Abstract
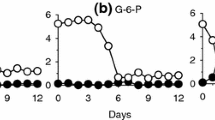
Batch culture experiments were performed to investigate potential effects of nutrient starvation on the allelochemical potency of the toxic dinoflagellate Alexandrium tamarense. Triplicate cultures with reduced nitrate (−N) or phosphate (−P) seed were compared to nutrient-replete (+N+P) cultures. Total depletion of the dissolved inorganic limiting nutrient, reduced cell quotas, changed mass ratios of C/N/P and reduced cell yield clearly indicate that treatment cultures at stationary phase were starved by either N or P, whereas growth cessation of +N+P cultures was probably due to carbon limitation and/or a direct effect of high pH. Pulsed addition of the limiting nutrient allowed −N and −P cultures to resume growth. Lytic activity of A. tamarense as quantified by a Rhodomonas bioassay was generally high (EC50 around 100 cells mL−1) and was only slightly modulated by growth phase and/or nutrient starvation. Lytic activity per cell increased with time in both +N+P and −P cultures but not −N cultures. P-starved stationary-phase cells were slightly more lytic than +N+P cultures, but this difference may be due to increased cell size and/or accumulation of extracellular compounds. In conclusion, only slight changes but no general and major increase in lytic activity in response to nutrient starvation was observed.





Similar content being viewed by others
References
Adolf JE, Bachvaroff TR, Krupatkina DN, Nonogaki H, Brown PJP, Lewitus AJ, Harvey HR, Place AR (2006) Species specificity and potential roles of Karlodinium micrum toxin. Afr J Marine Sci 28:415–419
Alpermann TJ, Tillmann U, Beszteri B, Cembella AD, John U (2010) Phenotypic variation and genotypic diversity in a planktonic population of the toxigenic marine dinoflagellate Alexandrium tamarense (Dinophyceae). J Phycol 46:18–32
Arzul G, Seguel M, Guzman L, Erard-LeDenn E (1999) Comparison of allelopathic properties in three toxic Alexandrium species. J Exp Mar Biol Ecol 232:285–295
Blanco J, Campos MJ (1988) The effect of water conditioned by a PSP-producing dinoflagellate on the growth of four algal species used as food for invertebrates. Aquaculture 68:289–298
Burkholder JA, Glibert PM, Skelton HM (2008) Mixotrophy, a major mode of nutrition for harmful algal species in eutrophic waters. Hamful Algae 8:77–93
Cembella AD (2003) Chemical ecology of eukaryotic microalgae in marine ecosystems. Phycologia 42:420–447
Cembella AD, Quilliam MA, Lewis NI, Bauder AG, Dell′Aversano C, Thomas K, Jellet J, Cusack RR (2002) The toxigenic marine dinoflagellate Alexandrium tamarense as the probable cause of mortality of caged salmon in Nova Scotia. Harmful Algae 1:313–325
Doucette GJ, Cembella AD, Martin JL, Michaud J, Cole TVN, Rolland RM (2006) Paralytic shellfish poisoning (PSP) toxins in North Atlantic right whales Eubalaena glacialis and their zooplankton prey in the Bay of Fundy, Canada. Mar Ecol Prog Ser 306:303–313
Durbin E, Teegarden G, Campbell R, Cembella A, Baumgartner MF, Mate BR (2002) North Atlantic right whales, Eubalaena glacialis, exposed to paralytic shellfish poisoning (PSP) toxins via a zooplankton vector, Calanus finmarchicus. Harmful Algae 1:243–251
Fistarol GO, Legrand C, Granéli E (2003) Allelopathic effect of Prymnesium parvum on a natural plankton community. Mar Ecol Prog Ser 255:115–125
Fistarol GO, Legrand C, Selander E, Hummert C, Stolte W, Granéli E (2004) Allelopathy in Alexandrium spp.: effect on natural plankton community and on algal monocultures. Aquat Microb Ecol 35:45–56
Flynn K, Franco JM, Fernandez P, Reguera B, Zapata M, Wood G, Flynn KJ (1994) Changes in toxin content, biomass and pigments of the dinoflagellate Alexandrium minutum during nitrogen refeeding and growth into nitrogen or phosphorus stress. Mar Ecol Prog Ser 111:99–109
Flynn K, Jones KJ, Flynn KJ (1996) Comparisons among species of Alexandrium (Dinophyceae) grown in nitrogen- or phosphorus-limiting batch culture. Mar Biol 126:9–18
Granéli E, Flynn K (2006) Chemical and physical factors influencing toxin content. In: Granéli E, Turner JT (eds) Ecology of harmful algae. Springer, Berlin, pp 229–241
