Abstract
In a growing context of green and circular economy, gaining knowledge of the composition of every crop is crucial, as this will allow for their full exploitation. Cherry (Prunus avium L.) is a widespread tree of particular interest for its fruits and its valuable timber. Its wood is rich in extractives and its characterization will allow to consider other applications for this feedstock. In this study, chipped cherry wood was extracted and chemically analysed to determine its total phenolic content, total condensed tannin, antioxidant capacity, and polysaccharide content through wet chemistry analysis. These investigations were coupled with 13C-NMR and FTIR spectrometry, with HPLC as well as elemental analysis to conduct a comprehensive chemical characterization. Thermogravimetric measurements were also taken to understand the behaviour of the extract when exposed to high temperature. The registered findings were benchmarked against commercial mimosa (Acacia mearnsii De Wild.) and chestnut (Castanea sativa Mill.) tannins which were selected as template for condensed and hydrolysable tannins, respectively. Cherry extract was found to be the poorest in phenolics which are mainly constituted of pyrogallic flavonoids strongly interconnected with significant amounts of polysaccharides.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Tannins are natural polyphenolic substances produced by plants to protect against UV, free-radicals, and biological decay agents such as animals, insects, fungi, and bacteria (Tondi et al. 2013). These compounds can be easily extracted from wood and commercially exploited for different purposes (Shirmohammadli et al. 2018; Pizzi 2019). Historically, the first application of these extracts is leather tanning (Falcão and Araújo 2018). Nowadays, the tannin extract is industrially extracted and it is mostly used to obtain vegetable-tanned leather (Auad et al. 2020). The high affinity of the polyphenolic structure of tannins with collagen, the skin's main protein, allows an irreversible change in structure resulting in an excellent stability of the leather. Soon, the critical protective abilities offered by tannins were also exploited in the medical field, thus benefiting from their antioxidant and free radical scavenger capabilities, inhibiting low-density lipoprotein oxidation (Cook and Samman 1996). In addition, the complexing properties of tannins can also be exploited in water remediation by trapping heavy metals (Yu et al. 2001). Another widespread application is in food industry. In particular, tannins are key constituents in popular beverages as tea and coffee, of fruit juices, and of alcoholic beverages as beer and wine (Li et al. 2020; Giacosa et al. 2021). Tannins are also present in significant quantities in distillates aged in wooden barrels such as brandy, whiskey, grappa, and cognac (De Rosso et al. 2020). In the case of wine, tannins are extracted from grapes and play a major role in wine quality as they contribute to colour, mouthfeel, stability, and longevity. Given their importance, especially for red wines, winemakers adopt processing techniques suitable to enrich the wine in tannins, for example by extending the skin maceration step, by adding tannin extracts to the wines (Harbertson et al. 2012), or by ageing the wines in barrels made with different wood types. The most common wood used to produce barrels for wine ageing is oak, although wood species as chestnut and cherry, the subject of this study, are also used. In winemaking, different wood types contribute differently to the ageing kinetic, to the type and amount of tannins released into the wines, and to the wine flavour (De Rosso et al. 2009), so that wood contact is also used as a tool to modify the sensorial properties of the wines. A similar approach is adopted in the production of Italian balsamic vinegar, where the aging in cherry barrels is part of the traditional production method (Guerrero et al. 2008). In these contexts, cherry wood exhibits high potentiality as it confers a distinctive flavour to the product (Versari et al. 2013; Rinaldi and Moio 2018). Considering the increasing sensibility for producing sustainable and renewable materials, tannins are increasingly attracting the interest of other engineering sectors. While their use as adhesives for wood is known since the middle of the last century (Pizzi 1980; Cesprini et al. 2021), their application as wood preservative is more recent (Tondi et al. 2012; Sommerauer et al. 2019) and both are suggestive alternatives to synthetic plastics and coatings (Cesprini et al. 2022). In the last decade, other engineered materials were produced from tannins: insulating foams have attracted the interest of green building construction (Tondi et al. 2008a, 2016), furanic bio-based plastics were successfully produced (Li et al. 2013) and also ultralight aerogels were synthesized (Amaral-Labat et al. 2013; Koopmann et al. 2021).
