Abstract
To investigate the effect of Fe-addition on the Cs+-adsorption ability (Cs+-AA) of woody charcoal, charcoal samples were prepared from Japanese cedar (JC) and Japanese oak (JO) wood impregnated with Fe3+ at 600 and 800 °C. The residual functional groups of the charcoal samples were examined using infrared-photoacoustic spectroscopy. OH groups were observed in the charcoal made at 600 °C, and no vibrational bands were detectable in the charcoal synthesized at 800 °C. The observation of Raman D-, G-, and G´-bands revealed that all charcoal samples contained sp2-carbon atoms but graphitization occurred only in the iron-loaded charcoal made at 800 °C. The Cs+-AA of the JC and JO charcoal samples were evaluated based on the adsorption isotherms of an aqueous CsCl (Cs+: 33 mg/L) solution. The addition of Fe3+ to wood had a negative effect on the Cs+-AA of the JO charcoal made at 600 °C, but a positive effect on the Cs+-AA of the JC charcoal prepared at 800 °C.
Similar content being viewed by others
Explore related subjects
Discover the latest articles, news and stories from top researchers in related subjects.Avoid common mistakes on your manuscript.
Introduction
Countries that use nuclear power for electricity generation must promptly remove radioactive contaminants when a serious accident occurs in their power plants. The past accidents, such as the Fukushima nuclear accident in 2011, highlighted a critical challenge associated with the decontamination of radioactive substances: the removal of radioactive cesium isotopes, 137Cs and 134Cs, which have half-lives of 30 and 2 years, respectively. These radioisotopes are formed as fission products in nuclear reactors. Cs-137 will lead to more grave consequences for the environment around the power plants involved in the accident because of its relatively long half-life.
Radioactive Cs+ emitted from nuclear reactors can be broadly classified into two types based on their water solubility. The first type is chemical species that are readily soluble in water. The other is insoluble or poorly soluble chemical species constituting partly spherical micro-sized cesium-bearing particles (Adachi et al. 2013). There has been no effective method to remove the micro-cesium particles, whereas a great number of studies have reported Cs+ adsorption from aqueous solution and most of them used porous inorganic materials, such as zeolite, alumina and clay, as adsorptive.
An adsorptive possible to be more inexpensively mass produced is required to remove radioactive Cs+ from soil and water when vast areas were contaminated. Charcoal produced from plant biomass is widely applied to the adsorptive removal of noxious and hazardous substances. Although the specific surface area of ordinary charcoal is much smaller than that of activated carbon, it has been generally accepted that woody charcoal exhibits high adsorption capabilities for a wide range of substances. Thus, cheap logs and lumber can be used to reduce the manufacturing costs of charcoal used as adsorptive.
In the last decade, the adsorption mechanisms of Cs+ from aqueous solutions on woody charcoal were studied under various conditions. It was confirmed that one of the primary interactions contributing to the capture of Cs+ on charcoal is electrostatic attraction (Yamauchi et al. 2014, 2015, 2016, 2017a; Yamagishi et al. 2017, 2019). Furthermore, it was reported that the hydroxy (OH) groups on charcoal surfaces play an important role in the adsorption of Cs+ on the charcoal synthesized at ≤ 500 °C (Yamauchi et al. 2017a; Yamagishi et al. 2019).
Although the Cs+-adsorption ability (Cs+-AA) is high enough to be usable for adsorptive when woody charcoal is synthesized under proper conditions, enhancing the Cs+-AA of woody charcoal to that of synthetic zeolite is difficult. However, woody charcoal has the advantage of substantial volume reduction by combustion. Volume reduction of adsorptive is essential for the long-term storage of radioactive substances. Woody biomass, which is used as the starting material of charcoal, is a typical renewable source. Moreover, even if logs or lumber are inappropriate for construction materials because of their insufficient strength, most of them can be used for manufacturing charcoal. Thus, the mass production of charcoal as adsorptive is expected to provide an efficient method for utilizing the substandard thinned and waste wood.
An important issue in the Japanese wood industry is making better use of thinned Japanese cedar (JC, Cryptomeria japonica) wood, whose production is the highest among the tree species planted in Japan. Charcoal made from JC wood is considered unsuitable as fuel because of its low density; therefore, no JC charcoal has been industrially produced. Hence, there are only a few studies on JC charcoal as adsorptive (Kurimoto et al. 2001; Pulido-Novicio et al. 2001).
The other substandard logs and lumber include ancient trees that had been buried or submerged by landslides or ground subsidence during long periods. In Japan, large quantities of the ancient trees have often been yielded in large-scale civil engineering works such as highway construction; however, the ancient logs are not entirely suitable for building materials because of aging degradation. A characteristic of the ancient wood is the enrichment of iron complexes with the wood constituents (Narita and Yatagai 2006; Yamauchi et al. 2017b, c, 2020; Kurimoto et al. 2020). The iron ions (Fe2+ or Fe3+) dispersed in the cell walls of wood are reduced to metallic iron through carbonization under inert gas, and the metallic iron acts as an effective catalyst for the formation of graphitic structures in charcoal (Suzuki et al. 2017, 2020; Yamagishi et al. 2020, 2022). Hence, the study of charcoal made from wood containing iron ions could unveil new uses for ancient wood.
