosales G, Martinez E, Chancerelle Y, Galand PE, Lerouvreur F, Nugues MM, Pochon X, Siu G, Steneck R, Planes S (2020) Contrasting patterns of mortality in Polynesian coral reefs following the third global coral bleaching event in 2016. Coral Reefs 39:939–952
Hughes TP, Baird AH, Dinsdale EA, Moltschaniwskyj NA, Pratchett MS, Tanner JE, Willis BL (2000) Supply-side ecology works both ways: the link between benthic adults, fecundity, and larval recruits. Ecology 81:2241–2249
Hughes TP, Kerry JT, Alvarez-Noriega M, Alvarez-Romeo JG, Anderson KD, Baird AH, Babcock RC, Beger M, Bellwood DR, Berkelmans R, Bridge TC, Butler IR, Byrne M, Cantin NE, Comeau S, Connolly SR, Cumming GS, Dalton SJ, Diaz-Pulido G, Eakin CM, Figueira WF, Gilmour JP, Harrison HB, Heron SF, Hoey AS, Hobbs JA, Hoogenboom MO, Kennedy EV, Kuo C, Lough JM, Lowe RJ, Liu G, McCulloch MT, Malcolm HA, McWilliam MJ, Pandolfi JM, Pears RJ, Pratchett MS, Schoepf V, Simpson T, Skirving WJ, Sommer B, Torda G, Wachenfeld DR, Willis BL, Wilson SK (2017) Global warming and recurrent mass bleaching of corals. Nature 543:373–377
Hughes TP, Anderson KD, Connolly SR, Heron SF, Kerry JT, Lough JM, Baird AH, Baum JK, Berumen ML, Bridge TC, Claar DC, Eakin CM, Gilmour JP, Graham NAJ, Harrison H, Hobbs JA, Hoey AS, Hoogenboom M, Lowe RJ, McCulloch MT, Pandolfi JM, Pratchett M, Schoepf V, Torda G, Wilson SK (2018a) Spatial and temporal patterns of mass bleaching of corals in the Anthropocene. Science 359:80–83
Hughes TP, Kerry JT, Baird AH, Connolly SR, Dietzel A, Eakin CM, Heron SF, Hoey AS, Hoogenboom MO, Liu G, McWilliam MJ, Pears RJ, Pratchett M, Skirving WJ, Stella JS, Torda G (2018b) Global warming transforms coral reef assemblages. Nature 556:492–496
Hughes TP, Kerry JT, Baird AH, Connolly SR, Chase TJ, Dietzel A, Hill T, Hoey AS, Hoogenboom MO, Jacobson M, Kerswell A, Madin JS, Mieog A, Paley AS, Pratchett MS, Torda G, Woods RM (2019) Global warming impairs stock-recruitment dynamics of corals. Nature 568:387–390
Johnston EC, Counsell CWW, Sale TL, Burgess SC, Toonen RJ (2020) The legacy of stress: coral bleaching impacts reproduction years later. Funct Ecol 34:2315–2325
Koester A, Migani V, Bunbury N, Ford A, Sanchez C, Wild C (2020) Early trajectories of benthic coral reef communities following the 2015/16 coral bleaching event at remote Aldabra Atoll. Seychells Sci Rep 10:17034
Levitan DR, Boudreau W, Jara J, Knowlton N (2014) Long-term reduced spawning in Orbicella coral species due to temperature stress. Mar Ecol Prog Ser 515:1–10
Loya Y, Sakai K, Yamazato K, Nakano Y, Sambali H, van Woesik, (2001) Coral bleaching: the winners and the losers. Ecol Lett 4:122–131
McClanahan TR (2014) Decadal coral community reassembly on an African fringing reef. Coral Reefs 33:939–950
McClanahan TR, Ateweberhan M, Darling ES, Graham NAJ, Muthiga NA (2014) Biogeography and change among regional coral communities across the Western Indian Ocean. PLoS ONE 9(4):e93385
McClanahan TR, Darling ES, Maina JM, Muthiga NA, D’agata S, Leblond J, Arthur R, Jupiter SD, Wilson SK, Mangubhai S, Ussi AM, Guillaume MMM, Humphries AT, Patankar V, Shedrawi G, Pagu J, Grimsditch G (2020) Highly variable taxa-specific coral bleaching responses to thermal stresses. Mar Ecol Prog Ser 648:135–151
Muko S, Arakaki S, Nagano M, Sakai K (2013) Growth form-dependent response to physical disturbance and thermal stress in Acropora corals. Coral Reefs 32:269–280
Murakami T, Ukai A, Kohno H, Mizutani A, Shimokawa S, Nakase K, Noguchi K, Yasuda T (2012) Relathionships between distributions of corals and physical environments in Amitori Bay, Iriomote Island Japan. Japan Soc Civil Eng B3 68(2):I_1133-I_1138 (in Japanese with English abstract)
Murakami T, Kohno H, Yamamoto Y, Mizutani A, Shimokawa S (2015) Numerical simulation for initial dynamic state of bundle based on filed observations of Acroporidae spawning in Amitori Bay, Iriomote Island, Japan. J Japan Soc Civil Eng B3 71(2):I_1225-I_1230 (in Japanese with English abstract)
Murakami T, Kohno H, Nakamura M, Tamamura N, Mizuztani A, Shimokawa S (2017) Bleaching in vertically distributed corals in Amitori Bay of Iriomtoe Iskand. Japan Soc Civil Eng B3 73(2):I_881-I_886 (in Japanese with English abstract)
