Abstract
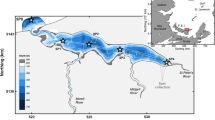
Mussel culture in coastal environments relies on the availability of food of sufficient quality and quantity. Both to determine this availability and to examine impacts that this aquaculture practice may have on the environment, it is important to have good knowledge of the type of plankton communities present in aquaculture sites. It is usually thought that phytoplankton make up the bulk of mussel diet in many of these sites. Here we show that the Grande-Entrée lagoon [Magdalen Islands, Gulf of St Lawrence (GSL), Canada], where commercial mussel culture has been on-going since 1980, differs from this pattern. Heterotrophic protists dominate for most of the summer-early fall season (apart from short diatom bursts), with a high average biomass of 160 mg C m−3. The dominance of small-sized phytoplankton cells (notably green algae), low nutrient concentrations (e.g. 0.3 μM NO −3 on average) and high biomass of heterotrophic protists (mostly naked ciliates and tintinnids) all point to the importance of the microbial food web in this shallow marine environment. Sustained cultivation of suspended mussels in the lagoon suggests that these heterotrophic protists could be an important source of food for the mussels, supplementing the small amount of phytoplankton present.









Similar content being viewed by others
References
Acri F (2004) Plankton communities and nutrients in the Venice Lagoon. J Mar Syst 51(1–4):321–329
Ansotegui A, Trigueros JM, Orive E (2001) The use of pigment signatures to assess phytoplankton assemblage structure in estuarine waters. Estuarine Coast Shelf Sci 52(6):689–703
Asmus RM, Asmus H (1991) Mussels beds, limiting or promoting phytoplankton? J Exp Mar Biol Ecol 148:215–232
Azam F, Fenchel T, Field JG, Gray JS, Meyer Reil L, Thingstad F (1983) The ecological role of water-column microbes in the sea. Mar Ecol Prog Ser 10:257–263
Bérard-Therriault L, Poulin M, Bossé L (1999) Guide to the identification of marine phytoplankton of the estuary and Gulf of St. Lawrence including certain protozoa. Can Spec Publ Fish Aquat Sci 128:1–387
Bernardy Aubry F, Acri F (2004) Phytoplankton seasonality and exchange at the inlets of the Lagoon of Venice (July 2001–June 2002). J Mar Syst 51(1–4):65–76
Beukema JJ, Cadée GC, Dekker R (2002) Zoobenthic biomass limited by phytoplankton abundance: evidence from parallel changes in two long-term data series in the Wadden Sea. J Sea Res 48(2):111–125
Cadée GC, Hegeman J (2002) Phytoplankton in the Marsdiep at the end of the 20th century; 30 years monitoring biomass, primary production, and Phaeocystis blooms. J Sea Res 48(2):97–110
Capriulo GM, Lints D, Lewinter M (1990) Ingestion rate and body size in phagotrophic organisms. Can J Zool 68(2):313–317
Charpy L (1996) Phytoplankton biomass and production in two Tuamotu atoll lagoons (French Polynesia). Mar Ecol Prog Ser 145(1–3):133–142
Chisholm SW (1992) Phytoplankton size. In: Falkowski PG, Woodhead AD (eds) Primary productivity and biogeochemical cycles in the sea. Environmental science research, vol. 43. Plenum, New York, pp. 213–238
Clarke KR, Gorley RN (2001) PRIMER v5, User Manual/Tutorial. PRIMER-E Ltd, Plymouth, UK
Clarke KR, Warwick RM (2001) Change in marine communities. An approach to statistical analysis and interpretation, 2nd edn. PRIMER-E Ltd, Plymouth, UK
Crosby MP, Newell RIE, Langdon CJ (1990) Bacterial mediation in the utilization of carbon and nitrogen from detrital complexes by the oyster, Crassostrea virginica (Gmelin). Limnol Oceanogr 35:625–639