The goal of the present study is to investigate the Cs+-AA of charcoal made from Fe3+-added wood in order to expand the usage of substandard lumber, such as the thinned JC trees and ancient wood. In this study, several charcoal samples were synthesized from ordinary and Fe3+-impregnated wood prepared from JC and Japanese oak (JO, Quercus serrata) trees. Based on the Cs+-adsorption isotherms and supplemental data of the charcoal samples, we assess the effects of carbonization temperature (CT) and Fe3+-addition on the Cs+-AA of JC and JO charcoal. The CTs were selected to be 600 and 800 °C because it is expected that the complete decomposition of functional groups originating from wood constituents and the reduction to Fe0 occur in the temperature range of 600–800 °C, based on the previous studies (Yamagishi et al. 2019, 2022, 2023; Yamauchi 2003; Yamauchi et al. 2017a).
Unless otherwise noted, “Fe3+-addition” implies “the addition of Fe3+ to wood used as the starting material.”
Materials and methods
Chemicals and preparation of aqueous cesium chloride solution
Special-grade cesium chloride anhydride (CsCl) and iron (III) nitrate nonahydrate (Fe(NO3)3·9H2O) were purchased commercially (FUJIFILM Wako Pure Chemical Co., Japan). After drying CsCl at 100 °C for 24 h, an aqueous CsCl solution was prepared to estimate the Cs+-AA of charcoal. The initial Cs+ concentration of the solution was 2.5 × 10–4 mol/L (Cs+: 33 mg/L). The water used in this study was deionized through a column packed with ion-exchange resins.
Impregnation with Fe3+ into wood
Air-dried chips of the JC and JO wood were ground into particles using a cutting mill. The wood particles that passed through an 8.6-mesh but not through a 16-mesh were used as the starting material of charcoal.
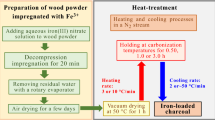
The left half of the flowchart in Fig. 1 shows the process of impregnation with Fe(NO3)3 solution under reduced pressure. The wood particles were dried in a small drying chamber at 105 °C for 1.0 h, and the mass of the dried wood was considered to be the true mass value. The Fe3+ concentration in aqueous solution was adjusted to 0.50 (solution α) or 1.17 w/w% (solution β). The Fe3+ contents in wood were set to be 3 w/w% or 7 w/w% by adding an appropriate amount of solution α or β, respectively. A small amount of water was added to immerse whole wood particles in the solution, if necessary. The Fe(NO3)3 solution containing wood particles was kept under reduced pressure in a vacuum glass vessel connected to a water-jet pump, for 20 min. Excess solvent water was slowly evaporated and the Fe(NO3)3 solution was completely infiltrated into the wood particles using a rotary evaporator at approximately 60 °C, under reduced pressure. A visual inspection after infiltration confirmed that no traces of the iron-salt powder were present on the inner wall of a flask connected to the evaporator and on the surfaces of the wood particles, indicating that almost all the Fe3+ ions were absorbed by the wood particles. The wet wood particles were spread on a filter paper and air-dried at room temperature for a few days.
Carbonization of wood particles
The carbonization process is demonstrated in the right half of the chart in Fig. 1. The carbonization tools used in this study are essentially the same as used previously (Yamagishi et al. 2022). A stainless-steel tube reactor including the wood particles was heated using a vertical electric furnace in a N2 gas stream (1 mL STP cm−2 min−1). We adopted a larger carbonization apparatus compared with that used previously. Therefore, 30–40 g of wood particles, which is approximately 10 times the weight treated in the previous apparatus, were able to be carbonized via a heat treatment. Heating and cooling rates were 10 °C/min and ~ 50 °C/min, respectively. Holding time at CT was set at 1.0 h for all charcoal samples. The carbonization procedure, including the cooling process, was carried out under the N2 gas flow.
The charcoal samples prepared from the JC and JO wood are denoted as JC-FeX1-X2 and JO-FeX1-X2, respectively. The letters X1 and X2 represent the Fe3+ content in raw wood particles and CT, respectively. For example, charcoal made from JC wood containing 3 w/w% of Fe3+ at 800 °C is written as JC-Fe3-800. In this study, charcoal synthesized from wood with and without Fe3+-addition are referred to as iron-loaded and ordinary charcoal, respectively.
Determination of cesium adsorbed on charcoal
The charcoal samples were ground in an agate mortar, and fine charcoal powder sieved through a 60-mesh was used as adsorbent. The charcoal powder was kept in a desiccator over silica gel, after drying at 105 °C for a day.
