Abstract
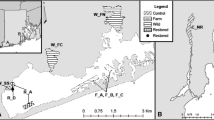
As a result of aquaculture activities, Pacific oysters Crassostrea gigas (Thunberg, 1793) have invaded European coasts. Using seven microsatellites, we found virtually no genetic differentiation between natural populations throughout the European range (from the south of the Wadden Sea (the Netherlands) to the south of France) and French cultivated oysters. The genetic homogeneity of Pacific oyster samples appears to be the result of repeated transfers from same seed stocks made for aquaculture and, to a lesser extent, widespread dispersal due to specific biological traits of this species. The only genetic differentiation of Sylt population in the north of the Wadden Sea (Germany) suggests a stronger, persistent impact of ongoing supply of new genetic material from hatchery production, corresponding to seeds selection made by breeders. All of our genetic data highlighted the importance of aquaculture practices on the genetic structure of the keystone invader C. gigas in Europe.


Similar content being viewed by others
References
Appleyard SA, Ward RD (2006) Genetic diversity and effective population size in mass selection lines of Pacific oyster (Crassostrea gigas). Aquaculture 254:148–159
Barret SCH, Husband BC (1990) The genetics of plant migration and colonisation. In: Brown AHD (ed) Plant population genetics, breeding, and genetic resources. Sinauer Associates, Sunderland, MA, USA, pp 254–277
Baumel A, Ainouche ML, Levasseur JE (2001) Molecular investigations in populations of Spartina anglica C.E. Hubbard (Poaceae) invading coastal Brittany (France). Mol Ecol 10:1689–1701
Belkhir K, Borsa P, Goudet J, Chikhi L, Bonhomme F (2004) GENETIX, logiciel sous WindowsTM pour la genetique des populations. Laboratoire Genome et Populations, CNRS UPR 9060, Universite de Montpellier II, Montpellier, France
Boudry P, Collet B, Cornette F, Hervouet V, Bonhomme F (2002) High variance in reproductive success of the Pacific oyster (Crassostrea gigas, Thunberg) revealed by microsatellite-based parentage analysis of multifactorial crosses. Aquaculture 204:283–296
Brookfield JFY (1996) A simple new method for estimating null allele frequency from heterozygote deficiency. Mol Ecol 5:453–455
Cardoso J, Langlet D, Loff JF, Martins AR, Witte JIJ, Santos PT, van der Veer HW (2007) Spatial variability in growth and reproduction of the Pacific oyster Crassostrea gigas (Thunberg, 1793) along the west European coast. J Sea Res 57:303–315
Carlton JT (1989) Man’s role in changing the face of the ocean: biological invasions and implications for conservation of near-shore environments. Conserv Biol 3:265–273
Carlton JT (1996a) Marine bioinvasions: the alteration of marine ecosystems by nonindigenous species. Oceanography 9(1):36–43
Carlton JT (1996b) Pattern, process, and prediction in marine invasion ecology. Conserv Biol 78:97–106
Castric V, Bernatchez L, Belkhir K, Bonhomme F (2002) Heterozygote deficiencies in small lacustrine populations of brook charr Salvelinus Fontinalis Mitchill (Pisces, Salmonidae): a test of alternative hypotheses. Heredity 89:27–35
Cavalli-Sforza LL, Edwards AWF (1967) Phylogenetic analysis: models and estimation procedures. Evolution 21:550–570
Clark JE, Langmo RD (1979) Oyster seed hatcheries on the US west coast: an Overview. Mar Fish Rev 41(12):10–16
Cornuet JM, Luikart G (1996) Description and power analysis of two tests for detecting recent population bottlenecks from allele frequency data. Genetics 144:2001–2014
Cornuet JM, Piry S, Luikart G, Estoup A, Solignac M (1999) New methods employing multilocus genotypes to select or exclude populations as origins of individuals. Genetics 153:1989
Davis MA, Thompson K (2000) Eight ways to be a colonizer; two ways to be an invader: a proposed nomenclature scheme for invasion ecology. ESA Bulletin 81:226–230
Di Rienzo A, Donnelly P, Toomajian C, Sisk B, Hill A, Petzl-Erler ML, Haines GK, Barch DH (1998) Heterogeneity of microsatellite mutations within and between loci, and implications for human demographic histories. Genetics 148:1269–1284
