Abstract
The carbon metabolism of two marine sponges, Haliclona oculata and Dysidea avara, has been studied using a 13C isotope pulse-chase approach. The sponges were fed 13C-labeled diatoms (Skeletonema costatum) for 8 h and they took up between 75 and 85%. At different times, sponges were sampled for total 13C enrichment, and fatty acid (FA) composition and 13C enrichment. Algal biomarkers present in the sponges were highly labeled after feeding but their labeling levels decreased until none was left 10 days after enrichment. The sponge-specific FAs incorporated 13C label already during the first day and the amount of 13C label inside these FAs kept increasing until 3 weeks after labeling. The algal-derived carbon captured by the sponges during the 8-h feeding period was thus partly respired and partly metabolized during the weeks following. Apparently, sponges are able to capture enough food during short periods to sustain longer-term metabolism. The change of carbon metabolic rate of fatty acid synthesis due to mechanical damage of sponge tissue was studied by feeding sponges with 13C isotope–labeled diatom (Pheaodactylum tricornutum) either after or before damaging and tracing back the 13C content in the damaged and healthy tissue. The filtration and respiration in both sponges responded quickly to damage. The rate of respiration in H. oculata reduced immediately after damage, but returned to its initial level after 6 h. The 13C data revealed that H. oculata has a higher metabolic rate in the tips where growth occurs compared to the rest of the tissue and that the metabolic rate is increased after damage of the tissue. For D. avara, no differences were found between damaged and non-damaged tissue. However, the filtration rate decreased directly after damage.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Sponges are known for their capacity to produce bioactive secondary metabolites. However, no sustainable production techniques have been developed yet to obtain sufficient quantities of these potential pharmaceuticals. In order to develop such techniques and to maximize productivity, we need to obtain a better understanding of feeding and metabolic rates in sponges. Sponges are heterotrophic organisms and pump large amounts of water through their body from which particles are filtered (Bergquist 1978). How and at which rate these particles are metabolized in sponges has not been studied widely (Schmidt 1970; Hahn et al. 1988). One of the problems in studying sponge metabolism is that particles filtered out of the water are not immediately metabolized. To understand sponge metabolism, it is necessary to be able to distinguish whether a compound is part of the taken food particles or of the sponge tissue.
This problem can be solved by looking at the metabolism of biomarkers, which are compounds only present in the sponge and not in the food. In a previous study, we found several fatty acids that could serve as a biomarker for sponges (Koopmans 2009). Among aquatic animals, sponges are specified by the greatest diversity of sterols and fatty acids (FAs), which have an unusual and sometimes unique structure (Litchfield et al. 1976; Thiel et al. 1999; Rod’kina 2005). Sponges contain large quantities of C22–C30 fatty acids with branched and odd-chains and hydroxyl-acids (Bergquist et al. 1984; Christie et al. 1992; Thiel et al. 1999; Nechev et al. 2002; Rod’kina et al. 2003). These very long-chain fatty acids are sometimes called ‘demospongic acids’ (Rod’kina 2005). Fatty acids are very diverse in structure and chain length, and some are unique for certain organisms. Consequently, many researchers have used FAs and combinations of FAs as biomarkers for algal or bacterial biomass (Dunstan et al. 1994; Boschker and Middelburg 2002; Kharlamenko et al. 2008). One of the problems we encountered in our previous study (Koopmans 2009) is that some of the demospongic acids were also found in suspended particles, implying either that these demospongic acids are not unique or that sponge detritus contributes substantially to suspended particle organic matter pools. This non-uniqueness of putative biomarkers is a common problem in organic geochemistry and microbial ecology (Boschker and Middelburg 2002). However, this can be solved by using isotopically labeled single algae species as a food source. 13C label appearing in sponge-specific fatty acids, which are not present in the added algae, can then be attributed to metabolism of the sponge.
Sponges possess an active homologation enzyme system using short-chain fatty acid precursors largely derived from exogenous sources as substrate (Djerassi and Lam 1991). Therefore, the composition of sponge FAs is probably related to the composition of food FAs and symbionts. Biosynthesis of fatty acids has been studied before in different sponges by using 14C-label (Raederstorff et al. 1987; Hahn et al. 1988). In Aplysina fistularis, it was found that 22MeC28:2(5,9) was elongated from 10MeC16:0 to 22MeC28:0 and then further desaturated (Raederstorff et al. 1987). Hahn et al. (1988) elucidated the biosynthesis of two common long-chain ‘demospongic acids’ in the sponge Microciona prolifera. They found that C14:0 was first elongated to C26:0, which was then further desaturated to form C26:2(5,9). Moreover, Hahn et al. (1988) showed that C26:3(5,9,19) had an exogenous palmitoleic acid (C16:1(9)) as precursor. Recently, a 13C labeling approach was used to study sponge carbon uptake from different food sources in a bacterial sponge in its natural habitat (de Goey et al. 2008). Fast uptake and conversion of food sources was found in the sponge and its symbionts. However, how long it takes for 13C label to be incorporated into the longer-chain FAs was not studied (de Goey et al. 2008).
Damaged sponge tissue regenerates at higher rates than the corresponding growth rates of the same sponge species (Ayling 1983; De Caralt et al. 2008). The rate of metabolism associated with regeneration and whether more energy is allocated toward the damaged tissue is not known.