Polypropylene tools (vessels, pipettes, and syringes) were used to handle CsCl solution to minimize the adsorption of Cs+ on the inner walls of the tools. The aqueous CsCl solution (50 mL) containing the powder adsorbent (0.10, 0.20, 0.40, or 0.80 g) was placed in a capped Erlenmeyer flask and circularly shaken at 22 ± 2 °C for 24 h. The charcoal powder was removed from the solution by filtration through a nylon-membrane microfilter. The pH of the filtrate was recorded using a pH meter. The Cs+ concentration in the filtrate was analyzed using inductively coupled plasma mass spectrometry (ICP-MS) (Agilent 7700x, Agilent Technologies, Inc., US), with an internal standard method (standard isotope: 115In). The ICP-MS analysis was repeated three times for each sample solution. All relative standard deviations were within 5.1%.
Assuming that the Cs+ concentration in the CsCl solution at equilibrium is equivalent to the residual Cs+ concentration in the filtrate, the concentration of Cs+ adsorbed on charcoal (W) was calculated by dividing the decrease (m) in the mass of Cs+ in solution by the mass (M) of the powder charcoal added. This relationship is defined by Eq. (1) as follows:
where the units of m and M are mg and g, respectively.
Raman spectroscopy
The charcoal powders were slightly ground and subjected to microscopic Raman measurements. Raman spectra were recorded using a Raman spectrometer (inVia Raman Microscope, Renishaw PLC, UK) with 532-nm laser excitation (2.33 eV laser energy). The laser power was set at 0.75 or 1.5 mW. Back-scattered Raman signals were collected through a microscope (50 × objective lens) with a spectral resolution of approximately 3 cm−1, and the exposure time was 50 s. More than 10 Raman spectra were obtained at different points for each charcoal sample, because the laser-irradiation spot size (diameter: ~ 2 μm) was too small for the sample size. Hence, one measurement might lead to an incorrect result owing to the inhomogeneity of the charcoal samples. The Raman shifts were calibrated with respect to the band at 520 cm−1 of a silicon wafer.
Infrared-photoacoustic spectroscopy
Infrared-photoacoustic (IR-PA) spectra were obtained at room temperature using a spectrometer (IRTracer-100, Shimadzu Corp., Japan) equipped with an IR-PA spectroscopy module (PA301, Gasera Ltd., Finland). The spectra were recorded from 4000 to 400 cm−1 with 512 accumulations. The spectral resolution was set at 8 cm−1. A thin aluminum cell (inner diameter: 10 mm, depth: 2 mm) was filled with the charcoal powder ground roughly, and PA signals were collected with flowing helium gas (~ 10 mL/min) through a cell bench. The moving-mirror velocity was set at 2.8 mm/s. The beam spot size of IR light on the powder samples was approximately 3 mm. Wavenumber was calibrated using the 1028, 1601, and 3060 cm−1 lines of polystyrene (polymerization degree: ~ 2000, FUJIFILM Wako Pure Chemical Co., Japan). Carbon black powder was used to obtain a reference spectrum.
Results and discussion
The isotherms of Cs+ adsorption on charcoal from aqueous CsCl solution, which consists of the plots of the Cs+ concentration (C: mg/L) in aqueous solution vs. the Cs+ concentration (W: mg/g) on the charcoal adsorbent, were generated for all charcoal samples. The isotherms showed that the Cs+-AA of JO-Fe0-600 charcoal was comparable with that of JO charcoal available commercially in Japan (Yamauchi et al. 2015) and exceeded greatly those of the other charcoal samples used in this study. Thus, we divide the charcoal samples into two groups: the JO and JC charcoal samples, and explain the Cs+-AA for each group.
Adsorption isotherms of JO charcoal
Figure 2 depicts the Cs+-adsorption isotherms of the four types of charcoal made from JO wood. As mentioned above, JO-Fe0-600 has the highest Cs+-AA for the CsCl solution (Cs+: 2.5 × 10–4 mol/L). The substantial decline of Cs+-AA due to an increase in the CT from 600 to 800 °C can be seen by comparing the isotherm of JO-Fe0-600 with that of JO-Fe0-800. The result of this comparison is consistent with the influences of CT on the Cs+-AA reported in our previous work (Yamauchi et al. 2017a, b, c). The increase in the CT also has a negative effect on the Cs+-AA of the iron-loaded JO charcoal.
As can be seen in Fig. 2, the effects of Fe3+-addition on the Cs+-AA are distinctly different between JO-Fe0-600 and JO-Fe0-800. The Cs+-AA of JO-Fe0-600 is substantially reduced by Fe3+-addition; however, no significant differences between JO-Fe0-800 and JO-Fe3-800 are observed in their adsorption isotherms.
