Abstract
Herbivorous fish can increase coral growth and survival by grazing down algal competitors. With coral reefs in global decline, maintaining adequate herbivory has become a primary goal for many managers. However, herbivore biomass targets assume grazing behavior is consistent across different reef systems, even though relatively few have been studied. We document grazing behavior of two scarid species in Antigua, Barbuda, and Bonaire. Our analyses show significant differences in intraspecific feeding rates, time spent grazing, and intensity of grazing across sites, which may alter the ecological impact of a given scarid population. We suggest several hypothesized mechanisms for these behavioral variations that would benefit from explicit testing in future research. As managers set targets to enhance herbivory on reefs, it is critical that we understand potential differences in scarid grazing impact. Our findings demonstrate the variability of grazing behavior across different reef sites and call for further investigation of the drivers and ecological implications of these inconsistencies.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Herbivorous fishes can play a critical role in coral reef ecosystems by suppressing algae and thereby facilitating coral growth, recruitment, and survival (Box and Mumby 2007; Rasher and Hay 2010; Steneck et al. 2014). In recent decades, coral reefs have become increasingly threatened by diverse anthropogenic impacts, including overfishing of herbivorous fish populations (Hughes et al. 2007; Edwards et al. 2014). Insufficient herbivory can allow algae to outcompete corals for space, ultimately driving shifts from coral to algal dominance that have occurred in much of the Caribbean region (Jackson et al. 2014). Coral reef fisheries thus cannot simply manage for sustainable yields as in traditional fisheries, but must also consider the levels of persistent herbivory required to maintain critical ecosystem functions. Increasing attention has been given to understanding how much herbivory is needed to sustain coral reef health and to identify thresholds of herbivore biomass that can be used to guide fisheries management targets (McClanahan et al. 2011; Adam et al. 2015; Karr et al. 2015).
However, all herbivorous fish are not the same in terms of their grazing impact on reefs, and variation can occur both among and within species. Studies have highlighted functional differences across species and size classes in terms of feeding morphology, selectivity, and foraging distribution within a reefscape (Bruggemann et al. 1994, 1996; Bonaldo and Bellwood 2008; Lokrantz et al. 2008; Ong and Holland 2010; Burkepile and Hay 2011; Rasher et al. 2013; Afeworki et al. 2013; Adam et al. 2015, 2018; Hoey 2018). Selective exclusions of different herbivorous fish species result in distinct algal communities (Burkepile and Hay 2011), highlighting the ecological significance of interspecific differences in functional roles among herbivores and suggesting that simple targets for total herbivore populations may be inadequate in managing for coral reef health.
In addition to established interspecific and size-specific differences, environmental contexts can also drive variability in herbivore behavior. Several empirical studies have demonstrated the sensitivity of behaviors such as foraging and movement to predator presence (Madin et al. 2010b; Davis et al. 2017); reef rugosity (Catano et al. 2014); grazing behaviors of proximate herbivores (Gil and Hein 2017); and the abundance, distribution, and nutritional content of algal resources (Tootell and Steele 2016; Davis et al. 2017). In some cases, these behavioral variations have been explicitly linked to larger ecosystem impacts. For example, fishing-induced declines in predatory fish abundances can increase grazing ranges of herbivorous fish and alter seascape-level algal distribution patterns (Madin et al. 2010b; DiFiore et al. 2019).
Despite a growing understanding of the ecological importance of herbivore behavior and its sensitivity to environmental conditions, many efforts to predict herbivore impact on reefs assume species- and size-specific feeding rates that remain constant across different reef environments, overlooking factors such as fishing pressure and habitat shifts that, as noted above, have the potential to dramatically alter grazing behaviors. For example, many reef models use grazing behavior data from one or two locations (e.g., Mumby et al. 2006; Bozec et al. 2016; Perry et al. 2018) which—while still valuable—may not accurately represent dynamics on other reefs. Other studies have struggled to establish links between herbivore biomass and reef health (e.g., McClanahan et al. 2011; Karr et al. 2015; Bruno et al. 2019), which could reflect variation in herbivore behavior that makes biomass an incomplete metric of herbivory. If herbivore feeding activity is suppressed under degraded reef conditions, such as low reef structural complexity or low fish populations, the biomass of herbivorous fish identified as capable of maintaining reef function in pristine systems may be insufficient in degraded environments. Insufficient herbivory may of course further reef degradation, potentially forming a reinforcing feedback loop (Mumby and Steneck 2008; Nyström et al. 2012; Bozec et al. 2013; Adam et al. 2015). Knowledge gaps around herbivore grazing behaviors are particularly important as we seek to manage individual reef ecosystems that vary greatly in terms of reef context and condition.
Here we assess both interspecific and intraspecific variability in multiple components of feeding behavior for two scarid (parrotfish) species across reef sites around three Caribbean islands and discuss potential underlying mechanisms, ecological consequences, and management implications. We provide a framework of hypothesized pathways in which human activities may alter reef function through impacts on herbivore behavior that motivate future research and can be used to guide management.