Tannins are mainly divided into condensed and hydrolysable, these having different chemical structures and consequently, different properties. The former consists of flavonoid oligomers/polymers with a variable degree of polymerization (Drewes and Roux 1963). The units are usually linked C4 to C6 or C4 to C8 to form various length chains depending on the type of tannin (Pizzi 1994). Hydrolysable tannins are esters of phenolic acids such as gallic, ellagic or more complex triaril acids with easy sugars such as glucose and xylose (Muller-Harvey 2001; Michel et al. 2011; Arbenz and Avérous 2015).
Since these families have different physical and chemical properties and are used industrially according to the characteristics required for the specific use, it is of fundamental importance to clarify the class to which a tannin extract belongs to direct its possible employment.
In this study, the cherry (Prunus avium L.) tannin extract is compared with two commercial tannin extracts. That of mimosa bark (Acacia mearnsii De Wild.) is taken as reference for condensed tannins (Missio et al. 2017), while the extract of chestnut wood (Castanea sativa Mill.) as the reference for the hydrolysable ones (Campo et al. 2016). Wet analytical chemical experiments, spectroscopic (13C-NMR and ATR FT-IR), elemental and thermal analyses (TGA) were applied to characterize the three extracts.
Experimental
2.1 Materials
Debarked cherry (Prunus avium L.) wood chips at 10% moisture content were provided by Silva Team (San Michele Mondovì, Italy). Industrial tannin powders of chestnut (Castanea sativa Mill.) and mimosa (Acacia mearnsii De Wild.) were supplied by Saviolife (Viadana, MN, Italy) and Silva Team (S. Michele Mondovì, CN, Italy), respectively, and were used as benchmark, respectively, for hydrolysable and condensed tannin in the investigation of the cherry extract. High-purity (≥ 99%) ethanol, sodium carbonate, sulphuric acid, sodium acetate trihydrate, acetic acid, 2,4,5-tripyridyl-s-triazine, HCl, ferric chloride, ammonium formate, pectin, and dextran were purchased from Sigma-Aldrich (St. Louis, MO, USA). Folin–Ciocalteu′s phenol reagent and vanillin were purchased from Merck (Darmstadt, Germany).
Equipment
The following equipment was used to perform extraction and characterization of the extracts. Spectroscopic investigations were carried out with a Varian Cary 50 Bio UV–Visible spectrophotometer; the solid-state NMR was performed with a Bruker AVANCE NEO 400 MHz NMR tool equipped with 4 mm CP-MAS probe while the FTIR analyses were done on a Nicolet, NEXUS, B70, FTIR instrument set up with ATR unit. The chromatography analyses were performed in Agilent 1260 series II quaternary pump LC (Agilent Technologies) equipped with G7162A refractive index detector (RID) and G7117C diode array detector (DAD). The separation was carried out using a gel permeation HPLC column (PL aquagel-OH, Agilent). Thermo Fischer Scientific FLASH 2000 element analyser equipped with MAS 200 R autosampler thermal conductivity detector (TCD), and two analytical chromatographic columns was used for elemental investigation. The thermal behaviour of the extracts was investigated with an SDT 2960 Simultaneous DSC-TGA TA instrument (Waters—TA instruments, New Castle, DE, USA).
Methods
Extraction of cherry tannin powder
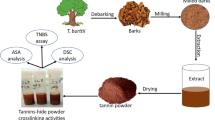
The cherry extract was obtained by a double extraction of the wood chips with boiling water kept at temperature with an external heater. Firstly, 250 g of chips were extracted with 1,500 g of deionized water for 1 h. The suspension was filtered and then the wet chips were further extracted using additional 1000 g of boiling water for 1 h. This second suspension was also filtered, and the two brown solutions obtained were then evaporated until dry in a rotavapor at 70 °C, and the remaining dry brown powder was collected and characterized.
Purification of the tannin extracts
The three tannin powders were submitted to a preliminary extraction to remove impurities. The process involved the dissolution of 100 mg of the three tannin powders in 5 mL of EtOH / H2O (80/20 v/v), followed by mechanical stirring for 30 min at 25 °C. The samples were then centrifuged for 15 min at 4 °C and 10,000 rpm, and the supernatants analysed to assess their antioxidant capacity, as well as their content in total polyphenols, condensed tannins, and polysaccharides. This procedure was similar to that presented by Katalinić et al. (2010).