Adsorption isotherms of JC charcoal
The Cs+-adsorption isotherms of the JC charcoal samples are plotted in Fig. 3. As observed from the isotherms of the JO charcoal samples (Fig. 2), the JC charcoal samples exhibit much weaker Cs+-AA than the corresponding JO charcoal samples, except for JC-Fe3-800. In particular, JC-Fe0-600 is decidedly inferior to JO-Fe0-600 in the Cs+-AA. In contrast, no marked differences between JC-Fe3-800 and JO-Fe3-800 can be observed in their Cs+-AA.
Both JC-Fe3-800 and JC-Fe7-800 are superior to JC-Fe3-600 in terms of the Cs+-AA. Interestingly, the effect of the CT increase on the iron-loaded JC charcoal is contrary to that on the iron-loaded JO charcoal. Moreover, Fe3+-addition has positive effects on the Cs+-AA of the ordinary JC charcoal made at 800 °C; however, there are no virtual differences between the Cs+-AA of JC-Fe3-800 and JC-Fe7-800. Furthermore, Fe3+-addition has positive effects on the JC charcoal when the CT was set at 800 °C, unlike the JO charcoal. Besides, the Cs+-AA of the ordinary JC charcoal is likely to be independent of the CT in the range of 600–800 °C.
The findings obtained from the Cs+-adsorption isotherms are summarized as follows:
-
(1)
When at the same carbonization conditions, the JO charcoal exhibits a stronger Cs+-AA than the JC charcoal, except for the iron-loaded charcoal samples made at 800 °C. JO-Fe0-600 has the strongest Cs+-AA among the charcoal samples prepared in this study, which is comparable with that of JO charcoal available commercially (Yamauchi et al. 2014).
-
(2)
The Cs+-AA of both the ordinary and iron-loaded JO charcoal become weaker with an increase in CT from 600 to 800 °C.
-
(3)
The addition of Fe3+ negatively affects the Cs+-AA of JO-Fe0-600, whereas no significant changes are observed in the Cs+-AA of JO-Fe0-800 after Fe3+-addition.
-
(4)
JC-Fe0-600, JC-Fe3-600, and JC-Fe0-800 belong to a group of weak Cs+-AA. There are no clear differences between the Cs+-AA of the three charcoal samples.
-
(5)
The Cs+-AA of JC-Fe3-800 and JC-Fe7-800 are superior to those of the other JC charcoal samples; however, the effects of increasing the Fe3+ content in wood from 3 to 7 w/w% are not confirmed. Most of the iron species in JC-Fe3-800 are Fe0 species (Yamagishi et al. 2022), whereas we have no experimental data for the chemical states of iron in JC-Fe7-800. Further research for iron species in JC-Fe7-800 is required to confirm the enhancing effects of Fe3+ ions in wood powder.
IR-PA spectra of JC and JO charcoal
We previously reported that the Cs+-AA of woody charcoal is not necessarily dependent on its specific surface area (Yamauchi et al. 2017a) and proposed that the surface OH groups on the charcoal synthesized at 300 °C and 500 °C play a primary role in adsorbing Cs+ ions from aqueous solution (Yamagishi et al. 2019). The IR-PA data (Yamauchi 2003; Yamagishi et al. 2023) suggested that wood constituents are not completely pyrolyzed even at 600 °C and functional groups originating from the constituents remain.
To investigate the adsorption isotherms in terms of residual functional groups, the charcoal samples were examined using IR spectroscopy. The IR-PA spectra of the JO and JC charcoal samples synthesized at 600 °C are shown in Fig. 4. Several vibrational bands, previously assigned to vibrational modes (Yamagishi et al. 2023), are observed in the four IR-PA spectra, indicating that many types of functional groups, including OH groups, remain in the four charcoal samples. The board bands observed from 3700 to 3000 cm−1 are assigned to the O–H stretching vibrational modes. The peak at ~ 3650 cm−1 corresponds to OH groups free from interactions with the neighboring atoms in Bellamy (1975) and Yamagishi et al. (2019). However, the bands at ~ 3050 cm−1 occurring in the region of O–H stretching modes are attributed to an aromatic C–H stretching vibration (Agarwal UP 2014; Yamagishi et al. 2023). The whole line-shapes of the IR-PA spectra indicate that the pyrolysis degrees of the four charcoal samples prepared at 600 °C are close to one another.
The IR-PA spectra of the charcoal samples prepared at 800 °C are not displayed in the figure because of no vibrational bands observed in the region of 4000–400 cm−1. Hence, we discuss the Cs+-AA based on the assumption that the JC and JO charcoal samples prepared at 800 °C contain few or no functional groups that can capture Cs+ ions.
A point worth noting in the IR spectra shown in Fig. 4 is the fact that OH bands are weak but clearly observable. More notably, as observed from their IR-band intensities, it is expected that the carbonization at 600 °C does not cause any significant difference between the amounts of residual OH groups of the four charcoal samples. Based on the IR-PA spectra, we could not confirm whether significantly more OH groups remain in the JO charcoal than in the JC charcoal.