Materials and methods
Study sites
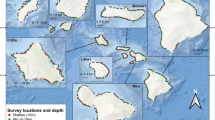
Caribbean reefs vary greatly in terms of anthropogenic impacts and reef condition. Our study focused on 13 reef sites off the islands of Bonaire, Antigua, and Barbuda (Fig. 1) that encompass a range of benthic and fish community conditions. Bonaire has among the highest live coral cover and herbivorous fish biomass in the Caribbean region (Jackson et al. 2014), likely a result of longstanding fishing restrictions that include the prohibition of parrotfish harvest (Steneck et al. 2019). Antiguan and Barbudan reefs are more representative of many Caribbean reefs today (Jackson et al. 2014), with relatively low coral cover and high algal abundances, as well as reduced fish stocks due to substantial local fishing pressure. All three islands receive regular wave and wind exposure from the east and northeast, and sites were selected on relatively sheltered western- and southwestern-facing shores for dive feasibility.
Surveys
All data were collected between March and August of 2017. Reef characteristics were assessed at 10 m depth to control for the influence of depth on algal growth rates, with behavioral observations of fish initiated between 8 and 12 m depths. Behavioral data were discarded if focal fish left a 5–15 m depth range during the observation period.
Behavioral observations
We observed grazing behavior of two dominant scarid species, Sparisoma viride and Scarus vetula, at each of our 13 study sites. We targeted these species because of their relative abundance as well as their contrasting grazing mechanisms. S. vetula takes relatively shallow, scraping bites and ingests mostly epilithic algae, or algae growing on a substrate’s surface. S. viride is an excavating grazer, taking deeper bites containing large amounts of both endolithic algae, which grow within the skeleton of a substrate such as dead coral or porous rock, and crustose algae, which form a thin crust on a substrate’s surface (Bruggemann et al. 1994). This analysis focuses only on initial phase (female) individuals due to their relatively high abundance and to eliminate potential interactions between territorial male behavior and feeding patterns. A size window of 15–30 cm forklength was used to reduce the potentially confounding effects of fish size on feeding behaviors.
Prior to data collection, three divers conducted underwater size calibrations with marked PVC reference pipes to ensure consistency and accuracy of fish forklength estimates. PVC dive sleeves (tubes on which data were recorded) were marked at 5 cm increments to provide size references during data collection. Practice dives were completed at the onset of the Bonaire, Antigua, and Barbuda data collection periods, during which divers took turns observing each other’s fish follows to ensure consistency in diver behavior and data notation.
Divers quantified grazing behavior by following individual fish for a 2-min observation period. Once a target fish was identified, we estimated fish size and allowed for a 15-s calibration period. We initiated all follows from a distance of at least 3.5 m based on previously established flight initiation distances (S. viride = 2.4 ± 0.4 m, S. vetula = 2.8 ± 0.4 m; Table S2) at spearfished sites in Antigua. Fish in Bonaire were assumed to have smaller flight initiation distances because of the longstanding and heavily enforced spearfishing ban, but we still initiated follows from a conservative 3.5 m distance. Divers maintained this distance unless the focal fish approached, in which case divers maintained their positioning. We discarded data from any incomplete follows (e.g., where visual contact could not be maintained for a full 2-min period) or follows where fish hid or fled from the observing diver. After each 2-min observation, divers moved slowly in a preestablished direction along the reef and identified a subsequent focal individual of a different species and/or estimated forklength to avoid repeated observations of the same individual (Nash et al. 2016).
During each follow period, divers recorded the commencement and cessation of grazing forays and the number of bites taken during each foray. Grazing forays were defined as a cluster of consecutive bites and were distinguished from a preceding foray by an elevation of the fish’s head > 45° above the substrate and active swimming to another location (Nash et al. 2012). We used these grazing data to quantify several components of feeding behavior: active bite rate, time spent grazing, feeding rate, and grazing intensity (Table 1). Active bite rate refers to the frequency of bites taken during periods of active feeding, while feeding rate refers to the frequency of bites taken during the entire duration of a follow, including time not spent feeding. Time spent grazing is reported as the fraction of the total observation period during which a fish was actively feeding. Grazing intensity refers to the average number of consecutive bites taken in a feeding foray before feeding ceases.
Reef community data
At each site we conducted fish, benthic, and rugosity surveys to quantify various components of reef condition (Table 2). We used a modified Atlantic Gulf Rapid Reef Assessment (AGRRA; Lang et al. 2010) protocol with 30 m by 4 m belt transects and 10 m point-intercept transects for fish and benthic surveys, respectively. We recorded all herbivorous and piscivorous fish encountered that were larger than 5 cm forklength. On benthic surveys, we assessed percent cover of live and dead coral, epilithic turf algae, macroalgae, and other benthic organisms by identifying the substrate under transect points at 10 cm intervals. In cases where multiple substrate types overlapped (e.g., dead coral covered by algae or other benthic organisms), we identified the substrate based on the uppermost layer. We measured the canopy height of turf and macroalgae at each point where it was present to a precision of 1 mm. To assess reef rugosity, we measured the length of a line run molded to the reef contour directly below each meter of the taut 10 m benthic transect tape (sensu Alvarez-Filip et al. 2009). We carried out between five and seven fish transects and between four and five benthic and rugosity transects per site.
