Abstract
Coastal kelp forest ecosystems create dynamic and productive habitats, supporting a wide range of epiphytic flora, invertebrates, fish and seabirds. Worldwide, kelp is harvested commercially, affecting kelp-associated animal communities. There is, however, limited knowledge of how fish and seabird respond to kelp harvest, highlighting the need to evaluate the ecological impact of harvest on all ecosystem levels. Using 6 years of GPS-tracking data, we examined the effects of kelp harvest on foraging behaviour of breeding European shags (Phalacrocorax aristotelis) from a colony in central Norway. We determined the spatial overlap between kelp harvest and foraging areas of shags and assessed the immediate, short- and long-term impacts of harvest on shag foraging behaviour. Our results demonstrated large spatial and temporal overlap in areas used by foraging shags and kelp harvest. We could not detect any clear alterations in the diving activity of shags due to kelp harvest. However, the broad temporal and spatial scale of our study constrained the detection of fine scale changes in shag behaviour in response to kelp harvest. Our study, nonetheless, identifies several issues that should be addressed before concluding on the effects of kelp harvest on seabird populations. This includes the need for experimental studies using directed and controlled harvest to investigate the effects of kelp harvest through the different trophic levels, including top predators. This is essential for ecosystem-based management of coastal resources, considering the many species composed in the coastal ecosystem.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Coastal marine ecosystems are ranked among the most productive ecosystems on earth, providing a range of resources to both humans and marine organisms (Costanza et al. 1997; Beaumont et al. 2008). At the same time these ecosystems are under pressure from human activities, contributing to their degradation and loss of habitat (Airoldi and Beck 2007; Halpern et al. 2008; Crain et al. 2009; Korpinen et al. 2013). In temperate and polar coastal ecosystems kelp forests create highly productive habitats (Mann, 1973; Steneck et al. 2002; Reed et al. 2008), facilitating a three-dimensional environment which supports a wide range of epiphytic flora and macrofauna (Steneck et al. 2002; Christie et al., 2003, 2009; Teagle et al. 2017). For instance, Christie et al. (2009) found that kelp forests along the Norwegian coast contained up to 300 different species of invertebrates and more than 100,000 individuals per square meter. Many of these invertebrates are important prey for a number of fish species, which use kelp forests as feeding and nursery areas and as refugia from predators (Norderhaug et al. 2005; Reisewitz et al. 2006; Bertocci et al. 2015). The high abundance of fish species attracts marine top predators, whose distributions are closely linked to that of kelp forests (Steneck et al. 2002; Fredriksen 2003; Reisewitz et al. 2006; Lorentsen et al. 2010; Christensen-Dalsgaard et al. 2017).
Throughout the world, various kelp species are harvested commercially for alginates, food, biofuel and other products (Vea and Ask 2011; Monagail et al. 2017). This has raised concerns regarding potential overexploitation and depletion of kelp as a natural resource (Ugarte and Sharp, 2001; Monagail et al. 2017) and highlights the need to evaluate the ecological impact of harvest on all trophic levels (Lorentsen et al. 2010). Kelp harvest targets large kelp plants, whereas the youngest and smallest plants remain within the trawl tracks after the harvest. The sudden increase in light exposure after harvest initiates a quick regrowth of these plants. Research has shown that kelp plants may reach preharvest size after four years. However, the recovery rates of individual kelp may not reflect recovery rates for the entire community, and the kelp-associated assemblages may take considerably longer to recover (Christie et al. 1998; Steen et al. 2016a, b).
Kelp harvest influence invertebrate and likely fish communities, but so far only a few studies have tried to estimate potential effects on the fish community (e.g. Lorentsen et al. 2010; Steen et al. 2012; 2016a, b). Most studies focus on single components of the kelp ecosystem restricted to small confined areas or species low in the food web (e.g. Christie et al. 1998). The small-scale variability in microbenthic assemblage properties is, however, often considerable (Frashetti et al. 2005), and a few small-scale samples may not be enough to detect the overall effect of harvesting (Stagnol et al. 2015). If kelp harvest impacts prey communities, it is likely to have effects throughout the food chain. Reductions in fish numbers after kelp removal have been demonstrated in different types of kelp forests (e.g. Bodkin 1988; Lorentsen et al. 2010), but these fish-kelp removal relationships are often species specific (O’Connor and Anderson 2010; Salter et al. 2010) and can depend on direct and indirect kelp canopy effects and fish species interactions (Steen et al. 2012; Norderhaug et al. submitted). Monitoring the impact of human exploitation, such as kelp harvest on an ecosystem, and separating human-induced changes from natural variability can be very challenging. It is nonetheless essential to assess the impacts of an anthropogenic disturbance such as kelp harvest across multiple trophic levels to understand the broader ecological implications of the activity on the coastal ecosystem. To our knowledge, only one study (Lorentsen et al. 2010) has tried to quantify the effects of kelp harvest on seabirds, demonstrating that great cormorants (Phalacrocorax carbo) preferentially foraged in unharvested areas and diving effort was higher when foraging in harvested compared to unharvested areas. Kelp harvest is only one of several human activities in the coastal zone threating seabirds worldwide (Dias et al. 2019), and understanding its impact is thus an essential piece of the puzzle in the protection of seabird species relaying on a functional kelp forest.