The aim of this paper is to get a better understanding of food uptake and metabolism of sponges and to find the effect of damage on sponge metabolism and growth. Sponges were fed 13C-labeled diatoms and label appearance and eventual loss in (sponge) fatty acids were followed over time. In addition, 13C-label incorporation into sponge fatty acids was also used to assess the effect of damaged sponge tissue on metabolic rate of the sponge. Different types of sponges were used in this study. Haliclona oculata occurs abundantly in the Oosterschelde, the Netherlands in temperatures ranging from 2–23°C. It is a finger sponge attached at one base, containing many spicules to build their strong skeleton. Dysidea avara is a widely distributed sponge species in the Mediterranean (Uriz et al. 1992), where temperature varies between 12–25°C. It is an encrusting sponge that can be found on rocky substrata. Also lobulated masses can be found. The skeleton is a meshwork of sponging fibers without spicules (Ribes et al. 1999), which makes it a more fragile sponge than H. oculata.
Materials and methods
Feeding experiment for metabolic rate
The sponge species used were Haliclona oculata and Dysidea avara. These sponges are not related and live in different habitats. H. oculata is a finger sponge and occurs widely in the Oosterschelde, a tidal inlet in the Netherlands (Lokkersnol, 51°38′58.07″N, 3°53′5.11″E), and grows attached to solid surfaces such as oyster shells. Dysidea avara is an encrusting sponge and occurs abundantly in the Mediterranean, Spain (Cala Montgo, 42°06′50.33″N, 3°10′02.10″E).
For metabolic rate studies, 27 sponge specimen of Haliclona oculata were collected on August 13, 2007, by scuba diving in the Oosterschelde at ~13 m depth, water temperature 20°C. Sponges were transported to the laboratory in aerated boxes in their natural seawater and placed within 2 h in three aquaria (water volume approximately 80 l) with continuous flowing natural seawater directly pumped from the Oosterschelde. On January 21, 2008, 18 sponge specimen of Dysidea avara were collected in the Mediterranean at ~8 m depth, water-temperature 14°C. Sponges were transported to two aquaria (water volume approximately 100 l) containing natural seawater close to the sea within 1 h after collection.
Haliclona oculata specimens were kept for 1 week in the new environment prior to the pulse labeling for acclimation. After 1 week, the sponges were fed with 300 ml of living 13C-labeled Skeletonema costatum per aquarium. The diatom S. costatum was cultured at 16°C in F2 medium in 6 l artificial seawater containing 25% 13C-labeled NaHCO3. The culture was centrifuged at 1,000*g and washed 3 times with filtered unlabeled seawater to remove any remaining 13C label after which they were diluted to 17,000 cells per ml to feed the sponges. Three samples of 1 ml were taken from the S. costatum culture to analyze algal fatty acids and 13C content. During labeling, the continuous flowing seawater was stopped and pumps were used to mix the seawater in the aquaria to prevent cells from settling on the bottom. After the pulse of 13C-labeled cells, we counted the cells every hour to determine sponge filtration rate. After 8 h, most cells were filtered out and the continuously flowing seawater was turned on again. Whole sponges were sampled from each aquarium at day 0 (before feeding), after 8 h (directly after labeling), and at days 1, 2, 3, 7, 10, 14, and 21. The sampled sponges were frozen in liquid nitrogen and stored at −80°C until freeze-drying for 24 h.
Dysidea avara specimens collected from the field were immediately fed with 300 ml Skeletonema costatum culture per aquarium. Sponge filtration was assessed by sampling particulate matter after 1 h and at the end of the feeding by analysis of particulate organic 13C content. Sponge feeding in the aquaria lasted for 8 h and afterward, the sponges were placed back into the sea at the site of collection. At day 0 (before feeding), after 8 h (directly after feeding), and at days 1, 2, 4, and 8 three (n = 3) sponge specimen were sampled for further analysis. The collected specimen were frozen on dry ice and in this way transported to the laboratory. The sponges were stored at −80°C until freeze-drying for 24 h.
Feeding experiment with damaged sponges
13C labeling experiments with damaged Haliclona oculata were done in situ in the Netherlands on September 26, 2007, water temperature 17°C. A platform was placed at the study site and 42 concrete pavement tiles (30-cm × 30-cm) were placed on top of the platform (Koopmans and Wijffels 2008). On every tile, a sponge specimen was attached. Twelve specimens were used in this experiment. These specimens had been attached to the tiles on the platform already for several months. The 13C labeling experiments with Dysidea avara were done in situ in Spain on January 22, 2008, with a water temperature of 14°C. On 21 January, 12 D. avara specimens were collected with their rocky substrate. The substrate was glued to tiles located close to the place of collection.