Granéli E, Johansson N (2003) Increase in the production of allelolpathic substances by Prymnesium parvum cells grown under N- or P-deficient conditions. Harmful Algae 2:135–145
Grasshoff K, Kremling K, Ehrhardt M (1999) Methods of seawater analysis. Willey-VCH, Weinheim
Gross EM, Legrand C, Rengefors K, Tillmann U (2012) Allelochemical interactions among aquatic primary producers. In: Brönmark C, Hansson LA (eds) Chemical ecology in aquatic systems. University Press, Oxford, pp 196–209
Hansen PJ (1989) The red tide dinoflagellate Alexandrium tamarense: effects on behaviour and growth of a tintinnid ciliate. Mar Ecol Prog Ser 53:105–116
Hansen PJ (2002) Effect of high pH on the growth and survival of marine phytoplankton: implications for species succession. Aquat Microb Ecol 28:279–288
Hansen PJ (2011) The role of photosynthesis and food uptake for the growth of mixotrophic dinoflagellates. J Eukaryot Microbiol 58:203–214
Hansen PJ, Lundholm N, Rost B (2007) Growth limitation in marine red-tide dinoflagellates: effects of pH versus inorganic carbon availability. Mar Ecol Prog Ser 334:63–71
Jacobson DM, Anderson DM (1996) Widespread phagocytosis of ciliates and other protists by mixotrophic and heterotrophic thecate dinoflagellates. J Phycol 32:279–285
Jeong HJ, Yoo YD, Park JY, Song JY, Kim ST, Lee SH, Kim KY, Yih WH (2005) Feeding by phototrophic red-tide dinoflagellates: five species newly revealed and six species previously known to be mixotrophic. Aquat Microb Ecol 40:133–150
Johansson N, Granéli E (1999a) Cell density, chemical composition and toxicity of Chrysochromulina polylepis (Haptophyta) in relation to different N:P supply ratios. Mar Biol 135:209–217
Johansson N, Granéli E (1999b) Influence of different nutrient conditions on cell density, chemical composition and toxicity of Prymnesium parvum (Haptophyta) in semi-continuous cultures. J Exp Mar Biol Ecol 239:243–258
John EH, Flynn KJ (2000) Growth dynamics and toxicity of Alexandrium fundyense (Dinophyceae): the effect of changing N:P supply ratios on internal toxin and nutrient levels. Eur J Phycol 35:11–23
Juhl AR (2005) Growth rates and elemental composition of Alexandrium monilatum, a red-tide dinoflagellate. Harmful Algae 4:287–295
Kattner G, Brockmann UH (1980) Semi-Automated Method for the determination of particulate phosphorus in the marine environment. Fresen Z Anal Chem 301:14–16
Keller MD, Selvin RC, Claus W, Guillard RRL (1987) Media for the culture of oceanic ultraphytoplankton. J Phycol 23:633–638
Lilly EL, Halanych KM, Anderson DM (2007) Species boundaries and global biogeography of the Alexandrium tamarense species complex. J Phycol 43:1329–1338
Ma H, Krock B, Tillmann U, Cembella A (2009) Preliminary characterization of extracellular allelochemicals of the toxic marine dinoflagellate Alexandrium tamarense using a Rhodomonas salina bioassay. Mar Drugs 7:497–522
Ma H, Krock B, Tillmann U, Cembella A (2010) Towards characterization of lytic compound(s) produced by Alexandrium tamarense. In: Ho KC, Zhou MJ, Qi YZ (eds) Proceedings of the 13th international conference on harmful algae. Environmental Publication House, Hong Kong, pp 142–146
Ma H, Krock B, Tillmann U, Bickmeyer U, Graeve M, Cembella A (2011a) Mode of action of membrane-disruptive lytic compounds from the marine dinoflagellate Alexandrium tamarense. Toxicon 58:247–258
Ma H, Krock B, Tillmann U, Muck A, Wielsch N, Svatos A, Cembella A (2011b) Isolation of activity and partial characterization of large non-proteinaceous lytic allelochemicals produced by the marine dinoflagellate Alexandrium tamarense. Harmful Algae 11:65–72
Matsuoka K, Cho HJ, Jacobson DM (2000) Observation of the feeding behaviour and growth rates of the heterotrophic dinoflagellate Polykrikos kofoidii (Polykrikaceae, Dinophyceae). Phycologia 39:82–86
Mortensen AM (1985) Massive fish mortalities in the Faroe Islands caused by a Gonyaulax excavata red tide. In: Anderson DM, White AW, Baden DG (eds) Toxic Dinoflagellates. Elsevier, Amsterdam, pp 165–170