In Norway, the kelp species Laminaria hyperborea has been harvested for the alginate industry since the 1970s (Vea and Ask 2011) and ~ 150 000 tons wet weight of kelp is landed annually (fishery statistics, www.fiskeridir.no). In this study we wanted to assess the potential impact of kelp harvest on a coastal seabird species, the European shag (Phalacrocorax aristotelis, hereafter shag). The shag is a nearshore-foraging, pursuit-diving seabird breeding throughout the northeast Atlantic (Cramp and Simmons 1977). It is a species of national responsibility (> 25% of the European population of the species is found in Norway) with an estimated population of 28,000 pairs (Fauchald et al. 2015), representing 35% of the NE Atlantic population (Mitchell et al. 2004). The shag can dive down to depths of more than 60 m, but in Norway has been shown to forage at an average depth < 16 m (Christensen-Dalsgaard et al. 2017). One of the largest Norwegian shag breeding colonies is situated at the islands of Sklinna off Central Norway (Fig. 1). The marine area around Sklinna was opened for exploratory kelp harvest in 2014 and commercial harvest in 2015. Following this, concerns have been raised on the potential impact of the kelp harvesting on the breeding population of shags on Sklinna as their foraging range is strongly associated with the distribution of kelp forests (Christensen-Dalsgaard et al. 2017). The primary prey for shags at Sklinna is 0- and 1-group (year) saithe Pollachius virens up to c. 200 mm in length, constituting a mean of 73% of their diet during 2011–2016 (Hillersøy and Lorentsen 2012; Lorentsen et al. 2019). Saithe utilize kelp forests until they reach 3 years of age (~ 300 mm in length) at which time they migrate to pelagic areas and join the spawning population (Olsen et al., 2010). The proportion of saithe in the diet of shags has a positive influence on shag breeding success (Bustnes et al. 2013; Lorentsen et al. 2015, 2019). Kelp harvest thus has a potential to influence breeding success in shags through a decrease in saithe abundance from pre- to postharvest (cf. Lorentsen et al. 2010), reducing the prey available to the shags.
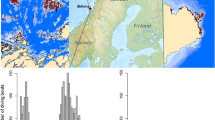
Map of Norway and overview of the study system. Star marks the colony on Sklinna. Left image: harvest sectors are shown as black lines, where each number shows the allowed harvest period (1: 1 Oct 2018–30 Sep 2019, 2: 1 Oct 2017–30 Sep 2018; 3: 1 Oct 2016–30 Sep 2017; 4: 1 Oct 2015–30 Sep 2016 and 5: 5 June 2015–30 Sep 2015). The grid cells where kelp is harvested (impact sites) are shown as dark grey cells, and the control areas are shown as light grey cells. The presence of kelp forest is shown as hatched green areas. All diving locations of shag included in the study are situated within the marked grid cells
To assess the potential effects of kelp harvest, we first determined the spatial overlap between kelp harvest and foraging areas of breeding shags. Following this we assessed the immediate, short- and long-term impacts of kelp harvesting on the foraging behaviour of breeding shags using a Before-After Control-Impact (BACI) design (Stewart-Oaten and Bence 2001; Smokorowski and Randall 2017). BACI is one of the most robust available designs for ecological monitoring studies that are not original designed to assess impacts from different kinds of exposures. We expected a spatial overlap in area use, as kelp harvesters often target areas with habitat characteristics similar to those which are preferred by foraging shags (cf. Christensen-Dalsgaard et al. 2017). If kelp harvest displaces the foraging birds or influences their foraging behaviour, this should be measurable when comparing shag feeding behaviour in postharvested areas with preharvested and unharvested areas. Two contrasting types of responses could be expected: (1) a reduction in diving activity post-harvest as a response to decreased abundance of prey, making the area unattractive as feeding habitat for shags. If this effect is only temporary, it may be caused by a direct disturbance from the kelp harvesting itself. If the effects are still detectable long term (≥ 3 years), this would indicate that the kelp’s function as shelter for small fish prey had not yet recovered, (2) an increase in diving activity post-harvest. This response could occur if shags need to increase their search effort to maintain prey intake rates, if fish abundance has decreased post-harvest, or that shags may be more attracted to the area due to increased access to prey and therefore conduct more dives.
Methods
Study system/species
The study was carried out in a shag colony at the islands of Sklinna (65°12′N, 10°59′E), off Central Norway (Fig. 1). In the period 2013 to 2018 an average of 2050 pairs of shags bred at Sklinna (range 1257–2570).
Data collection
To obtain data on feeding locations and diving behaviour for shags, birds were instrumented with GPS-loggers and time depth recorders (TDR). Breeding shags were opportunistically selected within the colony, and attempts were made to capture equal numbers of males and females when sampling the individuals. Adults were caught on the nest by hand or noose pole, and sex was determined by size and vocalization (cf. Cramp and Simmons 1977). The shags were instrumented with GPS-loggers (i-gotU GT-120, Mobile Action Technology, re-fitted in heat shrink tubes) and TDR’s (G5, CEFAS Technology). The GPS-loggers were attached to 3–4 middle tail feathers using strips of TESA ® tape. TDR-loggers were attached to the GPS-logger prior to instrumentation. The mean mass weight of the deployment when using both GPS- and TDR-loggers was 30.7 g (SD = 0.75, range 26.3–32.2), corresponding to 1.5% and 1.8% of mean body mass of males and females at Sklinna, respectively. The loggers were removed after approximately 3 days. Deployment of loggers normally required less than 3 min of handling and retrieval less than 10 min. Birds were fitted with loggers during late incubation and throughout the chick-rearing period (June–July). The GPS-loggers were programmed to take a location every 20–60 s, and the TDR-loggers were configured to record pressure every second. Cleaning and preparation of logger data followed Christensen-Dalsgaard et al. (2017) and Lorentsen et al. (2019).