Diederich S (2005) Differential recruitment of introduced Pacific oysters and native mussels at the North Sea coast: coexistence possible? J Sea Res 53:269–281
Diederich S, Nehls G, van Beusekom JEE, Reise K (2005) Introduced Pacific oysters (Crassostrea gigas) in the northern Wadden Sea: invasion accelerated by warm summers? Helgol Mar Res 59:97–106
Drinkwaard AC (1998) Introductions and developments of oysters in the North Sea area: a review. Helgol Meeresunters 52:301–308
Dutertre M, Beninger PG, Barillé L, Papin M, Haure J (2010) Rising water temperatures, reproduction and recruitment of an invasive oyster, Crassostrea gigas, on the French Atlantic coast. Mar Environ Res 69:1–9
Esnault S (2005) Etude chronologique des températures de la mer sur l’ouest de la France. Université de Bretagne Sud, Météo-France
Geller JB, Darling JA, Carlton JT (2010) Genetic perspectives on marine biological invasions. Ann Rev Mar Sci 2:367–393
Goulletquer P, Bachelet G, Sauriau PG, Noel P (2002) Open Atlantic coast of Europe-a century of introduced species into French waters. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive Aquatic Species of Europe: Distribution, Impacts and Management. Kluwer, Dordrecht, pp 276–290
Gower JC (1966) Some distance properties of latent root and vector methods used in multivariate analysis. Biometrika 53(3–4):325–338
Grizel H, Heral M (1991) Introduction into France of the Japanese oyster (Crassostrea gigas). Journal international pour l’exploration de la Mer 47:399–403
Hardie DC, Hutchings JA (2010) Evolutionary ecology at the extremes of species’ ranges. Environ Rev 18:1–20
Hedgecock D, Davis JP (2007) Heterosis for yield and crossbreeding of the Pacific oyster Crassostrea gigas. Aquaculture 272(1):S17–S29
Hedgecock D, McGoldrick DJ, Bayne BL (1995) Hybrid vigor in Pacific oysters: an experimental approach using crosses among inbred lines. Aquaculture 137:285–298
Huvet A, Lapègue S, Magoulas A, Boudry P (2000) Mitochondrial and nuclear DNA phylogeography of Crassostrea angulata, the Portuguese oyster endangered in Europe. Conserv Genet 1:251–262
Huvet A, Fabioux C, McCombie H, Lapegue S, Boudry P (2004) Natural hybridization between genetically differentiated populations of Crassostrea gigas and C. angulata highlighted by sequence variation in flanking regions of a microsatellite locus. Mar Ecol Prog Ser 272:141–152
Kang M, Buckley YM, Lowe AJ (2007) Testing the role of genetic factors across multiple independent invasions of the shrub Scotch broom (Cytisus scoparius). Mol Ecol 16:4662–4673
Kelly DW, Muirhead JR, Heath DD, Macisaac HJ (2006) Contrasting patterns in genetic diversity following multiple invasions of fresh and brackish waters. Mol Ecol 15:3641–3653
Lartaud F, de Rafelis M, Ropert M, Emmanuel L, Geairon P, Renard M (2010) Mn labelling of living oysters: artificial and natural cathodoluminescence analyses as a tool for age and growth rate determination of C. gigas (Thunberg, 1793) shells. Aquaculture 300:206–217
Launey S, Hedgecock D (2001) High genetic load in the Pacific oyster Crassostrea gigas. Genetics 159:255–265
Launey S, Ledu C, Boudry P, Bonhomme F, Naciri-Graven Y (2002) Geographic structure in the European flat Oyster (Ostrea edulis L.) as revealed by microsatellite polymorphism. J Hered 93:331–338
Lee CE (2002) Evolutionary genetics of invasive species. Trends Ecol Evol 17:386–391
Lejart M (2009) Etude du processus invasif de Crassostrea gigas en Bretagne: Etat des lieux, dynamique et conséquences écologiques. PhD thesis, Brest, France
Lenfant P (2002) Heterozygosity and fitness: the case of marine fish, Diplodus sargus (Linné, 1758). C R Biol 325:239–252
Li G, Hedgecock D (1998) Genetic heterogeneity, detected by PCR-SSCP, among samples of larval Pacific oysters (Crassostrea gigas) supports the hypothesis of large variance in reproductive success. Can J Fish Aquat Sci 55:1025–1033
Li G, Hubert S, Bucklin K, Ribes V, Hedgecock D (2003) Characterization of 79 microsatellite DNA markers in the Pacific oyster Crassostrea gigas. Mol Ecol Notes 3:228–232
Li R, Qi L, Yu R (2009) Parentage determination and effective population size estimation in mass spawning Pacific oyster, Crassostrea gigas, based on microsatellite analysis. J World Aquacult Soc 40:667–677