Analysis
To estimate fish biomass at each site, we calculated the weight of individual fish encountered on underwater surveys using published length–weight relationships (Bohnsack and Harper 1988). We classified potential predators as piscivores above 30 cm forklength based on approximations of predator gape size relative to the body depth of the smallest (15 cm forklength) S. viride and S. vetula individuals observed in this study (details provided in electronic supplementary material). While optimal prey size is likely smaller than a predator’s full gape (Mumby et al. 2006), consumption of prey up to gape size has been observed (Wainwright and Richard 1995; Nash et al. 2012). We used benthic point-intercept data to calculate the proportion of each transect composed of each benthic substrate type. To calculate rugosity, we generated a ratio of contoured to taut transect lines where 1 is a flat surface and increasing values indicate increasing complexity (Alvarez-Filip et al. 2009). Mean fish, benthic, and rugosity characteristics across transects for each site were integrated via principal component analysis (PCA) to characterize differences across our 13 study sites. All analyses were conducted in R 4.0.2 (R Core Team 2020).
When analyzing behavioral data, we excluded one site in Barbuda (Pallaster West) from S. viride analyses and two sites in Antigua (Rendezvous and Turtle Bay) and one in Barbuda (Pallaster East) from S. vetula analyses due to a low abundance of initial phase individuals at these sites. Thus, results are based on a total sample size of n = 194 and n = 163 individuals for S. viride and S. vetula, respectively, with means of 16.2 ± 1.6 and 16.3 ± 3.2 individuals observed per site (see Table S3). Grazing intensity was calculated by averaging the number of bites in a complete feeding foray for each individual fish followed. Site-level summary statistics were calculated using mean values of bite rates, time spent grazing, feeding rates, and grazing intensity. Variation among sites was quantified via Kruskal–Wallis analysis of variance, because heteroscedasticity of behavioral response variables violated parametric assumptions.
To evaluate the effects of reef condition, species, and fish size on grazing behaviors across sites, we used generalized additive mixed models (GAMMs) with Gaussian distributions using the mgcv package in R (Wood 2020). GAMMs allow for detection of nonlinear relationships among variables as well as the distinction among fixed and random effects (Zuur et al. 2009). Because explanatory fish and benthic community variables of interest exhibited collinearities (VIF (variable inflation factor) > 3; Zuur et al. 2007), PC1 and PC2 from our PCA were used to summarize variations in reef condition. Our models included combinations of PC1, PC2, species, and mean size (forklength) of focal fish for each species and site as explanatory variables, as well as interactions between species and PC1, species and PC2, and species and size to allow for different responses among species. All continuous variables (PC1, PC2, size, and any interactions) were included with smooth terms to allow for potential nonlinear relationships, while species, a categorical variable, was included with a parametric term. Island was included as a random effect. Mean feeding rates and grazing intensities were calculated for each species and site and included as the two behavioral response variables for our GAMMs. The number of knots, which correlates with the complexity of a GAMM’s fitted spline, was set to three to prevent overfitting while still accommodating potential nonlinear relationships (Zuur et al. 2009). We evaluated models using Akaike’s information criteria adjusted for small sample sizes (AICc) using the MuMIn package in R (Bartón 2020). Effective degrees of freedom (edf) are reported to quantify nonlinearities among continuous predictor and response variables (Hunsicker et al. 2016).
Results
Reef community composition
Reef characteristics varied substantially among sites (Fig. 2). PC1 and PC2 accounted for 55.6% and 24.6% of variation among sites, respectively. High PC1 values reflect primarily low coral cover, reef rugosity, and scarid biomass and high macroalgal cover and canopy height (Table 2), essentially indicating poor reef health. High PC2 values, on the other hand, primarily indicate high scarid density and low turf algal cover. Large predator biomass and density were highly correlated (R2 = 0.99), so only biomass was used to represent predator presence in the PCA. Bonaire sites were characterized by higher scarid and predator biomass and lower turf and macroalgal canopy height and macroalgal percent cover than Antiguan and Barbudan sites. Barbudan sites typically had higher scarid densities and reef rugosity and lower percent cover of turf algae than Antiguan sites, with both islands having similar levels of coral cover and scarid and predator biomasses.
Principal component analysis (PCA) of reef characteristics at 13 assessed sites across Antigua Barbuda, and Bonaire. Arrows represent the contributions of nine reef variables to the first and second principal components (PC1 and PC2). PC1 and PC2 account for 55.6% and 24.5% of variance in reef characteristics, respectively
Feeding behavior
Feeding rates, time spent grazing, and grazing intensity varied significantly (p < 0.001) across sites for both S. vetula and S. viride populations (Fig. 3). For S. vetula, feeding rates differed almost ninefold and grazing intensity differed nearly fivefold between the highest and lowest sites, while S. viride feeding rates and grazing intensities varied over fivefold and sixfold, respectively. Differences in site-level feeding rates reflected differences in the fraction of time that fish spent grazing as opposed to differences in bite rates while actively feeding, which did not differ significantly among sites (p > 0.5).