The spatial locations of dives were determined by relating each dive to the GPS location closest in time, restricted to maximum 30-s difference between the time of the GPS location and the time when the dive began or ended. This cut-off was applied to compensate for the fact that GPS devices did not record locations when submerged while retaining a high spatial resolution in the data. To minimize the risk of including washing dives as foraging dives, dives shallower than 1.5 m were excluded.
Kelp harvest
Kelp harvest occurred in the study area between 2014 and 2018. In this area, kelp harvest is organised in a 5-year cycle, where kelp is harvested in one sector for 1 year, followed by a 4-year fallow period. Each sector is one latitudinal minute high (i.e. 1843 m). Kelp trawlers operate at a depth of 2–20 m. The kelp is harvested with a 3-m-wide dredge pulled by a boat along the bottom, which rips the plants from the rock. These 3-m-wide openings in the kelp forest create a kelp matrix with a mix of patches of kelp and trawl tracks (Fig. 2). The proportion of kelp removed in the harvested areas depends on bottom topography and depth and likely varies from a few percent to large proportions.
Arial photograph illustrating recently harvested kelp forest. The light tracks are openings with a width of 2–3 m created by the harvest sledge. This photograph is from an area suitable for harvesting (not in the study area), and a large proportion of the kelp has been harvested. Photograph:
Information on the distribution and magnitude of kelp harvest was made available by the Directorate of Fisheries (www.fiskeridir.no). Vessels that harvest kelp are required to report the amount of kelp harvested once per day in an electronic catch diary. This information is however not connected to exact position of the harvesting event(s) carried out, which makes it methodically challenging to assess the accurate positions of the kelp harvest. Therefore, to estimate the areas of harvest, the assumption was made that it took place in areas where the boat travelled at low speed. To quantify the spatial and temporal distribution of harvest, information from the electronic catch diary was therefore coupled with tracking data, and positions report every ten minutes through AIS (Automatic Identification System) from the boat. The total harvest of kelp was subsequently divided by the number of tracking points recorded in grid cells of 1843 m * 780 m (Fig. 1) during the same period. This provided an estimate of the amount of kelp harvested in each grid cell. Data were made available to us from the Directorate of Fisheries as biomass of kelp harvested per grid cell per month. Since the boats are only allowed to harvest kelp within clearly defined zones, it was subsequently assumed that data recorded outside the valid zones were erroneous, and these data were excluded from the analysis.
Kelp harvest occurred between May and December. May, June and July, the months immediately before and during the period where the diving activity of shags was recorded, were kept as separate months in the analysis, while the biomass of kelp harvested during August to December, when no shags were monitored, was pooled. As the shags are not confined to the colony after the chicks have fledged in August, there is little use in keeping August to December separate, as the biomass removed will first affect the birds when they establish at the colony in spring.
In order to include grid cells with no harvest (i.e. control sites) but with kelp presence, the grid cells were expanded over the total area used by the diving shags (Fig. 1). All grid cells were then overlaid by a GIS layer, indicating the presence of kelp (obtained from https://kartkatalog.geonorge.no) to estimate the proportion of kelp in each grid cell. This GIS layer did not include biomass or harvest, but just indicated the areas where kelp was present.
Dive activity
Diving activity, measured as number of dives, of all individual shags pooled was summarized for each grid cell (harvested and non-harvested) for each year (2013–2018) based on the spatial location and time of the dive (see Christensen-Dalsgaard et al. 2017 for details on method). Diving data were available for the months of June and July which is the chick-rearing period for shags at Sklinna. The experimental design of shag instrumentation resulted in 80% of the diving activity occurring in July, and we therefore chose not to separate diving activity between June and July.
Total dive duration (i.e. sum of the duration of all single dives) and total dive depth (sum of maximum depth for all dives) were additionally used as an alternative measure of diving activity (Table S1). However, these three measures were strongly correlated (R = 0.93–0.97, Fig. S1) and generated similar results. We therefore present only the number of dives in the results, while the other measures are presented in the supplementary material.
Before-after, control-impact (BACI) analysis
To assess the impact of kelp harvest on foraging behaviour of shags, we used a BACI design where diving activity (i.e. number of dives) was used as the measured variable. In contrast to a controlled experiment, we were not able to randomly chose treatment sites, as kelp harvest follows set management restrictions and occurs continuously over a period of years. However, harvested areas can still represent impact sites and diving activity can be compared before and after the impact (i.e. harvest). To be able to account for natural environmental changes that may occur independent of harvest, impact sites must be compared to unharvested sites, which are referred to as control areas.