The uniformly 13C (I.E. 99.5%)-labeled biomass of Pheaodactylum tricornutum in lyophilized form was purchased from Protein Labelling Innovation (PLI), the Netherlands. The sponges on the platform were fed with this 13C-labeled biomass of P. tricornutum. A suspension containing 22.5 mg P. tricornutum in 15 ml of seawater was fed to the sponges for 25 min. For the in situ experiments, special experimental chambers made of polyvinylchloride (PVC) with 3,800 ml volume were developed. The chamber had an opening underneath to completely cover the sponge. The chamber was placed over the sponge, and by using elastic bands the chamber could easily be attached to the tiles. The chamber had two connections, one to connect an oxygen probe and a small sampling tube for adding the P. tricornutum solution. A magnetic stirrer inside the chamber continuously mixed the water inside to prevent particles from settling and to equalize oxygen distribution. Oxygen concentrations were measured using an Oxi 340i handheld oxygen meters (WTW, Weilheim, Germany). The oxygen concentration inside a chamber was logged every 10 s. The meter connected to the probe was in a waterproof box and taken underwater. Feeding experiments lasted for 25 min, to minimize the effect of oxygen depletion. In total, we used 3 experimental chambers. Since the maximal dive-time was 60 min, we could feed six sponges during one dive.
The finger sponge H. oculata was damaged using scissors, and all tips of the sponge were cutoff. D. avara was damaged by scraping of sponge tissue on one side of the sponge, keeping more than half of the sponge undamaged. To find differences between sponges that were damaged and not damaged, we used 12 sponge individuals, which were divided into four groups that were treated as described in Table 1. Thus, for each strategy, 3 sponge specimens were used. In this way, we could measure differences in uptake rate due to damage. One week after feeding, the sponges were collected. After collection, we separated the damaged tissue and the healthy part of the sponge body to be able to find differences in amount of 13C label in the different parts of the sponge. The collected specimen were frozen on dry ice and in this way transported to the laboratory in 2 h. The sponges were stored at −80°C until freeze-drying for 24 h. The dry sponges were grinded with mortar and pestle before further usage.
Total carbon and 13C content analysis
The freeze-dried sponges were grinded with mortar and pestle before further usage. The carbon content of all sponge individuals was measured using a Fisons NA 2500 element analyzer with auto sampler and Haysep-Q column 80–100 mesh I.D. 2 mm (Nieuwenhuize et al. 1994). In short, the dried sample was flash combusted in an oxygen-rich environment, and the generated CO2 and N2 were separated on a GC column and detected using a thermal conductivity detector. Moreover, the CO2 released was then transferred via a Conflo II interface to and isotope-ratio mass spectrometry to measure the carbon isotopic composition (EA-IRMS) (Middelburg et al. 2000).
Fatty acid and 13C analysis
About 100 mg sponge dry weight was used for lipid extraction using an adjusted Bligh and Dyer method (see also (Boschker et al. 1999; Middelburg et al. 2000). The total lipid fraction was derivatized by mild alkaline methanolysis to obtain fatty acid methyl esters (FAME). Both C12:0 and C19:0 were used as internal FAME standards. Identification of the fatty acids was done using equivalent chain length data with known standards measured on a non-polar column. Additional identification was done using GC–MS. Carbon isotopic composition of individual FAME was determined with a gas-chromatograph combustion-interface isotope-ratio mass spectrometer (GC-c-IRMS); a HP G1530 GC (Hewlett Packard) connected to Delta-plus IRMS via a type-III combustion interface from Thermo Finnigan (Bremen). Helium was used as a carrier gas (150 kPa) with an apolar column (Hewlett-Packard HP-5MS, 60 m*0.32 mm*0.25 μm). The column was kept at 70°C for 2 min, then temperature was programed from 70 to 150°C at 20°C min−1, subsequently from 150 to 290°C at 3°C min−1 and from 290°C to 325°C at 5°C min−1, temperature was kept at 325°C for 11 min. Fatty acid concentrations were determined by area correction according to the C19:0 internal standard. Stable carbon isotope ratios (13C/12C) for individual FA were calculated from FAME data by correcting for the one carbon atom in the methyl group that was added during derivatization (Middelburg et al. 2000).
Enrichment calculation
High-precision isotope ratios were determined as the relative difference in 13C/12C of a sample and the international standard for carbon of Vienna PeeDeeBelemnite (PDB). Isotope measurements are usually given in the δ-notation (in part per thousand) calculated using the following equation:
The absolute amounts of 13C-label (μg 13C g dw−1) in FA can be calculated from the concentration μg FA g dw−1 (C) and the increase in the fraction 13C after labeling (F13) relative to the control (F 13c ):
where \( {\text{F}}^{ 1 3} = {\text{R}}/\left( {{\text{R}} + 1} \right)\;{\text{and}}\;{\text{R}} = \left( {\delta^{ 1 3} {\text{C}}/ 1000 + 1} \right) \times {\text{R}}_{\text{PDB}} \)
Cell count
A Beckman coulter counter was used to count the cells during the pulse feeding of Haliclona oculata. Samples of 20 ml were taken after 0, 0.5, 1, 2, 3, 4, 5, 6, and 8 h from each aquarium. Only cells larger than 4 micron were counted.
Respiration rate
Respiration rates were measured for H. oculata at days −4, −3, −2, −1, 0, 1, 2, 7, and 14. Respiration experiments were performed in polyvinylchloride (PVC) experimental chambers with a volume of 765 ml and with an opening underneath to completely cover the sponge. Chambers were placed over the sponge and thus the sponge was not affected. An oxygen probe was connected to each chamber. A magnetic stirrer inside chambers continuously mixed the water inside to prevent particles from settling and to homogenize oxygen distribution. O2 concentrations were measured and logged every 10 s using two Oxi 340i handheld oxygen meters (WTW, Weilheim, Germany). Experiments lasted for 15 min, to minimize the effect of oxygen depletion on respiration rates.