To investigate potential drivers of these documented behavioral differences, we compared GAMMs with different combinations of reef condition and fish species and size predictors. We focused on drivers of feeding rate and grazing intensity, omitting time spent grazing because of its inherent link with overall feeding rate. Species and PC1 were included in all of the best models predicting both feeding rate and grazing intensity (Tables 3, S4). The role of species in driving feeding behaviors reflects known differences in grazing morphologies between S. viride and S. vetula, with S. vetula taking more frequent bites (Table S5). PC1 had an inverse relationship with both feeding rate and grazing intensity (see Figs. 2, 3), suggesting that scarids take fewer total bites and fewer bites per individual grazing foray on reefs characterized by low scarid and predator abundances, low coral cover and rugosity, and high turf and macroalgal cover. In the succeeding models for both feeding rate and grazing intensity, PC1 had a stronger negative effect for S. vetula than S. viride (Table S5). Fish size was included in the third best performing models for both feeding rate and grazing intensity, although it was not a significant predictor in either model (Table S5). Continuous predictor variables had linear relationships (edf = 1) in all selected models except for the second-best model predicting grazing intensity, in which the species and PC1 interaction term was slightly nonlinear (edf = 1.455 and 1.432 for S. vetula and S. viride, respectively; Table S5).
Discussion
Several underlying mechanisms linking environmental conditions to herbivore behavior may be driving observed differences among sites. Covariation among linked reef characteristics restrict us to using PCA indicators as opposed to specific reef traits in our GAMM analyses, and our correlational results do not allow us to determine causality (e.g., algal abundance may be causing differences in herbivore behavior, or vice versa). Despite these limitations, we present hypotheses for potential underlying mechanisms here (Fig. 4) and discuss relevant theory and ecological and management implications. While we cannot conclusively test these hypotheses within the scope of this study, we discuss preliminary evidence for each and highlight priorities for further research.
Hypothesized pathways in which various environmental factors may impact herbivore feeding behaviors, and potential feedback loops affecting overall grazing impact and coral health. Dashed lines indicate behaviorally mediated effects while solid lines represent density-mediated effects. Proposed mechanisms include social feeding dynamics (1), risk effects driven by predator presence (2) or reef rugosity (3), and bite content (4)
Social feeding or shared vigilance hypothesis: shared vigilance theory describes the benefits to prey of grouping together (e.g., schools, herds), as each individual can spend less time looking out for predators and more time carrying out other behaviors such as feeding (Pulliam et al. 1982; Roberts 1996; Lima and Bednekoff 1999). Assuming constant predator populations, as group size decreases, vigilance becomes more concentrated on each individual group member. A recent empirical study demonstrated that herbivorous reef fish use social cues from the density and behavior of other herbivores to determine whether or not to feed (Gil and Hein 2017). Individual fish were more likely to commence grazing as the presence of other feeding individuals increased. This ‘behavioral coupling’ may drive a potential reinforcing feedback loop in which higher herbivore abundances increase the feeding activity of each individual fish, further increasing the grazing impact of a given school (Fig. 4). While we cannot distinguish among correlated reef characteristics here, scarid biomass was one of the strongest contributors to PC1 (Table 2), a significant predictor of feeding rate and grazing intensity in all best performing GAMMs (Table 3). Scarid density contributed moderately to PC1 and strongly to PC2, which was not a significant predictor in any of the selected models. More data and dedicated studies are needed to distinguish the exact effects of herbivore abundance in determining feeding behaviors.
Predation risk hypothesis: as introduced above, predation risk can be an important driver of feeding behavior. While collective vigilance can moderate predation risk, predator presence is an ultimate determinant. Previous work has documented the suppressive effect of acute predator presence (typically simulated with large piscivore decoys) on herbivore feeding rates (Madin et al. 2010a; Rizzari and Frisch 2014; Catano et al. 2017; DiFiore et al. 2019). Reef rugosity may also impact predation risk by modifying prey refuge availability and visibility to predators (Alvarez-Filip et al. 2009). While predator biomass was a moderate driver of PC1, rugosity was among the strongest (Table 2), suggesting it may have played a role in predicting feeding rate and grazing intensity in our top performing models.
While not explicitly investigated here, risk of predation from spearfishers may play a substantial role in determining scarid grazing behavior. The larger S. vetula and S. viride individuals observed in our behavioral observations are approaching size refuge from most natural predators, but they would be likely targets for spearfishers in both Antigua and Barbuda. We did not quantify spearfisher presence across sites (beyond its enforced absence in Bonaire), so we cannot assess this potential effect here, but point out that it may have important implications for herbivore behavior. While declines in herbivorous fish abundances that could alter perceived risk and social feeding behaviors may be countered by simultaneous declines in natural predator abundance, spearfishing can have the effect of both reducing group size and increasing predation risk (Fig. 4). Similar to the social feeding hypothesis, spearfishing could have a double impact of both reducing herbivorous fish biomass and reducing the grazing impact of remaining fish by altering perceived risk environments. While several studies have documented the effects of spearfishing on fish flight behavior (e.g., Gotanda et al. 2009; Januchowski-Hartley et al. 2011, 2015), further work is needed to investigate the potential chronic effects of spearfishing on herbivore feeding behaviors.