To ensure that all grid cells included in the analyses were used by foraging shags, we excluded all cells (harvested or non-harvested) without any registered diving activity during the study period (2013–2018). To avoid the influence of few dives at the edge of a cell, diving activity was defined as minimum 10 dives within the appropriate cell. All remaining cells were classified as either impact sites, if they were harvested at some stage during the study period, or as control sites if they were never harvested. In order to make control sites comparable with impact sites with regard to habitat composition, we only kept sites where kelp was present following the mapping of kelp distribution. This procedure resulted in 77 control sites and 93 impact sites. The proportion of kelp areas was slightly higher in impact sites (mean = 36%, SD = 0.23) than in control sites (mean = 25%, SD = 0.18).
As kelp harvest was carried out continuously between 2014 and 2018, our data set did not allow for a balanced before and after design (Smokorowski and Randall 2017). To account for this unbalance, we ensured that the proportion of “before” and “after” within each year was similar in impact and control sites. Balanced distribution of “before” and “after” within years was important to account for, as recent work has shown that the spatial distribution of foraging distribution and diving activity of shags around Sklinna vary between years (Lorentsen et al. 2019), potentially independent of harvest. Thus, yearly variation in diving activity is assumed to be influenced by annual variations in abundance (= spawning success) of saithe, represented by the abundance of the younger age classes in the shag diet (e.g. Lorentsen et al. 2018). As available harvest data were summarized per month, it was not possible to classify whether shags had been diving before, during or after harvest that occurred in June or July. Due to this, we had to exclude sites in the years where harvest and dives occurred during the same months (79 of a total of 1020 year*site combinations).
Diving activity did not occur in all sites during the study period. This lack of diving activity some years is considered a true zero as the bird could, but did not, choose to dive there in some of the years, but did in other years. To deal with this excess of zeros, we decided to run the BACI analyses as a zero-inflated negative binomial model (ZINB) using the library “glmmTMB” (generalized linear mixed models using Template Model Builder; Brooks et al. 2017) in software R (https://www.r-project.org/). A ZINB model is a mixture model consisting of two parts: a binomial model used to model the excess zeros and a conditional count process, including expected zeros, modelled by a negative binomial GLM (Zuur 2009). The excessive variation in the count process made a negative binomial model (type 1) more appropriate than a Poisson model.
In the model we included, in addition to the interaction between impact (no treatment/treatment) and time (before/after) mandatory in a BACI design, the proportion of kelp as a quadratic term in both the binomial part and the count part of the model. By doing this we accounted for variation in diving activity related to the presence of kelp at sites, as diving activity of shags has been shown to be highly related to the occurrence of kelp (Christensen-Dalsgaard et al. 2017). To reduce the influence of a few sites with very high diving activity in the conditional part of the model, the response variable (number of dives) was square-root transformed. By using the library glmmTMB we were able to account for the spatial variation in diving activity observed between years, by including year as a random intercept in both parts of the ZINB model. Site ID was also included as a random intercept to account for repeated measurements in the same site over the years. The number of individual birds successfully equipped with loggers each year varied between 24 and 57 (mean = 30, SD = 15.9), and an increase in the number of individuals equipped increased the total number of dives conducted that year (Pearson’s correlation = 0.87, number of dives each year; range = 4917–14,677, mean = 8123, SD = 4006). Therefore, log (number of individual birds equipped) was used as an offset variable both in the zero-inflated and in the count process. Analysis of residuals of the models indicated no violation of assumptions.
To test for effects of harvest on different temporal scales, the BACI analyses were run on different subsets of the data depending on time since harvest (Table 1). All sites defined as “before” were kept constant for all subsets.
Potential effects of kelp harvest on shag diving activity may be related to the proportion of the kelp forest being removed. However, information on kelp biomass preharvest was not available so this could not be estimated. Biomass harvested at each site varied between 3 and 5133 tons (median = 283, SD = 880.6), but there was no clear relationship between kelp biomass harvested and proportion of kelp present at the site. The highest harvests (above the 95% quantile) were in sites with more than 60% kelp occurrence, but the smallest harvest (3–10 tons, below the 10% quantile) still occurred in sites with up to 64% kelp occurrence. To ensure that sites with low harvests were not driving our results, we additionally reran the models excluding impact sites with less than 10 tons of biomass harvested.
Results
As expected, there was a large spatial overlap between areas of kelp harvest and diving activity of shags (Fig. 1). After 4 years of harvest in the study area, more than half of the sites used for foraging by shags had been harvested at some stage (Fig. 3). Diving activity was registered in 26% of the sites during all 6 years included in the study, indicating fidelity at the population level to particular foraging grounds. Of these, 73% were impact sites where half of the diving activity occurred in postharvest years. This fidelity was not only driven by kelp occurrence as this was similar across sites used for foraging for at least 3 years or more (Fig. S2). There was a large spatial and temporal variation in diving activity, between and within sites, where number of dives varied between 0 and 1064 (mean = 79 ± 126 SD when excluding zeros, 37% of site*year combinations had no diving activity).
