Abstract
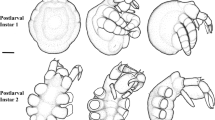
Laboratory rearing and reconstruction of Laodicea undulata (Hydrozoa) life cycle led to the discovery for the first time in Leptomedusae of the potential for ontogeny reversal, i.e. the medusa stage can asexually transform back into the polyp stage. In turn, each rejuvenated polyp stage can newly activate the standard developmental programme towards colony morphogenesis and budding of secondary medusae. These can be considered as clonemates of the initial medusa batch, since they originate by asexual processes. In combination with the ordinary medusa budding process, the potential for reverse development might represent a tool to increase jellyfish population growth rate during the favourable season, but eventually it does not avoid jellyfish to die. Comparably to polyembryony, reverse development leads to offspring multiplication from a single fertilization event, with a wider dispersal of each single genotype; eventually, it favours the enhancement of the overall genetic diversity at small spatial scale. The life cycle of L. undulata from the Mediterranean Sea is re-described, linking previously uncoupled descriptions of either the polyp or the early medusa stages. Taxonomic considerations of the genus Laodicea and a comparison among the known Mediterranean species are also provided.



Similar content being viewed by others
References
Agassiz L (1860) Contributions to the Natural History of the United States of America. Second monograph 3:i–xi, 1–301
Agassiz A, Mayer AG (1899) Acalephs from the Fiji Islands. Bull Mus comp Zool Harv 32:157–189
Babnik P (1948) Hidromeduze iz srednjega in junega Jadrana v letih 1939 in 1940 Hydromedusae from the middle and South Adriatic 1939 and 1940. Acta Adriat 3:275–340
Bavestrello G, Sommer C, Sarà M (1992) Bi-directional conversion in Turritopsis nutricula (Hydrozoa). In: Bouillon J, Boero F, Cicogna F, Gili JM, Hughes RG (eds) Aspects of Hydrozoan biology. Sci Mar 56:137–140
Berrill NJ (1953) Growth and form in gymnoblastic hydroids. VII. Growth and reproduction in Syncoryne and Coryne. J Morphol 92:273–302
Boero F (1984) The ecology of marine hydroids and effects of environmental factors: a review. Pubbl Stn Zool Napoli I Mar Ecol 5:93–118
Boero F, Sarà M (1987) Motile sexual stages and evolution of Leptomedusae (Cnidaria). Boll Zool 54:131–139
Boero F, Balduzzi A, Bavestrello G, Caffa B, Cattaneo Vietti R (1986) Population dynamics of Eudendrium glomeratum (Cnidaria: Anthomedusae) on the Portofino promontory (Ligurian Sea). Mar Biol 92:81–85
Boero F, Bouillon J, Piraino S (1992) On the origins and evolution of hydromedusan life cycles (Cnidaria, Hydrozoa). In: Dallai R (ed) Sex origin and evolution. Selected symposia and monographs UZI 6:59–68
Boero F, Gravili C, Pagliara P, Piraino S, Bouillon J, Schmid V (1998) The cnidarian premises of metazoan evolution: from triploblasty, to coelom formation, to metamery. Ital J Zool 65:5–9
Boero F, Bouillon J, Piraino S, Schmid V (2002) Asexual reproduction in the Hydrozoa (Cnidaria). In: Hughes RN (ed) Reproductive biology of invertebrates. Volume XI: Progress in asexual reproduction. Oxford and IBH, New Delhi, pp 141–158
Bouillon J, Boero F (2000) Phylogeny and classification of Hydroidomedusae. The Hydrozoa: a new classification in the light of old knowledge. Thalassia Salentina 24:1–46
Bouillon J, Medel MD, Pagès F, Gili JM, Boero F, Gravili C (2004) Fauna of the Mediterranean Hydrozoa. Sci Mar 68(2):1–449
Brinckmann-Voss A (1970) Anthomedusae/Athecata (Hydrozoa, Cnidaria) of the Mediterranean. Part I. Capitata. Fauna Flora Golfo di Napoli, Stazione Zoologica Anton Dohrn, Napoli, 39:1–96
Browne ET (1907) A revision of the medusae belonging to the family Laodiceidae. Ann Mag Nat Hist 20:457–480
Cornelius PFS (1995) North-West European Thecate Hydroids and their medusae. Part 1. Laodiceidae to Haleciidae. In: Barnes RSK, Crothers JH (eds) Synopses of the British fauna (new series). The Linn Soc London and Est Coas Sci Assoc Field Studies Council, Shrewsbury, 50:1–347
Craig SF, Slobodkin LB, Wray GA, Biermann CH (1997) The ‘paradox’ of polyembryony: a review of the cases and a hypothesis for its evolution. Evol Ecol 11:127–143
Forbes E, Goodsir J (1851) On some remarkable marine Invertebrata new to the British Seas. Trans R Soc Edinb 20:307–315
Frey J (1968) Die Entwicklungsleistungen der Medusenknospen und Medusen von Podocoryne carnea M Sars nach Isolation und Dissoziation. Wilhelm Roux’ Arch Entwicklungsmech Org 160:428–464