Raman spectra of JC and JO charcoal
We confirmed the formation of sp2-carbon by carbonization and investigated the structures of the carbon atoms in the JO and JC charcoal samples using characteristic Raman bands of sp2-carbon. The Raman D-, G-, and G′ (2D)-bands due to sp2-carbon are characteristic of many carbon materials except for diamond and diamond-like carbon. The D- and G′-bands are not ordinary Raman bands and their Raman shifts are dependent on excitation wavelengths.
Figure 5 depicts the typical Raman spectra of the charcoal samples made from JO (Fig. 5A) and JC (Fig. 5B) wood. The G- and D-bands are clearly observable in all the Raman spectra of the charcoal samples, revealing that sp2-carbon atoms were generated considerably when the temperature reached 600 °C. Moreover, an inspection of the Raman spectra shown in Fig. 5 indicates that a G´-band occurs in the spectra of the iron-loaded charcoal synthesized at 800 °C (JO-Fe3-800, JC-Fe3-800, and JC-Fe7-800). The occurrence of the G´-band corresponds to the formation of honeycomb structures composed of sp2-carbon atoms (Pimenta et al. 2007). Hence, the G´-bands observed in the Raman spectra of the iron-loaded charcoal demonstrate the formation of graphitic structures. Specifically, the Fe0 species reduced from Fe3+ ions acted as a catalyst for the ordering of sp2-carbon atoms in the iron-loaded charcoal made at 800 °C (Yamagishi et al. 2022).
pH of aqueous solutions containing charcoal
The pH of solution is an important factor affecting the Cs+-adsorption isotherms (Yamauchi et al. 2016, 2017a). The pH of the aqueous solutions varied with the weight of adsorbent charcoal during the Cs+-adsorption process, because the pH was affected by the amounts and properties of the chemical species eluted from the charcoal powder into the solution. Three findings can be seen in Table 1 summarizing the pH ranges used for preparing the adsorption isotherms.
-
(I)
The pH value for the JO charcoal is higher than that for the JC charcoal except for the iron-loaded charcoal made at 800 °C, under the same synthesis conditions.
-
(II)
The pH values for the charcoal samples made at 800 °C are higher than those for the charcoal samples made at 600 °C except for the ordinary JO charcoal. The CT increase has no virtual effect on the Cs+-AA of JO-Fe0-600.
-
(III)
The addition of Fe3+ raises the pH for the JC charcoal made at 800 °C; however, it has no marked effect on the pH for JO-Fe0-800 and JC-Fe0-600. The pH of the solutions containing JO-Fe3-600 is lower than that containing JO-Fe0-600. The effect of Fe3+-addition on the pH for the JO charcoal synthesized at 600 °C is opposite to that for the JC charcoal synthesized at 800 °C.
Before the addition of the adsorbent charcoal, the pH of the aqueous CsCl (2.50 × 10–4 mol/L) solution was 6.4. The factors affecting the pH of the solution are effluents from charcoal powders, which are group 1 and 2 metals and organic compounds originating from wood constituents (Yamauchi et al. 2016, 2017a). The metals are present as oxides in the charcoal, and the dissolution of the oxides in water increases the pH of the solution. In contrast, the organic compounds are expected to contribute to the reduction in the pH, because some of them probably contain phenolic OH or carboxy groups.
According to Tsuchiya et al. (2010), three metals, calcium, potassium, and magnesium were found as principal trace elements in the raw wood prepared from JC and JO trees planted in Japan. However, the contents of the metals contained in the JC and JO trees were substantially dependent on a production region and a part of tree. Thus, it is difficult to decide which tree species contains more metals belonging to 1 and 2 group.
Comprehensive explanation of Cs + -adsorption ability of charcoal samples
Effect of CT on Cs + -adsorption ability
First, we discuss the effects of CT on the Cs+-AA. As described above, JO-Fe0-600 displays the best Cs+-AA among the JO and JC charcoal samples, while the Cs+-AA of the ordinary JO charcoal substantially decreases with the CT increasing from 600 to 800 °C. The IR-PA spectra indicated that OH groups are almost completely decomposed at CT of 800 °C. Furthermore, since the pH values of JO-Fe0-600 and JO-Fe0-800 are in the same range, it is concluded that the reduction in the Cs+-AA is principally caused by the thermal decomposition of the OH groups. The adsorption isotherms shown in Fig. 2 demonstrate that the reduction in the Cs+-AA also occurs in the iron-loaded JO charcoal with the increase in CT; eventually, the Cs+-AA of JO-Fe3-800 is roughly equivalent to that of JO-Fe0-800. The increase in CT from 600 to 800 °C has essentially the same effects on the Cs+-AA of the iron-loaded JO charcoal as the ordinary JO charcoal. Thus, the major parts of the Cs+-AA of the ordinary and iron-loaded JO charcoal samples made at 600 °C are likely attributed to residual OH groups.