Proportion of grid cells with diving activity by shags per year in relation to harvest. Non-harvest sites (light grey) have not been harvested prior to diving activity, harvested sites previous years (grey) were harvest one time between 2014 and the year on the x-axis. Harvested sites same year (black) represent grid cell that were harvested during the same summer (June, July) as the diving activity occurred
BACI analyses
The BACI analysis showed that there was a tendency for increased diving activity in the period defined as “after” treatment, but this was observed for both impact (i.e. harvested) and control sites (non-harvested). There were no significant effects associated with the BACI interaction (treatment*time) for any of the temporal scales (short-, medium- or long-term effects 1 and 2) (Table 2, Figs. 4, S3). The proportion of kelp in the sites significantly increased the dive activity at all temporal scales (Table 2, Figs. S3 and S4).
BACI analysis of medium-term impact, showing the interaction between treatment (control vs. impact) and time (before vs. after) from a zero-inflated linear mixed-effects models fitted to diving activity (squared number of dives) of European shags at Sklinna. Impact is represented by harvest of kelp, and the after period is restricted to medium-term impact (1-year postharvest)
In the zero-inflated part of the model a significant BACI effect was observed for medium- (< 1 year after harvest) and long-term effects 1 and 2 (2–3 years after harvest, Table 1). Here the probability for zeros increased in control areas and decreased in impact areas after treatment, while the probability for zeros between control and impact was similar before treatment, i.e. the probability to observe diving activity was higher in harvested sites after treatment than in control sites (Table 2). This means that the shags had a higher probability of diving in postharvested sites but did not increase the numbers of dives.
The exclusion of impact sites with less than 10 tons of harvested biomass did not influence the results (Table S2). Similarly, the use of different measures of diving activity generated the same results as number of dives (Fig. S5).
Discussion
Our study clearly demonstrated large spatial and temporal overlap in area use by foraging shags and kelp harvest, underlining a potential for conflicts between the conservation of shags and industrial kelp fisheries. In spite of this, we did not observe any clear alterations in the diving activity of shags due to kelp harvest at the relatively broad scale of our analyses (grid cells of 1843 m * 780 m). Dive intensity was not influenced by harvest, but we did observe a slightly higher probability for diving to occur in harvested sites 1–3 years after harvest, compared to control sites. Disentangling potential effects of kelp harvest on shags is, however, not straightforward as shags do not directly depend on the kelp, but indirectly through their main prey, saithe, that form schools over kelp canopies (Norderhaug et al. 2005; Olsen et al. 2010). How shags are affected by the kelp harvest will therefore depend on the response of fish to harvest, both on a spatial and temporal scale.
Individual fish remaining in areas of harvest will likely become an easy prey target as the kelp forest opens up and potential hiding places are reduced. In addition, the stirring up of invertebrates in the hours following harvest could lead to increased food access, thereby attracting fish to the area. This may have a positive effect on intake rates for seabirds, at least initially and as long as the fish density is high enough so that they are profitable to hunt for. Similarly, if fish migrate from harvested areas to seek shelter in nearby kelp-forested areas, densities may increase locally also benefiting the seabirds food intake. Alternatively, fish can disperse over a wider area, as suggested by Bodkin (1988), forcing seabirds to compensate for local food depletion by intensifying their search effort within their foraging grounds and/or expanding their foraging range (Zador and Piatt 1999; Suryan et al. 2000; Burke and Montevecchi 2009; Lorentsen et al. 2019), increasing the cost associated with foraging. Finally, fish can change their distribution in the water column forcing seabirds to dive deeper for prey. In a recent study saithe redistributed in the water column when the kelp forest structure disappeared, using the water column all the way down to the sea floor postharvest (Norderhaug et al. submitted). There was no evidence in our results showing that shags at Sklinna were forced to alter their foraging range or to increase diving effort as they were using the same sites for foraging throughout the study area independent of harvesting regime (c.f. Table S1). The observed increase in number of dives in the time period after treatment occurred both in impact and in control sites, suggesting that it was not influenced by harvest but by other temporal ecological processes that were also taking place (c.f. Lorentsen et al 2019). If the increase was an effect of harvest, we would not have observed an increase in control site (i.e. non-harvested sites). However, the broad temporal and spatial design of our study restricts us from drawing any conclusions on changes in foraging behaviour to kelp harvest at a finer scale. Foraging shags may respond behaviourally to the matrix of kelp patches and 3-m-wide trawl tracks that harvest creates within our unit of measure (1843 m * 780 m sites) which would not be detectable in our data. In addition, the harvest only occurs in parts of the site leaving the rest of the site undisturbed with a potential increase in fish densities (cf. Figure 2). With access to more precise harvest data (i.e. exact time and place of harvest activity), information on the foraging behaviour of shags can be directly associated with harvest activities.
Alterations of foraging habitat can influence seabirds both on an individual and on a population level. Our study focused on foraging behaviour of individual shags following impacts on their foraging habitat. However, if kelp harvest leads to an overall reduction in fish productivity of the area due to removal of suitable habitat and food sources for their main prey, it can cause an increase in nutritional stress of the birds. The magnitude of this will depend on the total kelp removal in an area. For instance, comparison of modelled biomass to harvest statistics showed that only approximately 6% of kelp was removed by harvest in a 1150 km2 area over a 5-year period (Norderhaug et al. 2020; van Son et al. 2020). There are, however, no restrictions on how much is harvested within a given sector and the harvesting efficiency in some sectors might be higher, e.g. in areas with flat bottom topography which are most suitable for kelp trawling.