Bite content hypothesis: differences in feeding rates could also reflect differences in the content or quality of an individual bite. Significant negative relationships between PC1 and both feeding rates and grazing intensity in all top models reflect negative associations with the cover and canopy height of both turf and macroalgae (Table 2). In sites with higher algal canopies, scarids may be obtaining more biomass of food per bite than in areas with heavily cropped algae, requiring them to take fewer bites to obtain the same nutritional intake. However, previous empirical work done on the bite volume of both S. viride and S. vetula showed that bite content (biomass of algae removed) did not vary significantly with algal canopy height (Bruggemann et al. 1994). It is also possible that scarids need to employ more rapid feeding rates if forced to graze on material with lower nutritional quality. This could be the case in Bonaire, where higher parrotfish densities and lower algal cover may make high quality resources more limited. While actual bite content was not assessed here in terms of biomass nor nutritional quality, it would be valuable to investigate potential differences across sites as these could have implications for both fish growth and benthic dynamics.
Variation in herbivore feeding rates and grazing intensity may have important ecological consequences and management implications for coral reef systems. Feeding rates can directly influence the amount of algae removed from a given reef, while grazing intensity can influence the effectiveness of grazing in cropping algae. Grazing forays with more consecutive bites suggest more concentrated grazing, which are likely more effective in maintaining sufficiently low algal canopy heights and clearing substrate for growing or newly recruiting corals than bites dispersed throughout the reefscape. Experimental evidence has shown that more spatially concentrated grazing results in persistently suppressed algal canopy heights, suggesting that a given herbivore population’s ability to maintain algae in a cropped state depends on the distribution of grazing efforts (Williams et al. 2001).
Because of these ecological implications, behavioral variations may be important in guiding effective coral reef management. If feeding rate data from one area are used to predict the impact of a given species in other parts of the region, we may be overlooking important behavioral differences and misrepresenting grazing levels of different scarid populations. Feeding rates are used in herbivory models to calculate the grazing impact by a given fish community (usually expressed as the amount of reef surface area grazed per unit time). Studies examining relationships between herbivore biomass and reef health have had difficulty finding clear relationships (McClanahan et al. 2011; Karr et al. 2015; Bruno et al. 2019), possibly because these relationships are being distorted by differences in feeding behavior in different reef environments. Several hypothesized mechanisms predict lower feeding rates in more degraded reef conditions, implying that models using feeding rates from exemplary reefs may overestimate herbivory when applied to other systems. While these exact mechanisms are not tested here, we document some supporting trends and pose important questions for future research. Specifically, the relative roles of scarid abundance and scarid behavior, and the potentially reinforcing effects of these two factors, are critical areas for future empirical and modelling work.
While our study investigates the relationships between various reef traits and herbivore grazing behaviors, it is also important to note that we cannot establish causality here and that many of these relationships may be bidirectional or cyclical. For example, correlations between high algal abundances and low feeding rates may mean algal abundance is driving reduced feeding rates or that reduced feeding rates are increasing algal abundances, or that both relationships drive a reinforcing feedback loop. If mechanisms such as social feeding are responsible for variations in herbivore behavior, then links to algal communities could indicate the ecological implications of these behavioral impacts. Because algal variables were expectedly correlated with other reef characteristics such as rugosity and herbivore populations, we could not examine them here as potential response variables. Further investigation of these exact drivers is required to determine causality and investigate potential feedback loops in which grazing reductions attributed to more degraded reef conditions would further compound reef deterioration (Fig. 4). While additional experimental work is needed to distinguish underlying behavioral triggers, it is also likely that multiple mechanisms are acting in tandem as fishing, fish, and benthic characteristics are tightly linked in most reef systems.
Human activities are driving widespread changes in wildlife behaviors across marine and terrestrial ecosystems (Madin et al. 2015; Larson et al. 2016), which have the potential to affect critical ecological processes (Hebblewhite et al. 2005; Madin et al. 2010b; Ripple and Beschta 2012; Wilson et al. 2020). This study documents differences in multiple scarid feeding behaviors across various Caribbean reef systems and suggests possible sensitivity of these critical feeding behaviors to reef condition. Coral reef managers should be aware of pathways in which human activities may reduce herbivore grazing impacts and be conservative in setting targets for herbivorous fish biomass in areas where feeding behaviors may be suppressed. If behavioral variation among reef environments is ignored, managers of characteristically degraded areas may overestimate the grazing impact of a given herbivore population when using behavioral data from more ‘pristine’ systems, thereby underestimating the herbivore biomass needed to sufficiently suppress algae and insufficiently restricting fisheries. Managers may also inaccurately assume that increasing herbivore biomass will linearly increase herbivory, while in fact these relationships may be moderated by other environmental conditions. If social feeding dynamics are a strong driver of herbivory behaviors, cessation of spearfishing in reef areas would have disproportionately positive effects on herbivory, in that herbivore biomass would increase as would the grazing impact of each individual. However, further investigation of these specific drivers is needed before conclusive management recommendations can be made. Initial insights from this study suggest a need for increased incorporation of behavioral effects into ecosystem management and highlight critical areas for future research.
Data availability
All data are available at https://github.com/molwilson/scarid-behavior.
Code availability
All code is available at https://github.com/molwilson/scarid-behavior.