Lodge DM (1993) Biological invasions: lessons for ecology. Trends Ecol Evol 8:133–137
Magoulas A, Gjetvaj B, Terzoglou V, Zouros E (1998) Three polymorphic microsatellites in the Japanese oyster, Crassostrea gigas (Thunberg). Anim Genet 29:69
McGinnity P, Stone C, Taggart JB, Cooke D, Cotter D, Hynes R, McCamley C, Cross T, Ferguson A (1997) Genetic impact of escaped farmed Atlantic salmon (Salmo salar L.) on native populations: use of DNA profiling to assess freshwater performance of wild, farmed, and hybrid progeny in a natural river environment. ICES J Mar Sci 54:998–1008
Miossec L, Goulletquer P (2007) The Pacific Cupped Oyster Crassostrea gigas, a species introduced into Europe for aquaculture in the 70’s to become invasive in the late 90’s 5th internationale conference on marine bioinvasions, MIT, Cambridge (MA) USA
Moehler J, Wegner KM, Reise K, Jacobsen S (2011) Invasion genetics of Pacific oyster Crassostrea gigas shaped by aquaculture stocking practices. J Sea Res 66:256–262
Nehring S (1999) Oyster beds and Sabellaria reefs. In: De Jong F, Bakker JF, van Berkel CJM, Dankers NMJA, Dahl K, Gätje C, Marencic H and Potel P (eds) Wadden Sea Quality Status Report. Wadden Sea Ecosystem, pp 146–147
Nehring S (2006) NOBANIS–Invasive alien species fact sheet–Crassostrea gigas. http://www.nobanis.org/files/factsheets/Crassostrea_gigas.pdf. Accessed
Nehring S, Reise K, Dankers N, Kristensen PS (2009) Alien species. Thematic Report No.7. In: Marencic H, de Vlas J (eds) Quality Status report 2009. Wadden Sea Ecosystem Common Wadden Sea secretariat, Wilhemshaven
Nei M, Maruyama T, Chakraborty R (1975) The bottleneck effect and genetic variability in populations. Evolution 29:1–10
Petersen J, Ibarra A, May B (2010) Nuclear and mtDNA lineage diversity in wild and cultured Pacific lion-paw scallop, Nodipecten subnodosus (Baja California Peninsula, Mexico). Mar Biol 157:2751–2767
Piry S, Alapetite A, Cornuet JM, Paetkau D, Baudouin L, Estoup A (2004) GeneClass2: a software for genetic assignment and first-generation migrant detection. J Hered 95:536–539
Raymond M, Rousset F (1995) GENEPOP (Version 1.2): population genetics software for exact tests and ecumenicism. J Hered 86(3):248–249
Reise K (1998) Pacific oysters invade mussel beds in the European Wadden Sea. Senckenb Marit 28:167–175
Reise K, Dankers N, Essink K (2005) Introduced Species. In: Essink K, Dettman C, Farke H, Laursen K, Lüerβen G, Marencic H, Wiersinga W (eds) Wadden Sea quality status report 2004. Common Wadden Sea secretariat, Wilhelmshaven, pp 155–161
Reise K, Olenin S, Thieltges D (2006) Are aliens threatening European aquatic coastal ecosystems? Helgol Mar Res 60:77–83
Reusch TBH, Bolte S, Sparwel M, Moss AG, Javidpour J (2010) Microsatellites reveal origin and genetic diversity of Eurasian invasions by one of the world’s most notorious marine invader, Mnemiopsis leidyi (Ctenophora). Mol Ecol 19:2690–2699
Rico-Villa B, Bernard I, Robert R, Pouvreau S (2010) A Dynamic Energy Budget (DEB) growth model for Pacific oyster larvae, Crassostrea gigas. Aquaculture 305:84–94
Roman J (2006) Diluting the founder effect: cryptic invasions expand a marine invader's range. Proc R Soc Biol Sci Ser B 273:2453–2459
Roman J, Darling JA (2007) Paradox lost: genetic diversity and the success of aquatic invasions. Trends Ecol Evol 22:454–464
Rousset F (2008) GENEPOP’007: a complete re-implementation of the GENEPOP software for Windows and Linux. Molecular Ecology Resources 8:103–106
Royer J, Seguineau C, Park K-I, Pouvreau Sp, Choi K-S, Costil K (2008) Gametogenetic cycle and reproductive effort assessed by two methods in 3 age classes of Pacific oysters, Crassostrea gigas, reared in Normandy. Aquaculture 277:313–320
Schmidt A, Wehrmann A, Dittmann S (2008) Population dynamics of the invasive Pacific oyster Crassostrea gigas during the early stages of an outbreak in the Wadden Sea (Germany). Helgol Mar Res 62:367–376
Seamen MNL (1985) Ecophysiological investigation on the oyster Crassostrea gigas in Flensburg Fjord, Hamburg Bd