There are virtually no differences between the Cs+-AA of JC-Fe0-600 and JC-Fe0-800, and these two ordinary JC charcoal samples are inferior to JO-Fe0-800 in terms of Cs+-AA. This inferiority of JC-Fe0-600 and JC-Fe0-800 is most likely attributed to the lower pH of the solutions. The OH groups on the charcoal surface can take two chemical forms: undissociated (‒OH) and dissociated (‒O−) types; the dissociated OH groups electrostatically attract the Cs+ ions much more strongly than the undissociated OH groups (Yamagishi et al. 2019). Since the dissociation degree of the OH group decreases with a decrease in the pH of the aqueous solution, few OH groups may be dissociated on the JC-Fe0-600 surfaces in the aqueous solution. In other words, the OH groups of JC-Fe0-600 have only a minimal effect on the Cs+-AA, because almost all of them are electrically neutral. Additionally, more work focused on the turbostratic structures of sp2-carbon will be required to elucidate the Cs+-adsorption mechanism of the ordinary charcoal made at 800 °C.
It is worth noting that the pH values of JC-Fe3-800 and JC-Fe7-800 are much higher than those of JC-Fe3-600. We will discuss this remarkable result regarding the effect of the Fe3+-addition, in the next section.
Effect of Fe 3+ -addition on Cs + -adsorption ability
The influence of the CT on the iron-loaded JC charcoal was completely different from that on the iron-loaded JO charcoal. Here, we discuss the effects of Fe3+-addition on the Cs+-AA. We previously reported that most of the Fe3+ ions added to JC wood are changed into nano-sized particles of Fe2O3 at 600 °C and further reduced to Fe0 species (metallic iron and Fe3C) until reaching 800 °C (Yamagishi et al. 2022). Assuming that the same chemical changes of Fe3+ ions occur during the carbonization of JO wood impregnated with Fe3+, we can assess the effects of Fe-addition.
Fe3+-addition clearly weakens the Cs+-AA of the ordinary JO charcoal made at 600 °C, and this reduction is likely to be caused by the fall of the pH values. However, no reasonable explanation could be provided for the pH fall. There are no significant differences in the Cs+-adsorption isotherms and pH values between JO-Fe0-800 and JO-Fe3-800. In contrast to the JO charcoal, the Cs+-adsorption isotherms exhibit no marked effects of the Fe3+-addition on the JC charcoal made at 600 °C. Both the Cs+-AA of JC-Fe0-600 and JC-Fe3-600 are much smaller than those of the JO charcoal samples made at 600 °C, and the pH values for the two JC charcoal samples range from 6.7 to 7.5, suggesting that most of the OH groups on the surfaces of JC-Fe0-600 and JC-Fe3-600 are present as the undissociated type in aqueous solutions. Conversely, the Cs+-AA of the JC charcoal synthesized at 800 °C is enhanced by Fe3+-addition. This may be attributed to the rises in the pH values. These results for Fe3+-addition also support the view that the pH of solution is a key factor for controlling the Cs+-AA.
The correlation between Fe3+-addition and the pH of aqueous solution is necessary to be investigated in terms of two factors. One is the pH change caused by the iron species eluted from the charcoal. Nevertheless, it can be assumed that little or negligible amount of the iron species is extracted into the aqueous solution, because all the solutions are neutral or weak alkaline (6.7–10.6). Thus, the pH can scarcely be influenced by the chemical species of iron as effluent. The other is the variation in the surface potential of the iron species with the pH of the solution. It is essential for a better understanding of the effect of Fe3+-addition to estimate the surface potential of the iron species contained in the charcoal. However, it is extremely difficult to make an accurate estimate of the effects of surface potential, because the surface potential depends on many factors other than the pH of the solution, such as the particle sizes and structure of carbon atoms. Moreover, the iron atoms in the iron-loaded charcoal probably form several types of chemical species (Yamagishi et al. 2020, 2022), and the whole surfaces of iron-species particles do not have direct-contact with H2O molecules as solvent because they are partly coated with charcoal. For many types of solid substances, it is currently recognized that the surface potentials of the powders decrease with increasing the pH of the solution containing the powders; therefore, it can be assumed the potential of the JO and JC charcoal powders becomes electrically more negative with the rise in pH.
The addition of Fe3+ has a negative effect on the Cs+-AA of the JO charcoal made at 600 °C. This reduction in the Cs+-AA is due mainly to the fall of pH. A possible explanation for the pH fall is the effluents that originate from the pyrolyzed wood constituents rather than the iron species. It is anticipated that Fe3+-addition makes changes in the types and amounts of chemical species eluted from the pyrolyzed wood into the aqueous solution; however, the changes were not noticeable enough to appear in the IR-PA spectra of the charcoal samples. None of the pH fall due to Fe3+-addition is observed for the JC charcoal made at 600 °C, suggesting the significant differences in the effluents between JC-Fe3-600 and JO-Fe3-600.