Davenport J, Smith RJJW, Packer M (2000) Mussels Mytilus edulis: significant consumers and destroyers of mesozooplankton. Mar Ecol Prog Ser 198:131–137
De Jonge VN (1997) High remaining productivity in the Dutch western Wadden Sea despite decreasing nutrient inputs from riverine sources. Mar Pollut Bull 34(6):427–436
De Lafontaine Y, Demers S, Runge J (1991) Pelagic food web interactions and productivity in the Gulf of St. Lawrence, a perspective. In: Therriault JC (ed) The Gulf of St. Lawrence, small ocean or big estuary? Can Spec Publ Fish Aquat Sci 113:99–123
Del Giorgio PA, Gasol JM (1995) Biomass distribution in freshwater plankton communities. Am Nat 146(1):135–152
De Sève MA, Dunbar MJ (1991) Nutrient dynamics and biological variables of Ice Biota from the Gulf of St. Lawrence, Magdalen Islands area. In: Therriault JC (ed) The Gulf of St. Lawrence, small ocean or big estuary? Can Spec Publ Fish Aquat Sci 113:201–208
Dolan JR (1991a) Guilds of ciliate microzooplankton in the Chesapeake Bay. Estuarine Coast Shelf Sci 33(2):137–152
Dolan JR (1991b) Microphagous ciliates in mesohaline Chesapeake Bay waters, estimates of growth rates and consumption by copepods. Mar Biol 111(2):303–309
Doyon P, Ingram RG (2000) Seasonal upper-layer T-S structure in the Gulf of St. Lawrence during the ice-free months. Deep Sea Res II 47(3–4):385–413
Duarte CM, Cebrian J (1996) The fate of marine autotrophic production. Limnol Oceanogr 41(8):1758–1766
Dunbar MJ, MaClellan DC, Filion A, Moore D (1980) Biogeographic structure of the Gulf of St. Lawrence. Marine Sciences Centre, McGill University, Montreal, Canada
Dupuy C, Le Gall S, Hartmann HJ, Bréret M (1999) Retention of ciliates and flagellates by the oyster Crassostrea gigas in French Atlantic coastal ponds, protists as a trophic link between bacterioplankton and benthic suspension-feeders. Mar Ecol Prog Ser 177:165–175
Dupuy C, Vaquer A, Lam-Höai T, Rougier C, Mazouni N, Lautier J, Collos Y, Le Gall S (2000) Feeding rate of the oyster Crassostrea gigas in a natural planktonic community of the Mediterranean Thau Lagoon. Mar Ecol Prog Ser 205:171–184
Facca C, Sfriso A, Ghetti PF (2004) Phytoplankton community composition and distribution in an eutrophic coastal area (Venice lagoon, Italy). Acta Adriat 45(2):163–180
FAO (eds) (2004) The state of world fisheries and aquaculture (SOFIA). FAO, Rome
Gilabert J (2001a) Seasonal plankton dynamics in a Mediterranean hypersaline coastal lagoon, the Mar Menor. J Plankton Res 23(2):207–218
Gilabert J (2001b) Short-term variability of the planktonic size structure in a Mediterranean coastal lagoon. J Plankton Res 23(2):219–226
Gorham WT (1988) The energetic and nutritional contribution of glucose and glycine taken up from natural sea water by adult marine mussels. Mar Ecol 9(1):1–14
Hillebrand H, Dürselen CD, Kirschyel D, Pollingher U, Zohary T (1999) Biovolume calculation for pelagic and benthic macroalgae. J Phycol 35:403–424
Horner RA (eds) (2002) A taxonomic guide to some common marine phytoplankton. Biopress Limited, England
Jeffrey SW, Vesk M, Mantoura RFC (1997) Phytoplankton pigments, windows into the pastures of the sea. Nat Res 33(2):14–29
JGOFS (1994) Protocols for the joint global ocean flux study (JGOFS) core measurements. Report No. 19. Scientific Committee on Oceanic Research, International Council of Scientific Union, Bergen