As central-place foragers in the breeding season, shag foraging ranges are limited by the need to return to the colony at regular intervals to provision their chicks (cf. Orians and Pearson 1979). An overall reduced productivity of the surrounding kelp forests could therefore lead to a reduction in the breeding population size and productivity of the breeding shags in the surrounding colonies. It is however very challenging to separate the human-induced change from natural variability. Lorentsen et al. (2019) showed that there was a large variability in both breeding population size and productivity between years. This study, partly based on the same data as Lorentsen et al. (2019), indicates that this variation is, at least to some degree, independent of kelp harvest. Thus, strong effects of fish abundance and spatial distribution on shag diving activity may outrun small effects of kelp harvest.
Though spanning 6 years of data collection, our study will in some aspects be regarded as a short-term study, as we have only investigated the effects of the first round of kelp harvest in the area. Following the Norwegian harvesting regulations, the kelp harvest is organised in a 5-year cycle in the study area, where each sector is open for kelp harvesting for 1 year, followed by a 4-year fallow period. This is assumed to ensure the regrowth of kelp in each sector before it is re-harvested (Vea and Ask 2011). Research has, however, shown that the kelp-associated assemblages may take considerably longer to recover (Christie et al. 1998; Steen et al. 2016a, b). Thus, when the sector is reopened for kelp harvest after the fallow period the kelp forests ecosystem function within the trawl tracks has most likely not yet recovered. The long-term effects of the harvest on the whole kelp forest associated ecosystem, can therefore not be assessed until more research has been conducted.
Kelp is harvested industrially worldwide. A few studies have focused on kelp, other algae and invertebrates associated with kelps (e.g. Christie et al. 1998), but surprisingly few studies have been performed to detect effects on a scale relevant for species higher in the food web. If effective policies and management practices to reduce future impacts on the marine environment are to be developed, knowledge on impact pathways and environmental effects is essential. On the scale used in our study, we were not able to show an effect of the kelp harvest on shag foraging behaviour. However, we demonstrate significant overlaps in habitat used by shags and kelp harvesters, signifying the potential for conflict. Our work has identified several issues that should be addressed before concluding the effects of kelp harvest on seabird populations. Fine scale data on the distribution and density of kelp in seabird foraging areas, with associated data on fish distribution and biomass, are needed as well as high-resolution harvest data. Fine-scale data on seabird foraging activity, preferably using bird-borne cameras (cf. Watanuki et al. 2008), over extended periods would be valuable additional information. Most important, there is a need for experimental studies using directed and controlled harvest to investigate the effects of kelp harvest through the different trophic levels, including top predators. In Norway, kelp harvesters are in constant search for new areas, including access to protected areas. In order to assess the impacts of kelp harvest on threatened seabird species and other species dependent on kelp forest ecosystems, studies assessing the quantitative effects of this harvest are needed. We see such studies as essential in order to manage the services provided by kelp forests for the benefit of humans and the many species composed in the coastal ecosystem.
Data availability
The data used in this study are available from the corresponding author upon reasonable request.
References
Airoldi L, Beck MW (2007) Loss, status and trends for coastal marine habitats of Europe. Oceanogr Mar Bio 45:345–405
Beaumont NJ, Austen MC, Mangi SC, Townsend M (2008) Economic valuation for the conservation of marine biodiversity. Mar Pollut Bull 56:386–396
Bertocci I, Araújo R, Oliveira P, Sousa-Pinto I (2015) Potential effects of kelp species on local fisheries. J Appl Ecol 52:1216–1226
Bodkin JL (1988) Effects of kelp forest removal on associated fish assemblages in central California. J Exp Mar Biol Ecol 117:227–238
Brooks ME, Kristensen K, van Benthem KJ, Magnusson A, Berg CW, Nielsen A, Skaug HJ, Maechler M, Bolker BM (2017) glmmTMB balances speed and flexibility among packages for zero-inflated generalized linear mixed modeling. R J 9(2):378–400
Burke CM, Montevecchi WA (2009) The foraging decisions of a central place foraging seabird in response to fluctuations in local prey conditions. J Zool 278:354–361
Bustnes JO, Anker-Nilssen T, Erikstad KE, Lorentsen S-H, Systad GH (2013) Changes in the Norwegian breeding population of European shag correlate with forage fish and climate. Mar Ecol Prog Ser 489:235–244