Results and discussion
First, the results to determine metabolic rate are discussed for both Haliclona oculata and Dysidea avara, after which the damage experiments are discussed for both species.
Carbon metabolic rate
Both marine sponge species were fed 13C-labeled living diatoms (4 atom% 13C), and carbon uptake, assimilation, and conversion were followed over time. Dysidea avara from the Mediterranean, Spain, retained less 13C than Haliclona oculata from the Oosterschelde, the Netherlands, which may be partly the result of different feeding strategies and experimental procedures for both sponges. H. oculata was given 1 week to readjust from field to laboratory conditions. Labeling took place using actively growing Skeletonema costatum cells and the experiment lasted for 3 weeks. For D. avara, the experiment only lasted 1 week. And the feeding took place immediately after collection in a stirred non-flushed aquarium with live diatoms that were stored for 3 days. After the 8-h feeding period, D. avara sponges were placed back in their natural habitat. We will focus our discussion on the results for H. oculata because this experiment lasted longer, more 13C uptake occurred, and the diatoms provided were actively growing.
Haliclona oculata
During labeling, water samples were taken to follow diatom uptake. For the H. oculata experiment, we were able to immediately analyze the water samples by counting cells (Fig. 1). This allowed us to optimize experimental procedures and in particular the feeding period. More than 75% of the diatoms were filtered by the sponges within 8 h. Moreover, it also allowed estimating a filtration rate (F; l h−1 sponge−1) using the next equation:
where C represents the concentration of diatoms, V is the volume of water per sponge (l sponge−1) and t is time (h). The filtration rate based on the uptake of S. costatum cells found for H. oculata was 1.7 l h−1 sponge−1. This is an average value for 12 sponge individuals. This is somewhat lower than the value of 3.4 l h−1 sponge−1 found for H. oculata in its natural habitat (Koopmans et al. 2009). This may be due to the transfer to the aquaria causing the sponge to filter at a lower rate. Total 13C enrichment of the sponge specimen was followed in time (Fig. 2a). Most enrichment was found immediately after labeling and up to day 1, after that 13C content decreased gradually. Variability was high during the initial period (till day 1), which may be due to the difference in filtration activity by sponge individuals. Three different sponges, one from each aquarium, were collected each sampling, thus when a sponge did or did not filter a lot of material during the 8-h feeding period this had major consequences for 13C acquisition. After the first day, the 13C content decreased and thus H. oculata started to excrete or respire acquired carbon. This decrease was initially rapid and then more gradual. This initial rapid decrease may be due to the loss of diatom cells or rapid respiration of part of the 13C. The subsequent gradual decrease of 13C label in the sponge could be due to slow turnover of assimilated carbon. The decrease in 13C was expected as we found in our previous work that about 10% of carbon acquired was used for biomass assimilation and over 90% was used for respiration (Koopmans et al. 2009). After 3 weeks, still 22.3% of the carbon acquired within the 8-h pulse labeling period was inside H. oculata. Thus, we can distinguish between an initial phase with rapid 13C loss possibly due to direct loss of diatoms or rapid respiration and second phase with a slower 13C loss in which 13C label due to a slow turnover of assimilated 13C. Tracing 13C in FA will be instructive to further elucidate this.
For the H. oculata sponges, we also measured respiration rate several times. We found a slight decrease in respiration rate during the first week after collection (during acclimatization period) from 96.2 to 86.9 μmol O2 h−1 g dw−1, which qualitatively agrees with the somewhat low filtration rates in the aquaria. Two weeks after feeding the respiration rate was 82.5 μmol O2 h−1 g dw−1. These values are all within the range found for this species in its natural habitat (Koopmans et al. 2009), indicating that they were performing well in the aquaria. A respiration rate of 86.9 μmol O2 h−1 g dw−1 corresponds to a carbon usage for respiration of 65.2 μmol C h−1 g dw−1, assuming a respiratory quotient of 0.75 μmol C/μmol O2 (Hatcher 1989). Thus, per day 18.8 mgC g dw−1 was respired, which is in agreement with the decrease of 13C (Fig. 2).
Fatty acid enrichment
The enrichment of 13C in individual fatty acids of sponges was measured, and total quantity of label in FA is shown in Fig. 2b. Note that the y-axis is in μg 13C per gram dry weight and in Fig. 2a this is in milligram 13C per gram dry weight. Thus, only 10% of the incorporated 13C were recovered in the form of FAs. After 3 weeks, about 7.6% of the FAs labeled after the 8-h pulse were still present in the form of FAs (Fig. 2b). This is about 3 times less than we found for total carbon (22.3%; Fig. 2a). Thus, fatty acids were either excreted faster or respired more than other organic compounds to obtain energy.