Pedersen MF, Hansen PJ (2003) Effects of high pH on the growth and survival of six marine heterotrophic protists. Mar Ecol Prog Ser 260:33–41
Rengefors K, Legrand C (2001) Toxicity in Peridinium aciculiferum—an adaptive strategy to outcompete other winter phytoplankton? Limnol Oceanogr 46:1990–1997
Rost B, Richter KU, Riebesell U, Hansen PJ (2006) Inorganic carbon acquisition in red tide dinoflagellates. Plant, Cell Environ 29:810–822
Sheng J, Malkiel E, Katz J, Adolf J, Place AR (2010) A dinoflagellate exploits toxins to immobilize prey prior to ingestion. Proc Natl Acad Sci USA 107:2082–2087
Skovgaard A, Hansen PJ (2003) Food uptake in the harmful alga Prymnesium parvum mediated by excreted toxins. Limnol Oceanogr 48:1161–1166
Smayda TJ (1997) Harmful algal blooms: their ecophysiology and general relevance to phytoplankton blooms in the sea. Limnol Oceanogr 42:1137–1153
Smetacek V (2001) A watery arms race. Nature 411:745
Strom SL (2008) Microbial ecology of ocean biogeochemistry: a community perspective. Science 320:1043–1045
Tillmann U (2003) Kill and eat your predator: a winning strategy of the planktonic flagellate Prymnesium parvum. Aquat Microb Ecol 32:73–84
Tillmann U (2004) Interactions between planktonic microalgae and protozoan grazers. J Eukaryot Microbiol 51:156–168
Tillmann U, Hansen PJ (2009) Allelopathic effects of Alexandrium tamarense on other algae: evidence from mixed growth experiments. Aquat Microb Ecol 57:101–112
Tillmann U, John U (2002) Toxic effects of Alexandrium spp. on heterotrophic dinoflagellates: an allelochemical defence mechanism independent of PSP toxins. Mar Ecol Prog Ser 230:47–58
Tillmann U, John U, Cembella AD (2007) On the allelochemical potency of the marine dinoflagellate Alexandrium ostenfeldii against heterotrophic and autotrophic protists. J Plankton Res 29:527–543
Tillmann U, Alpermann T, John U, Cembella A (2008a) Allelochemical interactions and short-term effects of the dinoflagellate Alexandrium on selected photoautotrophic and heterotrophic protists. Harmful Algae 7:52–64
Tillmann U, John U, Krock B, Cembella A (2008b) Allelopathic effects of bioactive compounds produced by harmful algae. In Moestrup O (ed) Proceedings of the 12. International conference on harmful algae. International society for the study of harmful algae and intergovernmental oceanographic commission of UNESCO, 2008 Kopenhagen, pp 12–18
Tillmann U, Alpermann T, Purificacao R, Krock B, Cembella A (2009) Intra-population clonal variability in allelochemical potency of the toxigenic dinoflagellate Alexandrium tamarense. Harmful Algae 8:759–769
Uronen P, Lehtinen S, Legrand C, Kuuppo P, Tamminen T (2005) Haemolytic activity and allelopathy of the haptophyte Prymnesium parvum in nutrient-limited and balanced growth conditions. Mar Ecol Prog Ser 299:137–148
Verity PG, Smetacek V (1996) Organism life cycles, predation, and the structure of marine pelagic ecosystems. Mar Ecol Prog Ser 130:277–293
von Ehlert E, Jüttner F (1997) Phosphorus limitation and not light controls the extracellular release of allelopathic compounds by Trichormus doliolum (Cyanobacteria). Limnol Oceanogr 42:1796–1802
Weissbach A, Tillmann U, Legrand C (2010) Allelopathic potential of the dinoflagellate Alexandrium tamarense on marine microbial communities. Harmful Algae 10:9–18
Whittaker RH, Feeny PP (1971) Allelochemics: chemical interactions between species. Science 171:757–770
Yang I, Beszteri S, Tillmann U, Cembella A, John U (2011) Growth- and nutrient-dependent gene expression in the toxigenic marine dinoflagellate Alexandrium minutum. Harmful Algae 11:55–69
Acknowledgments
Thanks to Kai Uwe Ludwichowski for inorganic nutrient measurements, to Christiane Lorenzen and Sandra Murawski for help with C/N analysis and to Steve Pueppke for correcting the English.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by U. Sommer.
Rights and permissions
About this article
Cite this article
Zhu, M., Tillmann, U. Nutrient starvation effects on the allelochemical potency of Alexandrium tamarense (Dinophyceae). Mar Biol 159, 1449–1459 (2012). https://doi.org/10.1007/s00227-012-1924-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-012-1924-7