Murakami T, Kohno H, Nakamura M, Noritake K, Kinoshita H, Mizutani A, Shimokawa S (2020) Numerical analysis of dispersion-retention of coral larvae before and after large-scale coral bleaching in Amitori Bay Iriomote Island, Japan. J Japan Soc Civil Eng B3 76(2):I_840-I_845 (in Japanese with English abstract)
Nakamura M, Murakami T, Kohno H, Noda W, Matsushita Y, Mizutani A (2017) Coral recruitment on a local scale in Amitori Bay, Iriomote Island, estimated by settlement plates and numerical analysis. J Japan Soc Civil Eng B2 72(2):I_1279-I_1284 (in Japanese with English abstract)
Nakamura T, van Woesik R (2001) Water-flow rates and passive diffusion partially explain differential survival of corals during the 1998 bleaching event. Mar Ecol Prog Ser 212:301–304
Palumbi SR, Barshis DJ, Traylor-Knowles N, Bay RA (2014) Mechanisms of reef coral resistance to future climate change. Science 344:895–898
Papina M, Sakihama Y, Bena C, van Woesik R, Yamasaki H (2002) Separation of highly fluorescent proteins by SDS-PAGE in Acroporidae corals. Comp Biochem Physiol 131:767–774
Perry CT, Morgan KM (2017) Post-bleaching coral community change on southern Maldivian reefs: is there potential for rapid recovery? Coral Reefs 36:1189–1194
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to ImageJ: 25 years of image analysis. Nat Methods 9:671–675
Stobart B, Teleki K, Buckley R, Downing N, Callow M (2005) Coral recovery at Aldabra Atoll, Seychelles: five years after the 1998 bleaching event. Philos Trans R Soc A 363:251–255
van Woesik R, Sakai K, Ganase A, Loya Y (2011) Revisiting the winners and the losers a decade after coral bleaching. Mar Ecol Prog Ser 434:67–76
Ward S, Harrison O, Hoegh-Guldberg O (2000) Coral bleaching reduces reproduction of scleractinian corals and increases susceptibility to future stress. Proceedings 9th international coral reef symposium, Vol 2, Bali, 1123-1128.
Wilkinson CR (1998) Status of coral reefs of the world: 1998, global coral reef monitoring network, Australian Institute of Marine Science, Townsville, Australia.
Wilkinson CR (2000) Status of coral reefs of the world: 2000, global coral reef monitoring network, Australian Institute of Marine Science, Townsville, Australia.
Yokochi H, Shimoike K, Kajiwara K, Nomura K, Kitano Y, Matsumoto H, Shimada G, Sugihara K, Suzuki G, TachikawaH YH, Zayasu Y, Kimura T, Kohno H (2019) A preliminary report on hermatypic corals of Amitori Bay, Iriomote-jima, Ryukyu Islands, Japan. Study Rev Iriomote is ORRC Tokai Univ 2018:36–69 (in Japanese with English abstract)
Acknowledgements
Field surveys for this study were conducted with the assistance of Yusuke Matsushita, Wataru Noda, Naoya Tamamura, Yoshifumi Soyama, Youhei Takeshita, Kazuto Ohno, Kouzou Onaga, Akito Fukushima, Kaori Kurosawa, Ayano Kunishima, Hinano Kinoshita, Kohki Noritake, and Haruki Asahi, who were then students of the School of Marine Science and Technology, Tokai University. We extend our deepest gratitude for their kind support. We are grateful to Ken Sakihara for supporting our research.
Funding
This study was financially supported by JSPS KAKENHI Grant Numbers 16K07527 and by Tokai University General Research Organization Grant of 2016 to 2020.
Author information
Authors and Affiliations
Contributions
MN, TM, SS, and HK conceived the research ideas and methodology. MN, TM, and AM collected data in the field. MN led the writing of the manuscript, with all authors contributing significantly to the writing and giving final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
On behalf of all authors, the corresponding author declares that there are no conflicts of interest.
Ethical approval
All field work was done in Amitori Bay. This work did not cause damage to the coral communities in the bay. Sampling of recruits on the settlement panel pairs was approved by the Okinawa Prefectural Government.
Additional information
Responsible Editor: C. Wild.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Nakamura, M., Murakami, T., Kohno, H. et al. Rapid recovery of coral communities from a mass bleaching event in the summer of 2016, observed in Amitori Bay, Iriomote Island, Japan. Mar Biol 169, 104 (2022). https://doi.org/10.1007/s00227-022-04091-2
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-022-04091-2