The 13C content of specific fatty acids can be used to assess the fate of algal material and the metabolism of sponge fatty acids. Table 2 shows the percentage of the major fatty acids in the diatom used as food and in the two sponges. For H. oculata, we found some large sterol peaks at the end of the chromatogram, the major one could be attributed to campesterol ((24)-ergost-5-en-3beta-ol). The FAs C14:0, C16:3ω4, C16:1ω7, C16:0, C20:5ω3 have high concentrations in the diatom cultured and fed to the sponges and have rather low concentrations in the sponges. These FAs with high concentrations in diatoms and low concentrations in sponges can be used to follow how fast diatom carbon was metabolized by the sponges. Similarly, a number of FA were not found in diatom material added and were present in the sponges and these can be used to trace formation and transformation of FA by sponges. However, some of these sponge FAs may in fact reflect prokaryotic symbionts (branched FA such as iC17:0 and aiC17:0).
Figure 3 shows the absolute 13C content for the feed biomarkers per 1 ml S. costatum culture. The diatoms added were alive and not axenic to mimic natural conditions as much as possible. Therefore, some bacterial-derived FAs were present in the diatoms (Table 2). It is shown that most 13C is present in C16:1ω7, which is related to the high concentration of this FA in the diatom S. costatum (Table 2). When the diatom cells are present inside the sponge as intact cells, the 13C enrichment of C16:1ω7 will be higher inside the sponge as well. Figure 4a shows that after 8 h of feeding indeed the 13C enrichment of ‘sponge’ FA resembled that of S. costatum. Within the first day, FA enrichment levels did not change drastically, but a rapid decrease of 13C excess in diatom biomarkers was observed during the second day. Thus, the sponges either started to excrete the cells or to use the diatoms for their own metabolism for energy generation and build new biomass. These results confirm the results found by Schmidt (1970) as he found that feed particles were removed from the sponge body after 48 h. In our study, we were able to detect 13C enrichment in diatom FAs up to 10 days, indicating that it takes in the order of a week for sponges to process algal material after capture. Rapid collection of food followed by slow processes of algal material is an excellent strategy to survive in low-food environments or when food supply is pulsed.
Several long-chain FAs were abundant in H. oculata and they were not present in S. costatum (Table 2). Although in their natural habitat, these long-chain fatty acids are not sponge-specific biomarkers, in this case where we feed with specifically label diatoms, label incorporation in these fatty acids must be due to sponge metabolism. Figure 4b shows the incorporation of 13C into these long-chain FAs. It is clear that 13C incorporation in C24:1(17) started immediately, reached maximum levels at day one and then decreased gradually. The decrease of this intermediate compound was likely caused by the transformation to longer-chain FAs, like C26:3(5,9,19) and C28:3(5,9,21), which showed slower increase in label but incorporation continued up to the end of the experiment. Especially for C26:2(5,9) and C28:3(5,9,21), 13C enrichment was still increasing 3 weeks after labeling (Fig. 4b).
Dysidea avara
During the feeding of D. avara, we could not count the cell decrease in the water during incubations because of logistic restrictions, but we measured 13C of particulate organic carbon in the water, which decreased from 9.4 μg/l after 1-h feeding to 5.2 μg/l after 8-h feeding. Moreover, δ13C values decreased from 383 to 262‰ during the same time interval, which means that the labeled cells were preferentially filtered from the water. After 8 h, more than half of the added particles were still left, indicating that D. avara was less efficient than H. oculata in capturing particles, probably because D. avara filtered less water than H. oculata. The filtration rate found for D. avara was 0.85 l h−1 sponge−1; this is somewhat low but still in the range as reported before (Ribes et al. 1999). The low filtration rate was most likely caused by the stress inherent to collection. Besides this, there could also be a seasonal effect as H. oculata were sampled in summer, whereas D. avara was sampled in January.
Figure 2 shows the total 13C and the total fatty acid 13C enrichment for both sponge species. Although 13C acquisition was lower during the feeding period, retention of 13C was higher in D. avara than in H. oculata. After 8 days, 67.7% of total 13C excess and 46.7% of FA 13C excess was still present inside. D. avara appears to keep the acquired carbon longer in its body than H. oculata, both in terms of total 13C and FA 13C. D. avara lives in a more oligotrophic environment (i.e. Mediterranean) than H. oculata (The Netherlands) and efficient retaining captured algae is a sensible strategy to survive in food depleted settings. Besides; Ribes et al. (1999) found that D. avara obtains 85% of its ingested carbon from the fraction smaller than 5 μm and 15% from the fraction larger than 5 μm. Thus, the diatoms are not the main food for D. avara.
During the experiment with D. avara, some unexpected results appeared. Figure 5a shows that the highest 13C enrichment of diatom biomarkers was found after 1 day for D. avara. After incubation of the sponge with label in the laboratory for 8 h, they were placed back in the sea, but this could not be done immediately. As a consequence, label acquisition continued beyond 8 h. Following an expected decrease during the second day and a sudden and unexpected increase occurred after 4 days, which can also be seen in Fig. 2. The increase can only be explained by cross-contamination from another in situ labeling experiment done by us nearby. After 8 days, the enrichment levels of the diatom biomarkers decreased again but did not approach zero as expected. Another striking result is the low enrichment of two diatom FAs (Fig. 5a), as if during the culturing of the labeled cells these FAs were less formed and thus labeled.
In D. avara, less label was found in the long-chain sponge FAs (Fig. 5b), which is a result of the lower 13C uptake (Fig. 2). Enrichment level of C24 and C26 were similar; however, C24:1(17) showed a similar trend as the diatom biomarkers, namely the decrease after 2 days with an increase again after 4 days (Fig. 5). C24:1(17), an intermediate compound, was apparently turned over faster than the other long-chain FA.