Total polyphenol content
Determination of total phenolic content (TPC) was performed using the Folin–Ciocalteu assay (Singleton et al. 1999). In brief, 1 mL of diluted tannin extract, 1 mL of Folin reagent (Sigma-Aldrich) (diluted 1:10 with deionized water), and 0.8 mL of sodium carbonate solution (7.5%) were added. The mixture was left in the dark at 40 °C. After 30 min, the absorbance was read at 765 nm. Each measurement was repeated three times. The calibration curve was created with a serial dilution of gallic acid.
Total condensed tannin
The transformation of condensed tannin into anthocyanidols in the presence of sulphuric acid and vanillin allows to calculate the concentration of total condensed tannins (TCT) (Sepperer et al. 2019a). In brief, 50 μL of the ethanol tannin solution (1 mg/mL) was added to 2 mL of 4% methanol vanillin solution and 450 μL concentrated sulphuric acid. After 15 min, the absorbance was read at 527 nm and the results were expressed as relative ratio of equivalent catechin (μgCE/mg).
Antioxidant capacity
FRAP assay was performed for determination of the extracts’ antioxidant capacity (Galiñanes et al. 2015). FRAP reagent was prepared by mixing 25 mL of 0.3 M sodium acetate buffer (pH 3.6), 2.5 mL of 10 mM 2,4,5-tripyridyl-s-triazine HCl solution, and 2.5 mL of 20 mM ferric chloride. In brief, 100 μL of EtOH/H2O tannin extract was added to 990 μl FRAP reagent. After incubation (37 °C, 30 min), the absorbance was measured spectrophotometrically at 593 nm. The FRAP values were expressed in µg of ascorbic acid equivalents (AAE)/ mg tannins.
13C-NMR solid state
Solid-state 13C-NMR experiments of the three tannin extracts were performed on a Bruker AVANCE NEO 400 MHz NMR spectrometer using a 4-mm CP-MAS probe. The sample spinning frequency was 15 kHz. The 13C CP-MAS NMR experiments consisted of excitation of protons with p/2 pulse of 3.0 s, CP block of 2 ms, and signal acquisition with high-power proton decoupling. A total of ca. 2,000 to 14,000 scans were accumulated with the repetition delay of 5 s. The chemical shifts were referenced externally using adamantine. This analysis was performed according to the methodology described by Kotar et al. (2014).
FTIR ATR spectroscopy
The FTIR ATR spectra of the tannin powders were acquired with 32 scans from 4,500 to 600 cm−1, focusing the investigation between 1,800 and 600 cm−1. This analysis was conducted according to Sepperer et al. (2019b). The spectra were normalized and baseline corrected with the software OriginPro 8.5.0 SR1.
Determination of polysaccharides by high-resolution size-exclusion chromatography (HRSEC)
The polysaccharides quantity and molecular distribution were measured by analysing the tannin powders’ extracts by HRSEC (Ayestarán et al. 2004; De Iseppi et al. 2021). Briefly, 1 mg of sample was dissolved in 1 mL of running buffer (50 mmol/L aqueous solution ammonium formate) and sterile-filtered (0.22-μm acetate cellulose filters, Millipore) directly into HPLC glass vials. Samples were held at 4 °C prior to injection in a temperature controlled autosampler. Then, 10 μL of each sample were injected into the chromatographic system. The separation was carried out at 20 °C using a gel permeation column. The mobile phase was applied at a constant flow of 0.6 mL/min for 35 min, and the temperature of the RID cell was kept at 35 °C. The molecular weight distribution of the extracts’ polysaccharides was identified using a qualitative calibration curve made with 10 pullulan standards (Merck) at MW ranging between 342 and 805,000 Da, while pectin and dextran were used in the range between 0 and 2 g/L to create the calibration curve for polysaccharide quantification.
Thermogravimetry (TGA)
The thermal behaviour of tannin samples was investigated during heating through a temperature ramp of 10 °C/min up to 800 °C. The percentage weight loss of the samples as a function of temperature was then recorded. The tests were carried out in an inert environment with a flow of nitrogen. The first derivative (DTGA) of the thermograms was calculated to highlight the degradation peaks. Experiment and elaboration were done according to the method of Zanetti et al. (2014).
Organic elemental analyser “OEA”
The atomic composition of carbon, hydrogen, nitrogen, sulphur of the tannin powders was investigated. The Eager Xperience software was used for data analysis. Experiment and elaboration were done according to a recent work by Kapen et al. (2021).
Statistical analysis
Statistical analyses were performed using OriginPro 8.5.0 SR1 software (OriginLab Corp., Northampton, MA, USA). One-way analyses of variance (ANOVA) followed by post-hoc Tukey’s test (p ≤ 0.05) were used to ascertain significant differences within mean values.