Karl DM, Laws EA, Morris P, Williams PJL, Emerson S (2003) Global carbon cycle (communication arising)—metabolic balance of the open sea. Nature 426(6962):32
Kim KW, Garbary DJ, McLachlan JL (2004) Phytoplankton dynamics in Pomquet Harbour, Nova Scotia, a lagoon in the southern Gulf of St Lawrence. Phycologia 43(3):311–328
Koutitonsky VG, Tita G (2004) Étude du temps de renouvellement des eaux dans la lagune de Grande-Entrée, Îles-de-la-Madeleine. Rapport de recherche LHE 04-1. Laboratoire d’hydraulique environnementale. ISMER, Rimouski
Koutitonsky VG, Navarro N, Booth D (2002) Descriptive physical oceanography of great-entry lagoon, Gulf of St. Lawrence. Estuarine Coast Shelf Sci 54:833–847
Kreeger DA, Newell RIE (1996) Ingestion and assimilation of carbon from cellulosic bacteria and heterotrophic flagellates by the mussels Geukensia demissa and Mytilus edulis (Bivalvia, Mollusca). Aquat Microb Ecol 11:205–214
Kreeger DA, Newell RIE (2001) Seasonal utilization of different seston carbon sources by the ribbed mussel, Geukensia demissa (Dillwyn) in a mid-Atlantic salt marsh. J Exp Mar Biol Ecol 260:71–91
Lam-Hoai T, Rougier C, Lasserre G (1997) Tintinnids and rotifers in a northern Mediterranean coastal lagoon. Structural diversity and function through biomass estimations. Mar Ecol Prog Ser 152(1–3):13–25
Langdon C, Newell RIE (1990) Utilization of detritus and bacteria as food sources by two bivalve suspension-feeders, the oyster Crassostrea virginica and the mussel Geukensia demissa. Mar Ecol Prog Ser 58(3):299–310
Le Borgne R, Rodier M (1997) Net zooplankton and the biological pump, a comparison between the oligotrophic and mesotrophic equatorial Pacific. Deep Sea Res II 44(9–10):2003–2023
Lehane C, Davenport J (2006) A 15-month study of zooplankton ingestion by farmed mussels (Mytilus edulis) in Bantry Bay, Southwest Ireland. Estuarine Coast Shelf Sci 67:645–652
Loret P, Le Gall S, Dupuy C, Blanchot J, Pastoureaud A, Delesalle B, Caisey X, Jonquieres G (2000) Heterotrophic protists as a trophic link between picocyanobacteria and the pearl oyster Pinctada margaritifera in the Takapoto lagoon (Tuamotu Archipelago, French Polynesia). Aquat Microb Ecol 22(3):215–226
Lovejoy C, Vincent WF, Frenette J-J, Dodson JJ (1993) Microbial gradients in a turbid estuary: application of a new method for protozoan community analysis. Limnol Oceanogr 38(6):1295–1303
Lovejoy C, Legendre L, Therriault J-C, Tremblay J-E, Klein B, Ingram RG (2000) Growth and distribution of marine bacteria in relation to nanoplankton community structure. Deep Sea Res II 47(3–4):461–487
Mayzaud P, Koutitonsky VG, Souchu P, Roy S, Navarro N, Gomez-Reyer E (1992) L’impact de l’activité mytilicole sur la capacité de production du milieu lagunaire des Iles de la Madeleine. INRS-Océanologie Research Report FP707-8-5140. Rimouski, Canada
Mazzola A, Fabiano M, Pusceddu A, Sara G (2001) Particulate organic matter composition in a semi-enclosed marine system. Chem Ecol 17:315–334
Menden-Deuer S, Lessard EJ (2000) Carbon to volume relationships for dinoflagellates, diatoms, and other protist plankton. Limnol Oceanogr 45(3):569–579
Myrand B, Guderley H, Himmelman JH (2000) Reproduction and summer mortality of blue mussels Mytilus edulis in the Magdalen Islands, southern Gulf of St. Lawrence. Mar Ecol Prog Ser 197:193–207
Navarro N (1991) Océanographie physique descriptive de la lagune de la Grande-Entrée, Iles-de-la-Madeleine, Golfe du Saint-Laurent. MSc thesis, Université du Québec à Rimouski, Rimouski, p 143