Increased metabolic rate due to damaging
Filtration and respiration rate
H. oculata and D. avara are two very different sponge species with a different structure. Due to the difference in sponge structure also different damaging strategies were used. For H. oculata, we used scissors to cut off all tips of each sponge specimen used. For D. avara, we scraped off part of the tissue of one half of a sponge, leaving the other half intact. After the experiment, we collected the damaged and non-damaged parts of each sponge specimen separately and analyzed them on 13C content. For H. oculata, this was easier as the tips and rest of the body could easily be separated. For D. avara, this was less easy, because of the colonized organization it sometimes seemed that the damaged and healthy part started to act as two separate sponges. Consequently, it is difficult to compare the difference in damaged and non-damaged tissue of one sponge individual.
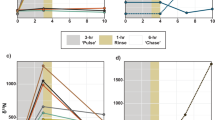
All sponges were fed with the same amount of 13C-labeled biomass P. tricornutum. For each sponge, we took a sample of water at the end of feeding from inside the experimental chamber, which was next tested on 13C content to determine the amount of 13C taken up by the sponge. We compared filter capacities of damaged sponges to that of the non-damaged sponges (Fig. 6). Figure 6 shows the 13C fraction in atom% in the SPM of the water inside the experimental chamber at the start and after 25-min feeding. The starting value was the same for all experiments. A decrease in 13C fraction means that the added diatoms are filtered preferentially over other carbon containing particles. Only for D. avara, the total carbon content was measured and actual filtration rates could be measured. This confirmed that a lower 13C fraction in the water at the end of the experiment means a higher filtration rate. The decrease in enrichment after 25 min is the same for the non-damage and feed-first experiment as was expected because both treatments mean the sponges are fed when not being damaged.
Fraction at the start of feeding and after 25 min of feeding for the different approaches. The start concentration is the same for both sponges. Non-damage and feed-first are non-damaged sponges while feeding, fast feed means immediate feeding after damage and late feed means that after damage a lag time was taken into account of 6 h (H. oculata) or 24 h (D. avara)
Directly after damage, H. oculata does not change filtration rate while D. avara immediately decreases filtration rate. After 6-h filtration rate for H. oculata increased to a higher value than the original value. D. avara responded faster than H. oculata to damage with a reduction of food uptake instead of an increase. Also at the late feed, still a reduction in food uptake was observed. However, for D. avara, late feeding occurred later (at 24 h) than for H. oculata (6 h). We were forced to use these different time intervals for late feeding due to bad weather conditions in the case of D. avara. Apparently, the response of H. oculata after damage is to increase food uptake for wound healing, while most likely the reduction of food uptake by D. avara is caused by killing part of the sponge tissue, leaving only half of the sponge alive for filtering. Respiration rate was also measured during feeding for H. oculata. Respiration rate immediately decreased after damage but was restored 6 h after damage (Fig. 7). The large increase in filtration rate 6 h after damage coincided with an increase of the respiration rate to the original level. The respiration rate measurements failed during the experiment with D. avara due to technical problems with the oxygen meters.
We only have respiration rate measurements for H. oculata, because the respiration rate measurements failed during the experiment with D. avara due to technical problems with the oxygen meters. Respiration rate for H. oculata immediately decreased after damage, but was restored 6 h after damage (Fig. 7). This only partly correlates with the filtration rate. The initial decrease in respiration due to damage was not accompanied with a decrease in filtration, which stayed the same. The following restoration of the respiration rate to the original level correlated with an increase in filtration rate.
13C label in whole sponges
Despite clear differences in 13C label uptake between damaged and non-damaged sponges, no significant differences in levels of 13C label inside the sponges were found 1 week after feeding (Figs. 8, 9). From the sponge individuals, we collected the damaged and non-damaged tissue separately. For H. oculata, this was easier as we damaged the tips of the sponge-fingers simply by cutting them off. For D. avara, we split all sponges in half, where one half was damaged and the other half was left intact. H. oculata contained higher concentrations of 13C label in the tips, both in the case of damaged and non-damaged sponges (Fig. 8). The damaged sponges had slightly more 13C in the tips than the non-damaged sponges, but the difference was not significant. This indicates that the food uptake in the tips is higher than in the rest of the body and that this may increase upon damage. This increase seems to indicate that growth occurs at the tips and that the metabolic activity in the tips is higher than in the rest of the tissue. H. oculata has two outliers in 13C uptake, the first fast feed sponge shows higher 13C content than the other sponges and the first late feed sponge shows a lower 13C content. The 13C fraction for D. avara showed large variations between the three individuals. No systematic difference was found between damaged and non-damaged parts for D. avara (Fig. 9). This could be explained by the fact that apparently D. avara lives more like colonies, and when part of the sponge is damaged, it seems to be left to die and the intact part is the part growing on.