Results and discussion
Chemical analysis
The cherry extract obtained from the extraction process presented a yield of 3% of dry extract on dry raw material. The quantification of the total phenolic content (TPC), total condensed tannins (TCT), antioxidant capacity (FRAP), and polysaccharide content (PS) of the three tannin extracts is shown in Table 1.
Mimosa and chestnut extracts contained comparable amounts of total polyphenols, with values that are in line with those reported in previous studies (Panzella et al. 2019; Sepperer et al. 2019a). Unsurprisingly, mimosa extract contained about 10 times more condensed tannins than chestnut, a finding in agreement with the literature as chestnut extracts mostly contain hydrolysable tannins (Campo et al. 2016; Missio et al. 2017). The cherry extract contained significantly less phenolic compounds than mimosa and chestnut (< 45%), but with a condensed tannin amount closer to that of mimosa, thus suggesting a strong flavonoid nature of its tannins. The antioxidant activity of the cherry extract is around half of that of mimosa, an occurrence attributable to its lower phenolic content. Indeed, different studies report a linear correlation between antioxidant capacity and TPC (Maksimovíc et al. 2005; Vázquez et al. 2008), and this is in agreement with the present findings. The chestnut extract shows the highest antioxidant capacity confirming the findings of a recent study where it was compared with quebracho using Trolox as reference (Molino et al. 2018). The fact that mimosa and chestnut extracts have similar total phenolic content but very different antioxidant activities suggests that hydrolysable tannins have significantly more antioxidant activity than the condensed ones (Vignault et al. 2018). The lower amount of condensed phenolics explains the limited antioxidant activity of the cherry extract. The HRSEC analysis of the three extracts allowed to determine their total polysaccharides content. The cherry extract exhibited the largest concentration in polysaccharides as it contained 88.5% more polysaccharides than mimosa, and 118.6% more polysaccharides than the chestnut extract. The chemical profile of the cherry extract was very different from the other two. Indeed, the ratio PS/TPC for cherry was almost 4 times higher than mimosa and chestnut, a value that contributes to justify the lower antioxidant capacity measured for cherry extract.
Elemental analysis
The elemental analysis of the three tannin powders is presented in Table 2.
Condensed tannins are generally richer in carbons than the hydrolysable ones (Zanetti et al. 2021), but the significative carbohydrate fraction in the cherry extract contributes to decrease the carbon fraction almost at the level of chestnut. Small amounts of nitrogen were detected, probably due to fixed proteins, while no sulphur was registered confirming that the industrial extracts were obtained by simple water extraction without sulphites.
Solid state 13C NMR spectroscopy
The 13C NMR spectra of the three tannin extracts are reported in Fig. 1. These spectra can be analysed dividing the spectral field into four main regions, based on the chemical shift of the different bondings:
-
190–135 ppm: signals due to Carom directly linked to oxygen;
-
135–90 ppm: signals due to Carom not directly bound to oxygen;
-
90–50 ppm: signals due to Caliph directly linked to oxygen;
-
50–10 ppm: signals due to Caliph not directly bound to oxygen.
Comparing the NMR spectra of cherry with the other two, two observations are striking: (i) The region between δ 90 and 60 ppm is very prominent and (ii) the region between δ 190 and 135 ppm is very similar to that of mimosa. These two findings are in line with the results observed after the wet chemistry tests: cherry extract results richer in polysaccharides and its polyphenolics principally belong to the condensed tannin family.