Christensen-Dalsgaard S, Mattisson J, Bekkby T, Gundersen H, May R, Rinde E, Lorentsen S-H (2017) Habitat selection of foraging chick-rearing European shags in contrasting marine environments. Mar Biol 164:196
Christie H, Jørgensen NM, Norderhaug KM, Waage-Nielsen E (2003) Species distribution and habitat exploitation of fauna associated with kelp (Laminaria hyperborea) along the Norwegian coast. J Mar Biol Assoc UK 83:687–699
Christie H, Norderhaug KM, Fredriksen S (2009) Macrophytes as habitat for fauna. Mar Ecol Prog Ser 396:221–233
Christie H, Fredriksen S, Rinde E (1998) Regrowth of kelp and colonization of epiphyte and fauna community after kelp trawling at the coast of Norway. Hydrobiologia 375(376):49–58
Costanza R, d'Arge R, de Groot R, Farber S, Grasso M, Hannon B, Limburg K, Naeem S, O'Neill RV, Paruelo J, Raskin RG, Sutton P, van den Belt M (1997) The value of the world's ecosystem services and natural capital. Nature 387:253–260
Cramp S, Simmons KEL (1977) The Birds of the Western Palearctic, vol I. Oxford University Press, Oxford
Crain CM, Halpern BS, Beck MW, Kappel CV (2009) Understanding and managing human threats to the coastal marine environment. Ann N Y Acad Sci 1162:39–62. https://doi.org/10.1111/j.1749-6632.2009.04496.x
Dias M, Martin PR, Pearmain EJ, Burfield IJ, Small C, Phillips RA, Yates O, Lascelles B, Borboroglu PG, Croxall JP (2019) Threats to seabirds: a global assessment. Biol Conserv 237:525–537. https://doi.org/10.1016/j.biocon.2019.06.033
Fauchald P, Anker-Nilssen T, Barrett RT, Bustnes JO, Bårdsen BJ, Christensen-Dalsgaard S, Descamps S, Engen S, Erikstad KE, Hanssen SA, Lorentsen S-H, Moe B, Reiertsen TK, Strøm H, Systad GH (2015) The status and trends of seabirds breeding in Norway and Svalbard. NINA report 1151. NINA, Trondheim, p 84
Fraschetti S, Terlizzi A, Benedetti-Cecchi L (2005) Patterns of distribution of marine assemblages from rocky shores: evidence of relevant scales of variation. Mar Ecol Prog Ser 296:13–29
Fredriksen S (2003) Food web studies in a Norwegian kelp forest based on stable isotope (δ13C and δ15N) analysis. Mar Ecol Prog Ser 260:71–81
Halpern BS, Walbridge S, Selkoe KA, Kappel CV, Micheli F, D'Agrosa C, Bruno JF, Casey KS, Ebert C, Fox HE, Fujita R, Heinemann D, Lenihan HS, Madin EMP, Perry MT, Selig ER, Spalding M, Steneck R, Watson R (2008) A global map of human impact on marine ecosystems. Science 319(5865):948–952. https://doi.org/10.1126/science.1149345
Hillersøy G, Lorentsen S-H (2012) Annual variation in the diet of breeding European Shag (Phalacrocorax Aristotelis) in Central Norway. Waterbirds 35:420–429
Korpinen S, Meidinger M, Laamanen M (2013) Cumulative impacts on seabed habitats: an indicator for assessments of good environmental status. Mar Pollut Bull 74:311–319
Lorentsen S-H, Anker-Nilssen T, Erikstad KE, Røv N (2015) Forage fish abundance is a predictor of timing of breeding and hatching brood size in a coastal seabird. Mar Ecol Prog Ser 519:209–220
Lorentsen S-H, Anker-Nilssen T, Erikstad KE (2018) Seabirds as guides for fisheries management; European shag (Phalacrocorax aristotelis) diet as indicator of saithe (Pollachius virens) recruitment. Mar Ecol Prog Ser 586:193–201. https://doi.org/10.3354/meps12440
Lorentsen S-H, Mattisson J, Christensen-Dalsgaard S (2019) Reproductive success in the European shag is linked to annual variation in diet and foraging trip metrics. Mar Ecol Prog Ser 619:137–147
Lorentsen S-H, Sjøtun K, Grémillet D (2010) Multi-trophic consequences of kelp harvest. Biol Conserv 143:2054–2062. https://doi.org/10.1016/j.biocon.2010.05.013
Mann KH (1973) Seaweeds: their productivity and strategy for growth. Science 182:975–981
Mitchell PI, Newton SF, Ratcliffe N, Dunn TE (2004) Seabird populations of Britain and Ireland. T. & A.D Poyser, London
Monagail MM, Cornish L, Morrison L, Araújo R, Critchley AT (2017) Sustainable harvesting of wild seaweed resources. Eur J Phycol 52(4):371–390. https://doi.org/10.1080/09670262.2017.1365273
Norderhaug KM, Christie H, Fosså JH, Fredriksen S (2005) Fish-macrofauna interactions in a kelp (Laminaria hyperborea) forest. J Mar Biol Assoc UK 85:1279–1286
Norderhaug KM, Filbee-Dexter K, Freitas C, Christensen L, Mellerud I, Thormas J, van Son T, Moy F, Vázquez Alonso M, Steen H (submitted) Ecosystem-level effects of large-scale disturbance in kelp forests. Submitted Mar Ecol Prog Ser
Norderhaug KM, Van Son, TC, Nikolioudakis N, Thormar J, Moy FE, Knutsen JA, Elvenes S, Steen H (2020) Biomassemodell for stortare—ressursmodell for fremtidens forvaltning. Rapport fra Havforskningen Nr. 2020–7 [in Norwegian with English summary] (under review)
O’Connor KC, Anderson TW (2010) Consequences of habitat disturbance and recovery to recruitment and the abundance of kelp forest fishes. J Exp Mar Biol Ecol 286:1–10
Olsen E, Aanes S, Mehl S, Holst JC, Aglen A, Gjøsæter H (2010) Cod, haddock, saithe, herring, and capelin in the Barents Sea and adjacent waters: a review of the biological value of the area. ICES J Mar Sci 67:87–101