References
Adam TC, Burkepile DE, Ruttenberg BI, Paddack MJ (2015) Herbivory and the resilience of Caribbean coral reefs: knowledge gaps and implications for management. Mar Ecol Prog Ser 520:1–20. https://doi.org/10.3354/meps11170
Adam TC, Duran A, Fuchs CE, Roycroft MV, Rojas MC, Ruttenberg BI, Burkepile DE (2018) Comparative analysis of foraging behavior and bite mechanics reveals complex functional diversity among Caribbean parrotfishes. Mar Ecol Prog Ser 597:207–220. https://doi.org/10.3354/meps12600
Afeworki Y, Zekeria ZA, Videler JJ, Bruggemann JH (2013) Food intake by the parrotfish Scarus ferrugineus varies seasonally and is determined by temperature, size and territoriality. Mar Ecol Prog Ser 489:213–224. https://doi.org/10.3354/meps10379
Alvarez-Filip L, Dulvy NK, Gill JA, Côté IM, Watkinson AR (2009) Flattening of Caribbean coral reefs: region-wide declines in architectural complexity. Proc Biol Sci R Soc 276:3019–3025. https://doi.org/10.1098/rspb.2009.0339
Bartón K (2020) MuMIn: multi-model inference. R package version 1.43.17
Bohnsack JA, Harper DE (1988) Length-weight relationships of selected marine reef. Fishes from the Southeastern United States and the Caribbean. Tech Memm NMFS-SEFC-215, NOAA, Miami, FL
Bonaldo RM, Bellwood DR (2008) Size-dependent variation in the functional role of the parrotfish Scarus rivulatus on the Great Barrier Reef, Australia. Mar Ecol Prog Ser 360:237–244. https://doi.org/10.3354/meps07413
Box SJ, Mumby PJ (2007) Effect of macroalgal competition on growth and survival of juvenile Caribbean corals. Mar Ecol Prog Ser 342:139–149
Bozec Y-M, Yakob L, Bejarano S, Mumby PJ (2013) Reciprocal facilitation and non-linearity maintain habitat engineering on coral reefs. Oikos 122:428–440. https://doi.org/10.1111/j.1600-0706.2012.20576.x
Bozec Y-M, O’Farrell S, Bruggemann JH, Luckhurst BE, Mumby PJ (2016) Tradeoffs between fisheries harvest and the resilience of coral reefs. Proc Natl Acad Sci 113:201601529–201601529. https://doi.org/10.1073/pnas.1601529113
Bruggemann J, Kuyper M, Breeman A (1994) Comparative analysis of foraging and habitat use by the sympatric Caribbean parrotfish Scarus vetula and Sparisoma viride (Scaridae). Mar Ecol Prog Ser 112:51–66
Bruggemann J, Van Kessel AM, Van Rooij JM, Breeman AM (1996) Bioerosion and sediment ingestion by the caribbean parrotfish Scarus vetula and Sparisoma viride: Implications of fish size, feeding mode and habitat use. Mar Ecol Prog Ser 134:59–71. https://doi.org/10.3354/meps134059
Bruno JF, Côté IM, Toth LT (2019) Climate change, coral loss, and the curious case of the parrotfish paradigm: why don’t marine protected areas improve reef resilience? Annu Rev Mar Sci 11:307–334. https://doi.org/10.1146/annurev-marine-010318-095300
Burkepile DE, Hay ME (2011) Feeding complementarity versus redundancy among herbivorous fishes on a Caribbean reef. Coral Reefs 30:351–362. https://doi.org/10.1007/s00338-011-0726-6
Catano LB, Shantz AA, Burkepile DE (2014) Predation risk, competition, and territorial damselfishes as drivers of herbivore foraging on Caribbean coral reefs. Mar Ecol Prog Ser 511:193–207. https://doi.org/10.3354/meps10921
Catano LB, Barton MB, Boswell KM, Burkepile DE (2017) Predator identity and time of day interact to shape the risk–reward trade-off for herbivorous coral reef fishes. Oecologia 183:763–773. https://doi.org/10.1007/s00442-016-3794-z
Davis K, Carlson PM, Bradley D, Warner RR, Caselle JE (2017) Predation risk influences feeding rates but competition structures space use for a common Pacific parrotfish. Oecologia 184:1–11. https://doi.org/10.1007/s00442-017-3857-9
DiFiore B, Queenborough S, Madin E, Paul V, Decker M, Stier A (2019) Grazing halos on coral reefs: predation risk, herbivore density, and habitat size influence grazing patterns that are visible from space. Mar Ecol Prog Ser 627:71–81. https://doi.org/10.3354/meps13074
Edwards CB, Friedlander AM, Green AG, Hardt MJ, Sala E, Sweatman HP, Williams ID, Zgliczynski B, Sandin SA, Smith JE (2014) Global assessment of the status of coral reef herbivorous fishes: evidence for fishing effects. Proc R Soc B Biol Sci 281:20131835. https://doi.org/10.1098/rspb.2013.1835
Gil MA, Hein AM (2017) Social interactions among grazing reef fish drive material flux in a coral reef ecosystem. PNAS 114:4703–4708. https://doi.org/10.1073/pnas.1615652114
Gotanda KM, Turgeon K, Kramer DL (2009) Body size and reserve protection affect flight initiation distance in parrotfishes. Behav Ecol Sociobiol 63:1563–1572. https://doi.org/10.1007/s00265-009-0750-5