Focusing on the low field chemical shifts, the differences between the classes of condensed (mimosa) and hydrolysable (chestnut) tannins are clearly visible. Firstly, chestnut shows the peak due to the carbonyl group of gallic acid at δ 168 ppm while mimosa and cherry do not present evident C=O signals but only weak and broad signals between 200 and 190 ppm typical for aldehydes. These signals can be attributed to: (i) small amount of galloylated catechin, and (ii) possible oxidized or open forms of sugar portions. In the region between δ 160 and 140 ppm, signals characteristic of the Carom − O bond occur. The 13C NMR spectra of mimosa and cherry show two signals at δ 154 and δ 144 ppm attributed to the C–OH of the A− and B− ring of the flavonoids (Navarrete et al. 2010; Vázquez et al. 2013). The relative intensity of these two peaks is opposite between the two tannins. Area integration ratio of the two signals (area δ 144 ppm/area δ 154 ppm) for the spectra of cherry is 1.195, whereas 0.841 was obtained for mimosa. This suggests that cherry presents proportionally more OH in the B-ring (or less OH in the A ring) than mimosa. The literature describes mimosa to be mainly constituted of robinetinidin (two − OR groups in the A ring and three − OH in B ring) (Pizzi 1994), and hence, we suppose the flavonoid of cherry will be constituted of A-rings even poorer of − OH and pyrogallic like B-rings. In the area of the spectrum between δ 135 and 90 ppm, other interesting features can be highlighted. C1' and C5' of the B-ring of flavonoids resonate at δ 130 and 116 ppm, respectively (Davis et al. 1996), and they result visible for cherry and mimosa. The signals of cherry tannin in this area appear to be shifted to higher fields in comparison with that of mimosa. These shifts suggest a less cross-linked structure (Tondi 2017). In particular, mimosa shows two peaks between 105 and 95 ppm, while the ones of cherry appear at δ 100 and 90 ppm. The two signals occurring at higher frequencies are due to the interflavonoid bonds C4–C8 and C4–C6, while the ones at lower frequencies can be assigned to free C6, C8, C10 (Wawer et al. 2006; Navarrete et al. 2010). Cherry tannin shows the signal relative to free carbons with higher intensity. Conversely, the region between δ 90 and 50 ppm is dominated by C–O absorptions mostly due to carbohydrates, however also the C2 and C3 of the A-ring of the flavonoids (Zhang et al. 2010) resonate in this region. In this case, the relative signal intensity for cherry tannin is much higher than for the other two samples. This is due to a higher portion of carbohydrates whose carbons resonate mostly between 70 and 80 ppm. A further identifying marker for the presence of saccharide in cherry tannin is the signal at around δ 56 ppm (Konai et al. 2017), which is also observable for chestnut because it contains a glycosidic unit in its structure (Navarrete et al. 2010; Santos et al. 2017).
ATR FTIR spectroscopy
The ATR FTIR spectroscopy was also applied as it is an effective technique to investigate tannin extracts. The region of major interest for studying these compounds is from 1800 to 600 cm−1. Figure 2 shows the three spectra of tannin powders.
At a first glance, it is possible to notice that the profile of the cherry spectrum is similar to that of mimosa; in particular, the C=C aromatic stretching region at around 1600 cm−1 so as the major band around 1000 cm−1. This immediately allows to confirm the main observations obtained from the 13C-NMR analysis.
Looking at the profiles in detail, the region between 1730 and 1700 cm−1 is related to the C=O stretching of carbonyl group which is due to gallic and ellagic acid, typical moieties of hydrolysable tannins (Michel et al. 2011; Falcão and Araújo 2014). The spectrum of cherry presents a very weak peak in this region, suggesting the presence of small amounts of carbonyl groups due to the presence of galloylated catechins related to condensate tannins (Hagerman et al. 1997), or to possible oxidized or open forms of small carbohydrates (Konai et al. 2017) as already assumed for the low chemical shield signal in 13C-NMR.
C=C aromatic stretching vibrations absorb between 1620 and 1500 cm−1, and all the extracts present relatively similar signals at 1604 cm−1 (symmetric stretching) and around 1510 cm−1 (asymmetric) (Tondi and Petutschnigg 2015). Only the small shoulder at 1535 cm−1 of mimosa could provide some structural information. Model compounds suggest that this type of signal is more frequent in species containing aromatic rings with two hydroxyl groups. Flavonoids such as profisetinidin or procyanidin with a majority of di-hydroxy-aromatic rings should be responsible for the presence of the signal (Haslam 1998).
The region between 1500 and 1100 cm−1 is generally dominated by C–H bending and C–O stretching vibrations, often overlapping and hence hard to attribute uniquely. However, it is possible to observe that at 1310 and 1174 cm−1, the two dominant signals attributed to C–O stretching of gallic acid and its ester (typical for hydrolysable tannins) (Ricci et al. 2015; Konai et al. 2017) do not occur in cherry.
The area between 1100 and 600 cm−1 is characterized by the overlap of several bands, the assignment of which is very difficult. This region is strongly dominated by the broad band at around 1030 cm−1 which can be attributed to the C–O stretching of the methoxy groups with C–C, C–OH, C–H of the sugar fractions (Schwanninger et al. 2004). This band is prominent for cherry which confirms the presence of important fractions of sugars.