13C label in specific fatty acids
Whether 13C label is present as algal cells or metabolized algal biomass can be seen in the specific fatty acid content. The fatty acid (FA) C16:0 is a biomarker for diatoms (Dunstan et al. 1994). This biomarker was still present in the sponge body after 7 days (Fig. 10). For H. oculata, more 13C label was present in this FA in the tips of the damaged sponges, in contrast to the non-damaged sponges where this was not the case. This confirms the finding that damaged tips took up more 13C label (Fig. 8). The FA C26:2(5,9) is very abundant in both sponges and non-existent in P. tricornutum (data not shown). In the tips of both damaged and non-damaged H. oculata, more enrichment in this sponge biomarker was observed than in the rest of the body (Fig. 10). The difference between tips and body was larger for damaged sponges with the exception of one individual at the late feed. Apparently, more 13C label was converted in the damaged tips when compared to non-damaged tips. This is in accordance with the fact that H. oculata grows at the tips (Kaandorp 1991), which leads to more 13C label in the tips. Regeneration is mostly found to be a faster process in sponges than normal growth (Ayling 1983; De Caralt et al. 2003, 2008; Duckworth 2003), which is confirmed here by the higher enrichment found in the tips of damaged sponges. For D. avara, the variation in 13C fraction is again very high, which makes it impossible to see significant differences between damaged and non-damaged parts of the sponges and between damaged and non-damaged sponges. D. avara shows the same trend in the food and sponge FAs as for the total amount of 13C in the sponges (Fig. 10). Remarkable for D. avara was that the food biomarkers were still highly present after 1 week, while the sponge components were not that high enriched. For H. oculata, this was just the other way around, where higher levels of enrichment were found for the sponge component and not for the food components. The latter was also found before, where less 13C label was found in the larger FAs in D. avara than in H. oculata. However, the absolute values of enrichment did not differ too much in the sponge FAs for both H. oculata and D. avara. Apparently, the food FAs are metabolized slower or stored in D. avara.
Conclusion
Two sponge species (Haliclona oculata and Dysidea avara) fed with 13C-labeled diatoms became strongly enriched within 8 h after taking up the labeled cells. The sponge remained strongly labeled during the first 2–3 days because diatoms, as inferred from intact diatom fatty acids, were still present. However, 10 days after labeling, diatom-specific-labeled FA could not be detected anymore indicating that diatom cells were either excreted or converted. Sponge-specific fatty acids were produced from day one onwards and 3 weeks after labeling 13C levels still increased in the longer-chain fatty acids (FAs) in Haliclona oculata. Thus, food particles taken up at a certain moment are still being processed 3 weeks later.
Both sponge species responded quickly to damage. Immediately after damage H. oculata respired less, but respiration rate recovered to the original rates within 6 h. Filtration rate initially stayed the same but increased to a higher level within 6 h after damage. In contrast, D. avara immediately decreased filtration rate after damage, which was partly but not fully restored 1 day later. One week after labeling relatively less 13C-labeled algal biomarkers were found in H. oculata compared to 13C label in sponge biomarkers. D. avara had comparable enrichment in sponge biomarkers but contained clearly more algal biomarkers indicating that D. avara either stored algal components and/or respired them less fast than H. oculata, whereas assimilation into biomass was comparable.
Damaged and non-damaged tissue was collected separately. In H. oculata, a higher fraction of 13C label was found in the tips of both damaged and non-damaged sponges, indicating that growth rate is higher in the tips. The sponge biomarkers were higher enriched in the tips of the damaged sponges compared to the non-damaged sponges, indicating a higher growth and metabolic rate in the tips after damage. For D. avara, large variation in levels of 13C label was found in healthy and damaged tissue, and significant differences were not found.
The differences found between the sponges can be explained by the different growth forms of the two sponge species. H. oculata is a finger sponge and grows mostly at the tips. D. avara is an encrusting sponge that lives more like colonies and when part of the sponge is damaged it seems to be left to die and the intact part is the part that continues to grow.
References
Ayling AL (1983) Growth and regeneration rates in thinly encrusting demospongiae from temperate waters. Biol Bull 165:343–352
Bergquist PR (1978) Sponges. Hutchinson & Co Ltd, London
Bergquist PR, Lawson MP, Lavis A, Cambie RC (1984) Fatty acid composition and the classification of the porifera. Biochem Syst Ecol 12:63–84
Boschker HTS, Middelburg JJ (2002) Stable isotopes and biomarkers in microbial ecology. FEMS microbiology 40:85–95
Boschker HTS, de Brouwer JFC, Cappenberg TE (1999) The contribution of macrophyte-derived organic matter to microbial biomass in salt-marsh sediments: Stable carbon isotope analysis of microbial biomarkers. Limnol Oceanogr 44:309–319