Hebblewhite M, White C, Nietvelt C, McKenzie J, Hurd T, Fryxell J, Bayley S, Paquet P (2005) Human activity mediates a trophic cascade caused by wolves. Ecology 86:2135–2144
Hoey AS (2018) Feeding in parrotfishes: the influence of species, body size, and temperature. Biology of parrotfishes. CRC Press, Boca Raton, pp 119–133
Hughes TP, Rodrigues MJ, Bellwood DR, Ceccarelli D, Hoegh-Guldberg O, McCook L, Moltschaniwskyj N, Pratchett MS, Steneck RS, Willis B (2007) Phase shifts, herbivory, and the resilience of coral reefs to climate change. Curr Biol 17:360–365. https://doi.org/10.1016/j.cub.2006.12.049
Hunsicker ME, Kappel CV, Selkoe KA, Halpern BS, Scarborough C, Mease L, Amrhein A (2016) Characterizing driver-response relationships in marine pelagic ecosystems for improved ocean management. Ecol Appl 26:651–663. https://doi.org/10.1890/14-2200
Jackson EJ, Donovan M, Cramer K, Lam V (2014) Status and trends of Caribbean coral reefs: 1970-2012. Global Coral Reef Monitoring Network, IUCN, Gland, Switzerland
Januchowski-Hartley FA, Graham NAJ, Feary DA, Morove T, Cinner JE (2011) Fear of fishers: human predation explains behavioral changes in coral reef fishes. PLoS ONE. https://doi.org/10.1371/journal.pone.0022761
Januchowski-Hartley FA, Graham NAJ, Cinner JE, Russ GR (2015) Local fishing influences coral reef fish behavior inside protected areas of the Indo-Pacific. Biol Conserv 182:8–12. https://doi.org/10.1016/j.biocon.2014.11.024
Karr KA, Fujita R, Halpern BS, Kappel CV, Crowder L, Selkoe KA, Alcolado PM, Rader D (2015) Thresholds in Caribbean coral reefs: implications for ecosystem-based fishery management. J Appl Ecol 52:402–412. https://doi.org/10.1111/1365-2664.12388
Lang J, Marks K, Kramer P, Kramer P, Ginsburg R (2010) AGRRA protocols version 5.4
Larson CL, Reed SE, Merenlender AM, Crooks KR (2016) Effects of recreation on animals revealed as widespread through a global systematic review. PLoS ONE 11:e0167259–e0167259. https://doi.org/10.1371/journal.pone.0167259
Lima SL, Bednekoff PA (1999) Temporal variation in danger drives antipredator behavior: the predation risk allocation hypothesis. Am Nat 153:649–659. https://doi.org/10.1086/303202
Lokrantz J, Nyström M, Thyresson M, Johansson C (2008) The non-linear relationship between body size and function in parrotfishes. Coral Reefs 27:967–974. https://doi.org/10.1007/s00338-008-0394-3
Madin EMP, Gaines SD, Warner RR (2010a) Field evidence for pervasive indirect effects of fishing on prey foraging behavior. Ecology 91:3563–3571. https://doi.org/10.1890/09-2174.1
Madin EMP, Gaines SD, Madin JS, Warner RR (2010b) Fishing indirectly structures macroalgal assemblages by altering herbivore behavior. Am Nat 176:785–801. https://doi.org/10.1086/657039
Madin EMP, Dill LM, Ridlon AD, Heithaus MR, Warner RR (2015) Human activities change marine ecosystems by altering predation risk. Glob Change Biol. https://doi.org/10.1111/gcb.13083
McClanahan TR, Graham NAJ, MacNeil MA, Muthiga NA, Cinner JE, Bruggemann JH, Wilson SK (2011) Critical thresholds and tangible targets for ecosystem-based management of coral reef fisheries. Proc Natl Acad Sci USA 108:17230–17233. https://doi.org/10.1073/pnas.1106861108
Mumby P, Steneck R (2008) Coral reef management and conservation in light of rapidly evolving ecological paradigms. Trends Ecol Evol 23:555–563. https://doi.org/10.1016/j.tree.2008.06.011
Mumby PJ, Dahlgren CP, Harborne AR, Kappel CV, Micheli F, Brumbaugh DR, Holmes KE, Mendes JM, Broad K, Sanchirico JN, Buch K, Box S, Stoffle RW, Gill AB (2006) Fishing, trophic cascades, and the process of grazing on coral reefs. Science 311:98–101. https://doi.org/10.1126/science.1121129
Nash KL, Graham NAJ, Januchowski-Hartley FA, Bellwood DR (2012) Influence of habitat condition and competition on foraging behaviour of parrotfishes. Mar Ecol Prog Ser 457:113–124. https://doi.org/10.3354/meps09742
Nash K, Abesamis R, Graham N, McClure E, Moland E (2016) Drivers of herbivory on coral reefs: species, habitat and management effects. Mar Ecol Prog Ser 554:129–140. https://doi.org/10.3354/meps11795
Nyström M, Norström AV, Blenckner T, de la Torre-Castro M, Eklöf JS, Folke C, Österblom H, Steneck RS, Thyresson M, Troell M (2012) Confronting feedbacks of degraded marine ecosystems. Ecosystems 15:695–710. https://doi.org/10.1007/s10021-012-9530-6
Ong L, Holland KN (2010) Bioerosion of coral reefs by two Hawaiian parrotfishes: species, size differences and fishery implications. Mar Biol 157:1313–1323. https://doi.org/10.1007/s00227-010-1411-y