Simon-Bouhet B, Garcia-Meunier P, Viard F (2006) Multiple introductions promote range expansion of the mollusc Cyclope neritea (Nassariidae) in France: evidence from mitochondrial sequence data. Mol Ecol 15:1699–1711
Smaal A, Kater B, Wijsman J (2009) Introduction, establishment and expansion of the Pacific oyster Crassostrea gigas in the Oosterschelde (SW Netherlands). Helgol Mar Res 63:75–83
Storey JD (2002) A direct approach to false discovery rates. J R Stat Soc Ser B (Statistical Methodology) 64:479–498
Syvret M, Fitzgerald A, Hoare P (2008) Development of a Pacific oyster aquaculture protocol for the UK—technical report
Todd CD, Lambert WJ, Thorpe JP (1998) The genetic structure of intertidal populations of two species of nudibranch molluscs with planktotrophic and pelagic lecithotrophic larval stages: are pelagic larvae “for” dispersal? J Exp Mar Biol Ecol 228:1–28
Troost K (2010) Causes and effects of a highly successful marine invasion: case-study of the introduced Pacific oyster Crassostrea gigas in continental NW European estuaries. J Sea Res 64:145–165
Tsutsui ND, Suarez AV, Holway DA, Case TJ (2000) Reduced genetic variation and the success of an invasive species. Proc Natl Acad Sci USA 97:5948–5953
Tsutsui ND, Suarez AV, Holway DA, Case TJ (2001) Relationships among native and introduced populations of the Argentine ant (Linepithema humile) and the source of introduced populations. Mol Ecol 10:2151–2161
Van Oosterhout C, Hutchinson WF, Wills DPM, Shipley P (2004) MICRO-CHECKER: software for identifying and correcting genotyping errors in microsatellite data. Mol Ecol Notes 4:535–538
Voisin M, Engel CR, Viard F (2005) Differential shuffling of native genetic diversity across introduced regions in a brown alga: aquaculture vs. maritime traffic effects. Proc Natl Acad Sci 102:5432
Voisin M, Daguin C, Engel C, Grulois D, Javanaud C, Viard F (2007) Introduction and establishment processes of marine species: a study case with theJapanese brown kelp Undaria pinnatifida. J Soc Biol 201:259–266
Wehrmann A, Schmidt A (2005) Die Einwanderung der Pazifischen Auster in das Niedersächsische Wattenmeer. Frankfurt
Wehrmann A, Herlyn M, Bungenstock F, Hertweck G, Millat G (2000) The distribution gap is closed—First record of naturally settled pacific oysters Crassostrea gigas in the East Frisian Wadden Sea, North Sea. Senckenb Marit 30:153–160
Weir BS, Cockerham CC (1984) Estimating F-statistics for the analysis of population structure. Evolution 38:1358–1370
Williamson M (1996) Biological invasions. Chapman & Hall, London
Williamson MH, Fitter A (1996) The characters of successful invaders. Conserv Biol 78:163–170
Wolff WJ, Reise K (2002) Oyster imports as a vector for the introduction of alien species into Northern and Western European coastal waters. In: Leppäkoski E, Gollasch S, Olenin S (eds) Invasive aquatic species of Europe: distribution, impacts and management. Kluwer, Dordrecht, pp 193–205
Wrange AL, Valero J, Harkestad LS, Strand Ø, Lindegarth S, Christensen HT, Dolmer P, Kristensen PS, Mortensen S (2009) Massive settlements of the Pacific oyster, Crassostrea gigas, in Scandinavia. Biol Invasions 12:1145–1152
Acknowledgments
We are grateful to Audrey Rohfritsch, Sylvie Lapègue, Christopher Sauvage, Solène Coedel, Julien Normand, Nicole Faury and Serge Heurtebise for their help for microsatellite genotyping. We are indebted to Douve van den Ende and Karsten Reise for providing samples from the Netherlands and Germany, respectively. We also acknowledge Edouard Lavergne for his help for multidimensional scaling analysis (MDS) in R. This research program was financially supported by the national program PROGIG (Prolifération de Crassostrea gigas, LITEAU II) and by the ANR project 08-0334-01 “Hi-Flo”. We also thank Leon Meyer and Helen McCombie for correcting the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by S. Uthicke.
Rights and permissions
About this article
Cite this article
Meistertzheim, AL., Arnaud-Haond, S., Boudry, P. et al. Genetic structure of wild European populations of the invasive Pacific oyster Crassostrea gigas due to aquaculture practices. Mar Biol 160, 453–463 (2013). https://doi.org/10.1007/s00227-012-2102-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-2102-7