Giard A (1898) Sur l’éthologie du Campanularia caliculata Hincks (Stolonisation et allogonie). C R Soc Biol 5:17–20
Gili JM, Hughes RG (1995) The ecology of marine benthic hydroids. Oceanogr Mar Biol Annu Rev 33:351–426
Gravier N (1970) Libération des médusoides par Macrorhynchia philippina Kirchenpauer, 1872 (Hydrozoa, Plumulariidae). Recl Trav Stn Mar Endoume 10:253–257
Hadzi J (1909) Einige Kapitel aus der Entwicklungsgeschichte von Chrysaora. Arb Zool Inst Univ Wien 17:17–44
Hincks T (1868) A history of the British hydroid zoophytes. John Van Voorst, Paternoster Row, London
Kramp PL (1927) The hydromedusae of the Danish waters. K Dan Vidensk Selsk Biol Skr Afd 8(12):1–290
Kramp PL (1959) The Hydromedusae of the Atlantic Ocean and adjacent waters. Dana-Reports Carlsberg Found 46:1–283
Kubota S (2005) Distinction of two morphotypes of Turritopsis nutricula Medusae (Cnidaria, Hydrozoa, Anthomedusae) in Japan, with reference to their different abilities to revert to the hydroid stage and their distinct geographical distributions. Biogeography 7:41–50
Kubota S, Mizutani S (2003) Strange fates of degenerated medusae of Turritopsis nutricula (Hydrozoa, Anthomedusae, Clavidae) from northern Japan. Nanki Seibutu 45:107–109
Metschnikoff E (1886) Embryologische Studien an Medusen. Ein Beitrag zur Genealogie der Primitiv-Organe. Verlag Alfred Hölder, k.k. Hof-u Universitäts-Buchhändler, Wien
Piraino S, Todaro C, Geraci S, Boero F (1994) Ecology of the bivalve-inhabiting hydroid Eugymnanthea inquilina in the coastal sounds of Taranto (Ionian Sea, SE Italy). Mar Biol 118:695–703
Piraino S, Boero F, Aeschbach B, Schmid V (1996) Reversing the life cycle: medusae transforming into polyps and cell transdifferentiation in Turritopsis nutricula (Cnidaria, Hydrozoa). Biol Bull 190:302–312
Piraino S, De Vito D, Schmich J, Bouillon J, Boero F (2004) Reverse development in Cnidaria. Can J Zool 82:1748–1754
Russell FS (1936) On the hydroid of Laodicea undulata (Forbes and Goodsir). J Mar Biol Assoc UK 20:581–588
Russell FS (1938) The Plymouth offshore medusae fauna. J Mar Biol Assoc UK 22:411–440
Russell FS (1953) The medusae of the British Isles. Anthomedusae, Leptomedusae, Limnomedusae, Trachymedusae and Narcomedusae. Cambridge University Press, Cambridge
Schmid V (1972) Untersuchungen über Dedifferenzierungsvorgänge bei Medusenknospen und Medusen von Podocoryne carnea M Sars. Wilhelm Roux’ Arch Entwicklungsmech Org 169:281–307
Schmid V (1992) Transdifferentiation in medusae. Int Rev Cytol 142:213–261
Schmidt HE (1973) Hydromedusae from the eastern Mediterranean Sea. Isr J Zool 22:151–167
Schuchert P (2004) Revision of the European athecate hydroids and their medusae (Hydrozoa, Cnidaria): families Oceanidae and Pachycordylidae. Rev Suisse Zool 111:315–369
Spring J, Yanze N, Middel AM, Stierwald M, Gröger H, Schmid V (2000) The mesoderm specification factor twist in the life cycle of jellyfish. Dev Biol 228:363–375
Stefani R (1959) Sulla variabilità ecologica di un Idrozoo (Campanularia caliculata Hincks). Boll Zool 26:115–120
Yanze N, Spring J, Schmidli C, Schmid V (2001) Conservation of Hox/ParaHox-related genes in the early development of a cnidarian. Dev Biol 236:89–98
Werner B (1954) On the development and reproduction of the anthomedusan, Margelopsis haeckeli Hartlaub. Trans NY Acad Sci 16:143–146
Werner B (1963) Effect of some environmental factors on differentiation and determination in marine Hydrozoa, with a note on their evolutionary significance. Ann NY Acad Sci 105:461–488
Acknowledgments
Financial supports were provided by MURST (60%, COFIN, and FIRB Projects), the Administration of the Province of Lecce, ICRAM (Project “Identificazione e distribuzione delle specie non indigene nel Mediterraneo”), the European Union (Marie Curie contract no. HPMD-CT-2001-00099, and the MARBEF network), the NSF of the USA (PEET project on the Hydrozoa). We are grateful to Cristina Di Camillo for drawings of L. undulata. Special thanks are due to Adam Benovic and Alenka Malej for their kind translation of part of the original paper by Babnik (1948).
Author information
Authors and Affiliations
Corresponding author
Additional information
Communicated by R. Cattaneo-Vietti, Genova
Rights and permissions
About this article
Cite this article
De Vito, D., Piraino, S., Schmich, J. et al. Evidence of reverse development in Leptomedusae (Cnidaria, Hydrozoa): the case of Laodicea undulata (Forbes and Goodsir 1851). Marine Biology 149, 339–346 (2006). https://doi.org/10.1007/s00227-005-0182-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00227-005-0182-3