Perry CT, Alvarez-Filip L, Graham NAJ, Mumby PJ, Wilson SK, Kench PS, Manzello DP, Morgan KM, Slangen ABA, Thomson DP, Januchowski-Hartley F, Smithers SG, Steneck RS, Carlton R, Edinger EN, Enochs IC, Estrada-Saldívar N, Haywood MDE, Kolodziej G, Murphy GN, Pérez-Cervantes E, Suchley A, Valentino L, Boenish R, Wilson M, Macdonald C (2018) Loss of coral reef growth capacity to track future increases in sea level. Nature 558:396–400. https://doi.org/10.1038/s41586-018-0194-z
Pulliam HR, Pyke GH, Caraco T (1982) The scanning behavior of juncos: a game-theoretical approach. J Theor Biol 95:89–103. https://doi.org/10.1016/0022-5193(82)90289-2
R Core Team (2020) R: a language and environment for statistical computing. R Foundation for Statistical Computing, Vienna, Austria
Rasher DB, Hay ME (2010) Chemically rich seaweeds poison corals when not controlled by herbivores. Proc Natl Acad Sci USA 107:9683–9688. https://doi.org/10.1073/pnas.0912095107
Rasher DB, Hoey AS, Hay ME (2013) Consumer diversity interacts with prey defenses to drive ecosystem function. Ecology 94:1347–1358. https://doi.org/10.1890/12-0389.1
Ripple WJ, Beschta RL (2012) Trophic cascades in Yellowstone: The first 15 years after wolf reintroduction. Biol Conserv 145:205–213. https://doi.org/10.1016/j.biocon.2011.11.005
Rizzari J, Frisch A (2014) Not worth the risk: apex predators suppress herbivory on coral reefs Justin. Oikos 123:829–836. https://doi.org/10.1111/oik.01318
Roberts G (1996) Why individual vigilance declines as group size increases. Anim Behav 51:1077–1086. https://doi.org/10.1006/anbe.1996.0109
Steneck RS, Arnold SN, Boenish R, de León R, Mumby PJ, Rasher DB, Wilson MW (2019) Managing recovery resilience in coral reefs against Climate-induced bleaching and hurricanes: a 15 year case study from Bonaire, Dutch Caribbean. Frontiers Marine Sci. https://doi.org/10.3389/fmars.2019.00265
Steneck RS, Arnold SN, Mumby PJ (2014) Experiment mimics fishing on parrotfish: Insights on coral reef recovery and alternative attractors. Mar Ecol Prog Ser 506:115–127. https://doi.org/10.3354/meps10764
Tootell JS, Steele MA (2016) Distribution, behavior, and condition of herbivorous fishes on coral reefs track algal resources. Oecologia 181:13–24. https://doi.org/10.1007/s00442-015-3418-z
Wainwright PC, Richard BA (1995) Predicting patterns of prey use from morphology of fishes. Environ Biol Fishes 44:97–113. https://doi.org/10.1007/BF00005909
Williams I, Polunin N, Hendrick V (2001) Limits to grazing by herbivorous fishes and the impact of low coral cover on macroalgal abundance on a coral reef in Belize. Mar Ecol Prog Ser 222:187–196. https://doi.org/10.3354/meps222187
Wilson MW, Ridlon AD, Gaynor KM, Gaines SD, Stier AC, Halpern BS (2020) Ecological impacts of human-induced animal behaviour change. Ecol Lett 23(10):1522–1536
Wood S (2020) mgcv: Mixed GAM computation vehicle with automatic smoothness estimation. R package version 1.8.33
Zuur AF, Ieno EN, Smith GM (2007) Analyzing ecological data. Springer, Berlin
Zuur AF, Ieno EN, Walker N, Saveliev AA, Smith GM (2009) Mixed effects models and extensions in ecology with R. Springer Science & Business Media, Berlin
Acknowledgements
We thank J. Mason, R. Boenish, and A. Casagrande for their assistance in reef surveys and behavioral observation dives, N. Psihoyos and R. Boltar for the use of their boats in Antigua and Barbuda, and STINAPA for all of their research support in Bonaire. We thank three anonymous reviewers for their constructive feedback on a previous version of this manuscript. We gratefully acknowledge the National Science Foundation GRFP program and The Schmidt Family Foundation for funding this work.
Funding
This work was supported by a National Science Foundation Graduate Research Fellowship and U.C. Santa Barbara Schmidt Research Accelerator Award to M. Wilson.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest. All data were obtained via observational and non-extractive methods and were collected in accordance with local research protocols. Data are publicly available at https://github.com/molwilson/scarid-behavior.
Additional information
Responsible Editor: A. Gori.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewers: undisclosed experts.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Wilson, M.W., Gaines, S.D., Stier, A.C. et al. Variation in herbivore grazing behavior across Caribbean reef sites. Mar Biol 168, 53 (2021). https://doi.org/10.1007/s00227-021-03844-9
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-021-03844-9