In contrast, Fe3+-addition has the positive effects on the Cs+-AA of JC-Fe3-800 and JC-Fe7-800. A likely explanation for these effects is the rise in pH, as listed in Table 1. Moreover, it is necessary to consider the effects of the graphitization of sp2-carbon as well as the surface potential due to the iron species. Carbon atoms consisting of graphitic structures are expected to attract Cs+ ions more strongly amorphous carbon because aromatic rings can form weak hydrogen bonds. Thus, it is possible that graphitic structures formed through the catalytic reaction with Fe0 species have some positive effects on the Cs+-AA of the iron-loaded charcoal made at 800 °C.
Consequently, we concluded that the dissociation degree of OH groups is a factor governing the Cs+-AA when charcoal has the OH groups detectable using IR spectroscopy. Our experimental data revealed that pH plays a key role in the adsorption of Cs+ from aqueous solution onto woody charcoal.
Conclusion
The results obtained in this study present useful findings regarding the adsorption of Cs+ from aqueous solution onto charcoal.
The pH of solution is a vital factor governing the Cs+-AA of woody charcoal. When the OH groups in woody charcoal remained to some extent, the Cs+-AA was highly dependent on the amount of dissociated OH groups (‒O−), which increased with increasing the pH of solution. The Cs+-AA of the charcoal made at 800 °C also increased with increasing the pH of solution, suggesting that the surface potential of charcoal with few or no functional groups is an important factor for the Cs+-AA. Furthermore, it can be expected that the pH values depend strongly on effluents from woody charcoal; therefore, the effluents make a significant contribution to governing the pH around Cs+ ions in soil.
The Cs+-AA of the JO charcoal made at 600 °C was negatively influenced by Fe3+-addition; however, the effect of Fe3+-addition on the Cs+-AA of the JC charcoal made at 800 °C was positive. Hence, we conclude that the pH survey of aqueous solution with the charcoal powder are essential for utilization of iron-loaded charcoal made from ancient buried wood as adsorptive. Moreover, the selection of tree species and setting of the CT are also important.
Data availability
The datasets used and analyzed in the current study are available from the corresponding author on reasonable request.
Abbreviations
- JC:
-
Japanese cedar
- JO:
-
Japanese oak
- CT:
-
Carbonization temperature
- Cs+ AA:
-
Cs+-adsorption ability
- IR-PA:
-
Infrared-photoacoustic
- JC-FeX1-X2 and JO-FeX1-X2 :
-
is a code expressing charcoal samples made from JC and JO wood, respectively. The letters of X1 and X2 represent Fe3+ content (w/w%) in starting material wood and CT (°C), respectively
References
Adachi K, Kajino M, Zaizen Y, Igarashi Y (2013) Emission of spherical cesium-bearing particles from an early stage of the Fukushima nuclear accident. Sci Rep 3:2554. https://doi.org/10.1038/srep02554
Agarwal UP (2014) 1064 nm FT-Raman spectroscopy for investigations of plant cell walls and other biomass materials. Front Plant Sci 5:490. https://doi.org/10.3389/fpls.2014.00490
Bellamy LJ (1975) The infra-red spectra of complex molecules. Chapman and Hall Ltd, London, pp 107–128
Kurimoto Y, Doi S, Aoyama M (2001) Removal of trichloroethylene from aqueous solution by pyrolyzed Japanese cedar bark. J Wood Sci 47:76–79. https://doi.org/10.1007/BF00776650
Kurimoto Y, Yamauchi S, Takayama T, Sakai Y (2020) Coloring mechanisms of ancient buried wood: Japanese cedar trees excavated from the foothills of Mt. Chokai J Wood Sci 66:24–29. https://doi.org/10.1186/s10086-020-01870-7
Narita H, Yatagai M (2006) Terpenes from bogwood of Cryptomeria japonica D. Don and characterization of its ash. Org Geochem 37:818–826. https://doi.org/10.1016/j.orggeochem.2006.03.001
Pimenta MA, Dresselhaus G, Dresselhaus MS, Cançado LG, Jorio A, Saito R (2007) Studying disorder in graphite-based systems by Raman spectroscopy. Phys Chem Chem Phys 9:1276–1294. https://doi.org/10.1039/B613962K
Pulido-Novicio L, Kurimoto Y, Aoyama M, Seki K, Doi S, Hata T, Ishihara S, Imamura Y (2001) Adsorption of mercury by sugi wood carbonized at 1000°C. J Wood Sci 47:159–162. https://doi.org/10.1007/BF00780567
Suzuki K, Saito Y, Kita H, Sato K, Konno T, Suzuki T (2017) Production of carbon nanoshell chains by the co-catalyzed carbonization of wood. TANSO 277:55–62. https://doi.org/10.7209/tanso.2017.55