Christie WW, Brechany EY, Stefanov K, Popov S (1992) The fatty acids of the sponge Dysidea fragilis from the Black Sea. Lipids 27:640–644
De Caralt S, Agell G, Uriz MJ (2003) Long-term culture of sponge explants: conditions enhancing survival and growth, and assessment of bioactivity. Biomol Eng 20:339–347
De Caralt S, Uriz MJ, Wijffels RH (2008) Grazing, differential size-class dynamics and survival of the Mediterranean sponge Corticium candelabrum. Mar Ecol Prog Ser 360:97–106
De Goey JM, Moodley L, Houtekamer M, Carballeira NM, van Duyl FC (2008) Tracing 13C-enriched dissolved and particulate organic carbon in the bacteriacontaining coral reef sponge Halisarca caerulea: evidence for DOM feeding. Limnol Oceanogr 53:1376–1386
Djerassi C, Lam W (1991) Sponge phospholipids. Acc Chem Res 24:69–75
Duckworth AR (2003) Effect of wound size on the growth and regeneration of two temperate subtidal sponges. J Exp Mar Biol Ecol 287:139–153
Dunstan GA, Volkman JK, Barrett SM, Leroi JM, Jeffrey SW (1994) Essential polyunsaturated fatty acids from 14 species of diatom (Bacillariophyceae). Phytochemistry 35:155–161
Hahn S, Stoilov TB, Ha TBT, Readerstorff D, Doss GA, Li H, Djerassi C (1988) Biosyntethic studies of marine lipids. 17. The course of chain elongation and desaturation in long-chain fatty acids. J Am Chem Soc 110:8117–8124
Hatcher A (1989) RQ of benthic marine invertebrates. Mar Biol 102:445–452
Kaandorp JA (1991) Modelling growth forms of the sponge Haliclona oculata (Porifera, Demospongiae) using fractal techniques. Mar Biol 110:203–215
Kharlamenko VI, Kiyashko SI, Rod’kina SA, Imbs AB (2008) Determination of food sources of marine invertebrates from a subtidal sand communtiy using analyses of fatty acids and stable isotopes. Russ J Mar Biol 34:101–109
Koopmans M (2009) Growth and metabolism of sponges. Bioprocess Engineering, Wageningen, pp 61–80. ISBN: 978-90-8585-441-8
Koopmans M, Wijffels RH (2008) Seasonal growth rate of the sponge Haliclona oculata (demospongiae: Haplosclerida). Mar Biotechnol 10:502–510
Koopmans M, Martens D, Wijffels RH (2009) Growth efficiency and carbon balance for Haliclona oculata. Mar Biotechnol (in press)
Litchfield C, Greenberg AJ, Noto G, Morales RW (1976) Unusually high levels of C24–C30 fatty acids in sponges o f the class demospongiae. Lipids 11:567–570
Middelburg JJ, Barranguet C, Boschker HTS, Hesse C, Moens T, Heip CHR (2000) The fate of intertidal microphytobenthos carbon: an in situ 13C labelling study. Limnol Oceanogr 45:1224–1234
Nechev J, Christie WW, Robaina R, de Diego F, Popov S, Stefanov K (2002) Lipid composition of the sponge verongida aerophoba from the Canary Islands. Eur J Lipid Sci Technol 104:800–807
Nieuwenhuize J, Maas YEM, Middelburg JJ (1994) Rapid analysis of organic carbon and nitrogen in particulate materials. Mar Chem 45:217–224
Raederstorff D, Shu AYL, Thompson JE, Djerassi C (1987) Biosyntethic studies of marine lipids. 11 1 Synthesis, biosynthesis, and absolute configuration of the internally branched demospongic acid 22-Methyl-5, 9-octacosadienoic acid. J Org Chem 52:2337–2346
Ribes M, Coma R, Gili JM (1999) Natural diet and grazing rate of the temperate sponge Dysidea avara (Demospongiae, Dendroceratida) throughout an annual cycle. Mar Ecol Prog Ser 176:179–190
Rod’kina SA (2005) Fatty acids and other lipids of Marine sponges. Russ J Mar Biol 31:S49–S60
Rod’kina SA, Latyshev NA, Imbs AB (2003) Fatty acids from the sea of Japan Sponge Halichondria panicea. Russ J Bioorg Chem 29:382–386
Schmidt I (1970) Phagoeytosis and pinocytosis in spongillids. Z vergl Physiologie 66:398–420
Thiel V, Jenisch A, Wörheide G, Löwenberg A, Reitner J, Mechaelis W (1999) Mid-chain branched alkanoic acids from “living fossil” demosponges: a link to ancient sedimentary lipids? Org Geochem 30:1–14
Uriz MJ, Rosell D, Martin D (1992) The sponge population of the Cabrera archipelago (Balearic islands): characteristics, distribution, and abundance of the most representative species. Mar Ecol 13:101–117
Acknowledgments
This study was funded by NWO-ALW (814.02.010). We thank the Waterboard Zeeuwse Eilanden and the province of Zeeland for issuing the necessary permits, and Maria Uriz for providing us with the permits to collect sponge specimens in name of CSIC-CEAB. We are grateful to A. Kliphuis, J. Lindenbergh, B. van Vark, E. van Vark, P. Lamers, and A. Bal for helping with placing the platform and the monitoring. Thanks to the Netherlands institute for ecology for providing equipment for the extraction and analysis of the fatty acids and M. Houtekamer and P. van Breugel for their analytical assistance.
Open Access
This article is distributed under the terms of the Creative Commons Attribution Noncommercial License which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by H.O. Pörtner.
Rights and permissions
Open Access This is an open access article distributed under the terms of the Creative Commons Attribution Noncommercial License (https://creativecommons.org/licenses/by-nc/2.0), which permits any noncommercial use, distribution, and reproduction in any medium, provided the original author(s) and source are credited.
About this article
Cite this article
Koopmans, M., van Rijswijk, P., Martens, D. et al. Carbon conversion and metabolic rate in two marine sponges. Mar Biol 158, 9–20 (2011). https://doi.org/10.1007/s00227-010-1538-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-010-1538-x