Reed DC, Rassweiler A, Arkema KK (2008) Biomass rather than growth rate determines variation in net primary production by giant kelp. Ecology 89:2493–2505
Reisewitz SE, Estes JA, Simenstad CA (2006) Indirect food web interactions: sea otters and kelp forest fishes in the Aleutian archipelago. Oecologia 146:623–631. https://doi.org/10.1007/s00442-005-0230-1
Salter ZT, Harvey ES, Kendrick GA, Murray K (2010) The effect of kelp bed disturbance on the abundance and feeding behaviour of fishes on high-relief reefs. Mar Freshw Behav Phy 43:109–125
Smokorowski KE, Randall RG (2017) Cautions on using the before-after-control-impact design in environmental effects monitoring programs. Facets 2:212–232
Stagnol D, Michel R, Davoult D (2015) Unravelling the impact of harvesting pressure on canopy-forming macroalgae. Mar Freshwater Res 67:153–161
Steen H, Bodvin T, Moy FE (2012) Visuell registrering av fisk etter prøvehøsting av stortare i Nord-Trøndelag i 2011. Fisken og havet Nr. 1–2012 [in Norwegian with English summary]
Steen H, Bodvin T, Moy FE, Gustad E, Øverbø Hansen H, Jelmert A, Baardsen P (2016) Effekter av stortarehøsting i Nordland i 2016. Rapport fra Havforskningen Nr. 38–2016 [in Norwegian with English summary]
Steen H, Moy F, Bodvin T, Husa V (2016) Regrowth after kelp harvesting in Nord-Trøndelag, Norway. ICES J Mar Sci 73:2708–2720
Steneck RS, Graham MH, Bourque BJ, Corbett D, Erlandson JM, Estes JA, Tegner MJ (2002) Kelp forest ecosystems: biodiversity, stability, resilience and future. Environ Conserv 29:436–459
Stewart-Oaten A, Bence JR (2001) Temporal and spatial variation in environmental impact assessment. Ecol Monogr 71(2):305–339
Suryan RM, Irons DB, Benson J (2000) Prey switching and variable foraging strategies of Black-Legged Kittiwakes and the effect on reproductive success. Condor 102:374–384
Teagle H, Hawkins SJ, Moore PJ, Smale DA (2017) The role of kelp species as biogenic habitat formers in coastal marine ecosystems. J Exp Mar Biol Ecol 492:81–98
Ugarte RA, Sharp G (2001) A new approach to seaweed management in Eastern Canada: the case of Ascophyllum nodosum. Cah Biol Mar 42:63–70
van Son TC, Nikolioudakis N, Steen H, Albretsen J, Rugaard Furevik B, Elvenes S, Moy F, Norderhaug KM (2020) Achieving reliable estimates of the spatial distribution of kelp biomass. Front Mar Sci 7:107. https://doi.org/10.3389/fmars.2020.00107
Vea J, Ask E (2011) Creating a sustainable commercial harvest of Laminaria hyperborean. Norway J Appl Phycol 23(489):494
Watanuki Y, Daunt F, Takahashi A, Newell M, Wanless S, Sato K, Miyazaki N (2008) Microhabitat use and prey capture of a bottom-feeding top predator, the European shag, shown by camera loggers. Mar Ecol Prog Ser 356:283–293
Zador SG, Piatt JF (1999) Time-budgets of common murres at a declining and increasing colony in Alaska. Condor 101:149–152
Acknowledgements
Open Access funding provided by Norwegian institute for nature research. We thank all of our field assistants for invaluable help in the field. Special thanks to Per Finne from the Directorate of Fisheries, who prepared and systematized the information on kelp harvest. We thank Elizabeth Morgan and three anonymous referees who provided useful comments and suggestions to improve the manuscript.
Funding
The study was supported by the Norwegian Environment Agency and the Norwegian Institute for Nature Research. The work at Sklinna is part of the SEAPOP program (www.seapop.no), which is financed by the Norwegian Ministry of Climate and Environment via the Norwegian Environment Agency, the Norwegian Ministry of Petroleum and Energy via the Norwegian Research Council and the Norwegian Oil and Gas Association.
Author information
Authors and Affiliations
Contributions
SCD and SHL conceived the idea; SCD, JM and SHL contributed to the study conception and design; SHL collected GPS data; JM performed the analysis; and SCD and JM wrote the manuscript. All authors contributed critically to drafts and gave final approval for publication.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving animals were in accordance with the ethical standards of the institution. All protocols pertaining to handling and instrumentation of European shag were approved by the Norwegian Environment Agency and the Norwegian Animal Research Authority (FOTS ID: 3238, 5148 and 8616).
Consent to participate
Not applicable.
Consent for publication
Not applicable.
Code availability
The codes used in this study are available from the corresponding author upon reasonable request.
Additional information
Responsible Editor: V. Paiva.
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Reviewed by undisclosed experts.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Christensen-Dalsgaard, S., Mattisson, J., Norderhaug, K.M. et al. Sharing the neighbourhood: assessing the impact of kelp harvest on foraging behaviour of the European shag. Mar Biol 167, 136 (2020). https://doi.org/10.1007/s00227-020-03739-1
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00227-020-03739-1