Suzuki K, Saito Y, Okazaki N, Suzuki T (2020) Graphite-shell-chains selectively and efficiently produced from biomass rich in cellulose and chitin. Sci Rep 10:12131. https://doi.org/10.1038/s41598-020-69156-y
Tsuchiya Y, Shimogaki H, Abe H, Kagawa A (2010) Inorganic elements in typical Japanese trees for woody biomass fuel. J Wood Sci 56:53–63. https://doi.org/10.1007/s10086-009-1055-z
Yamagishi T, Kurimoto Y, Yamauchi S (2017) A method for visualizing cesium adsorbed on wood charcoal using SEM-EDX. Wood Sci Technol 51:413–429. https://doi.org/10.1007/s00226-016-0875-4
Yamagishi T, Nishikiori K, Kurimoto Y, Yamauchi S (2019) Cesium-adsorption mechanisms of woody charcoal discussed on the basis of its functional groups and nanostructures. J Wood Sci 65:26–34. https://doi.org/10.1186/s10086-019-1805-5
Yamagishi T, Yamauchi S, Suzuki K, Suzuki T, Kurimoto Y, Takayama T, Sakai Y (2020) Mössbauer and Raman spectroscopic characterization of iron and carbon in iron-loaded Japanese cypress charcoal. J Wood Sci 66:82–89. https://doi.org/10.1186/s10086-020-01930-y
Yamagishi T, Yamauchi S, Shibutani S, Suzuki H, Takayama T, Sakai Y (2022) Mössbauer and Raman characterization of iron-loaded woody charcoal: effects of Fe3+-dispersion in wood on reduction of Fe3+ and graphitization in carbonization. J Wood Sci. https://doi.org/10.1186/s10086-022-02014-9
Yamagishi T, Sakai Y, Takayama T, Shibutani S, Yamauchi S (2023) Characterization of iron-loaded charcoal using infrared-photoacoustic spectroscopy: factors governing graphitization. Wood Sci Technol 57:229–252. https://doi.org/10.1007/s00226-022-01436-4
Yamauchi S (2003) Infrared photoacoustic spectra of Japanese cedar (Cryptomeria japonica D. Don) wood and bark heat-treated at temperatures ranging from 200 °C to 1100 °C. Eurasian J For Res 6:75–78
Yamauchi S, Yamagishi T, Kirikoshi K, Yatagai M (2014) Cesium adsorption from aqueous solutions onto Japanese oak charcoal I: effects of the presence of group 1 and 2 metal ions. J Wood Sci 60:473–479. https://doi.org/10.1007/s10086-014-1431-1
Yamauchi S, Yamagishi T, Kirikoshi K, Yatagai M (2015) Cesium adsorption from aqueous solutions onto Japanese oak charcoal II: effects of metal ions eluted from the charcoal. J Wood Sci 61:185–191. https://doi.org/10.1007/s10086-014-1450-y
Yamauchi S, Yamagishi T, Kirikoshi K, Yatagai M (2016) Cesium adsorption from aqueous solutions onto Japanese oak charcoal III: effects of water-extraction treatment. J Wood Sci 62:562–566. https://doi.org/10.1007/s10086-016-1578-z
Yamauchi S, Yamagishi T, Kirikoshi K, Yatagai M (2017a) Factors governing cesium adsorption of charcoals in aqueous solution. J Wood Sci 63:183–191. https://doi.org/10.1007/s10086-016-1604-1
Yamauchi S, Kurimoto Y, Sakai Y (2017) Mössbauer characterization of iron in ashes made from the ancient buried woods excavated in the foothills of Mt. Chokai. J Nucl Radiochem Sci 17:1–7. https://doi.org/10.14494/jnrs.17.1
Yamauchi S, Kurimoto Y, Sakai Y (2017) Mössbauer characterization of iron in ancient buried trees excavated from the foothills of Mt. Chokai. J Nucl Radiochem Sci 17:23–29. https://doi.org/10.14494/jnrs.17.23
Yamauchi S, Kurimoto Y, Takayama T, Sakai Y (2020) Mössbauer and Raman characterization of ash produced by burning ancient buried Japanese cedar wood and investigation for its unusual color tone. J Radioanal Nucl Chem 326:753–764. https://doi.org/10.1007/s10967-020-07359-3
Funding
Open Access funding provided by The University of Tokyo. This work was financially partly supported by the Grant-in Aid for Scientific Research (Grant Numbers 21K05714 and 23K05330) from the Japan Society for the Promotion of Science.
Author information
Authors and Affiliations
Contributions
TY and SY designed this study and wrote the initial draft of the manuscript. SS and HS synthesized the charcoal samples and prepared the Cs+-adsorption isotherms. TY and SY were major contributors in obtaining and analyzing IR-PA and Raman spectra. All authors have contributed to data collection and interpretation, and critically reviewed the manuscript. All authors read and approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no competing interests.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Yamagishi, T., Shibutani, S., Suzuki, H. et al. Cesium adsorption ability of charcoal made from Japanese cedar and Japanese oak wood: effect of Fe3+-addition to starting materials. Wood Sci Technol 58, 725–740 (2024). https://doi.org/10.1007/s00226-024-01530-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-024-01530-9