Niquil N, Pouvreau S, Sakka A, Legendre L, Addessi L, Le Borgne R, Charpy L, Delesalle B (2001) Trophic web and carrying capacity in a pearl oyster farming lagoon (Takapoto, French Polynesia). Aquat Living Resour 14:165–174
Page HM, Hubbard DM (1987) Temporal and spatial patterns of growth in mussels Mytilus edulis on an offshore platform. Relationships to water temperature and food availability. J Exp Mar Biol Ecol 111(2):159–179
Parsons TR, Maita Y, Lalli CM (eds) (1984) A manual of chemical and biological methods for seawater analysis. Pergamon, Toronto
Pérez-Ruzafa A, Gilabert J, Gutierrez J, Fernandez A, Marcos C, Sabah S (2002) Evidence of a planktonic food web response to changes in nutrient input dynamics in the Mar Menor coastal lagoon, Spain. Hydrobiologia 475(1):359–369
Roditi HA, Fisher NS, Sanudo-Wilhelmy SA (2000) Uptake of dissolved organic carbon and trace elements by zebra mussels. Nature 407(6800):78–80
Roy S, Mayzaud P, Souchu P (1991) Environnement physico-chimique et trophique d’un site mytilicole, Île de la Madeleine (Québec), II—Matière particulaire, composition biochimique et productivité primaire. In: Therriault J-C (ed) Le golfe du Saint-Laurent, petit océan ou grand estuaire. Can Spec Publ Fish Aquat Sci 113:219–230
Roy S, Chanut JP, Gosselin M, Sime-Ngando T (1996) Characterization of phytoplankton communities in the lower St. Lawrence Estuary using HPLC-detected pigments and cell microscopy. Mar Ecol Prog Ser 142:55–73
Sakka A, Legendre L, Gosselin M, Niquil N, Delesalle B (2002) Carbon budget of the planktonic food web in an atoll lagoon (Takapoto, French Polynesia). J Plankton Res 24(4):301–320
Savenkoff C, Vézina AF, Roy S, Klein B, Lovejoy C, Therriault JC, Legendre L, Rivkin R, Bérubé C, Tremblay JE, Silverberg N (2000) Export of biogenic carbon and structure and dynamics of the pelagic food web in the Gulf of St. Lawrence—Part 1. Seasonal variations. Deep Sea Res II 47:585–607
Schumann R, Hammer A, Gors S, Schubert H (2005) Winter and spring phytoplankton composition and production in a shallow eutrophic Baltic lagoon. Estuarine Coast Shelf Sci 62(1–2):169–181
Sévigny J-M, Sinclair M, El-Sabh MI, Poulet S, Coote A (1979) Summer plankton distributions associated with the physical and nutrient properties of the northwestern Gulf of St. Lawrence. J Fish Res Board Can 36(2):187–203
Sfriso A, Facca C, Ghetti PF (2003) Temporal and spatial changes of macroalgae and phytoplankton in a Mediterranean coastal area, the Venice lagoon as a case study. Mar Environ Res 56(5):617–636
Sheldon RW, Nival P, Rassoulzadegan F (1986) An experimental investigation of a flagellate-ciliate-copepod food chain with some observations relevant to the linear biomass hypothesis. Limnol Oceanogr 31(1):184–188
Sherr EB, Sherr BF (1994) Bacterivory and herbivory, key roles of phagotrophic protists in pelagic food web. Microb Ecol 28:233–235
Sherr EB, Sherr BF, Fallon RD, Newell SY (1986) Small, aloricate ciliates as a major component of the marine heterotrophic nanoplankton. Limnol Oceanogr 31(1):177–183
Sime-Ngando T, Juniper K, Vézina A (1992) Ciliated protozoan communities over Cobb Seamount. Increase in biomass and spatial patchiness. Mar Ecol Prog Ser 89(1):37–51
Sime-Ngando T, Gosselin M, Roy S, Chanut J-P (1995) Significance of planktonic ciliated protozoa in the Lower St. Lawrence Estuary, comparison with bacterial, phytoplankton, and particulate organic carbon. Aquat Microb Ecol 6(3):243–258
Sinclair M (1978) Summer phytoplankton variability in the lower St Lawrence Estuary. J Fish Res Board Can 35(9):1171–1185
Smaal AC (1991) The ecology and cultivation of mussels: new advances. Aquaculture 94:245–261
Smetacek V (1981) Annual cycle of protozooplankton in the Kiel Bight. Mar Biol 3(1):1–11
Souchu P, Mayzaud P (1991) Inorganic nutrients in precipitation over the Magdalen Islands area (Quebec, Canada) and their impact on the primary productivity of the lagoons. Atmos Res 26:543–554
Souchu P, Mayzaud P, Roy S (1991) Environnement physico-chimique et trophique d’un site mytilicole, Îles de la Madeleine (Québec), I—evolution estivale des composés de l’azote, du phosphore et du silicium. In: Therriault J-C (ed) Le golfe du Saint-Laurent, petit océan ou grand estuaire? Publ spéc can sci halieut aquat 113:209–218
Stoecker DK, Capuzzo JM (1990) Predation on protozoa, its importance to zooplankton. J Plankton Res 12:891–988
Strickland JDH, Parsons TR (1972) A practical handbook of seawater analysis. Bull Fish Res Board Can 167:1–311
Tamigneaux E, Mingelbier M, Klein B, Legendre L (1997) Grazing by protists and seasonal changes in the size structure of protozooplankton and phytoplankton in a temperate nearshore environment (western Gulf of St. Lawrence, Canada). Mar Ecol Prog Ser 146:231–247
Therriault JC, Levasseur M (1985) Control of phytoplankton production in the lower St. Lawrence Estuary, light and freshwater runoff. St. Lawrence Estuary. Nat Can (Rev Écol Syst) 112:77–96
Tian RC, Vezina A, Legendre L, Ingram RG, Klein B, Packard T, Roy S, Savenkoff C, Silverberg N, Therriault JC, Tremblay JE (2000) Effects of pelagic food–web interactions and nutrient remineralization on the biogeochemical cycling of carbon: a modeling approach. Deep Sea Res II 47(3–4):637–662
Tomas CR (eds) (1993) Marine phytoplankton: a guide to naked flagellates and coccolithophorids. Academic, San Diego
Winter JE (1978) A review on the knowledge of suspension-feeding in lamellibranchiate bivalves, with special reference to artificial aquaculture systems. Aquaculture 13:1–33
Wong WH, Levinton JS, Twining BS, Fisher N (2003) Assimilation of micro- and mesozooplankton by zebra mussels: a demonstration of the food web link between zooplankton and benthic suspension feeders. Limnol Oceanogr 48(1):308–312
Zapata M, Rodriguez F, Garrido JL (2000) Separation of chlorophylls and carotenoids from marine phytoplankton, a new HPLC method using a reserved phase C8 column and pyridine-containing mobile phases. Mar Ecol Prog Ser 195:29–45
Acknowledgments
We thank O. Pitre for his help with sampling, F. Blouin and S. Leblanc for technical advice and help in the field and in the laboratory, G. Tita for his help. This study was funded by an Action concertée en Sciences et Technologies de la Mer (Fonds Québécois de la Recherche sur la Nature et les Technologies) grant to V. Koutitonsky, S. Roy et al., by ISMER, by RAQ (Réseau Aquaculture Québec) and by SODIM (SOciété de Développement de l’Industrie Maricole). We also thank Québec Océan for financial support of A. Trottet.
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. J. Thompson, St. John's.
Rights and permissions
About this article
Cite this article
Trottet, A., Roy, S., Tamigneaux, E. et al. Importance of heterotrophic planktonic communities in a mussel culture environment: the Grande Entrée lagoon, Magdalen Islands (Québec, Canada). Mar Biol 151, 377–392 (2007). https://doi.org/10.1007/s00227-006-0494-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-006-0494-y