Summarizing, the FTIR also confirms results of the wet chemistry test of Table 1 as well as that of 13C-NMR, indicating that cherry extract is constituted of higher amounts of carbohydrates, and that its polyphenols mostly belong to the condensed family.
Polysaccharides
The chromatography study of tannin extracts allowed to investigate their polysaccharide content and nature, as well as the interaction that might occur between polyphenols and carbohydrate moieties.
Figure 3 shows that the three extracts contained carbohydrates. The chromatographic profile of the extracts was similar, while the concentration of carbohydrates clearly differed, with cherry being the richest in polysaccharides while chestnut the poorest (see Table 1). Interestingly, when looking at the molecular weight distribution of the carbohydrate material, it is visible that most of it is comprised between 1 and 20 kDa, that is the interval in which the two main peaks are eluted for all extracts. In particular, the highest peak is entirely eluted in the oligosaccharide region between 18 and 20 min (1–7.5 kDa). Considering the ratio between the two main peaks (3 kDa/9 kDa), cherry showed an intermediate value (2.5), mimosa the highest (3.8) and chestnut the lowest (1.3), suggesting that their carbohydrates composition also presents differences. Additionally, cherry was the only extract which has a small fraction coming out of the column earlier (10 min) meaning that the extract also contains polysaccharide moieties of high molecular mass (> 1,500 kDa).
To study whether polysaccharides could be covalently bonded with polyphenols in the extracts, the high-resolution size exclusion chromatography (HRSEC) analyses also included the recording of the 210 nm absorbance (Fig. 4).
Comparing the chromatograms obtained with the detector sensitive for polysaccharides (RID) with those obtained from the detector sensitive for aromatics (DAD, 210 nm) it is visible that all the peaks found with the RID are also found with the DAD, while some of the peaks visible in DAD cannot be seen with the RID detector (Fig. 4). This is an indication of the likely interaction occurring between carbohydrate and phenolic material in the extracts. Indeed, the chromatograms suggest that polysaccharides are always eluted together with phenolic compounds, so an association between these two classes of molecules can be hypothesized. Despite there are no experiments that can undoubtedly prove the nature of the interaction between phenolic and carbohydrate moieties, all the experiments performed in this study and in previous ones (Bianchi et al. 2015; Missio et al. 2017; Sepperer et al. 2019a, b) seem to highlight that the interaction between these bonds are covalent because it is almost impossible to selectively separate the two families of compounds. However, it is also clear that parts of the flavonoids, especially those with lower molecular weight, are present in free form.
Results are in line with previous data on extracts composition. Indeed, the ratios RID/DAD in both peaks are the highest for mimosa and the lowest for chestnut. This observation further highlights that cherry extract is more similar to mimosa and that the main fractions are richer in carbohydrates.
In summary, the above reported data indicates that total phenolic content is similar between chestnut and mimosa, this means that the chestnut extract is made of polyphenols covalently combined with small moieties of carbohydrates, while mimosa is made by a mixture of macromolecules: sugar-free shorter chains and heavier fraction dominated by polyphenolics combined with some saccharide unit. Cherry has the strongest carbohydrate content and also sugar-free short chain, meaning that average to high molecular mass units are constituted of (covalently) bonded polyphenolic and saccharide moieties (here saccharides are proportionally more than in mimosa).
A schematic representation of these structures is reported in Fig. 5.
Thermogravimetric analysis (TGA)
The weight loss (TGA) and the first derivate weight loss (D-TGA) are reported as a function of the temperature in Fig. 6.
The three thermograms have similar trend of a relatively constant degradation. Cherry degrades slightly faster and reaches 36% of its original weight at 800 °C. Mimosa and chestnut are more stable until 500 °C, then chestnut degrades reaching similar weight loss to cherry, while mimosa is more stable registering a weight of 41% at the end of the experiment.
A significant degradation occurs at 260 °C for all three extracts. This signal is probably due to the decarboxylation of the RCOOH groups with the release of carbon dioxide (Garro Galvez et al. 1997). Chestnut differs from the mimosa in the presence of a second peak at around 298 °C which suggest that gallic acid derivatives may require higher temperatures to complete decarboxylation. The behaviour of cherry tannin seems to be intermediate. Degradation starts between 150 and 200 °C because of the presence of polysaccharide which starts degrading at lower temperature than the polyphenolic compounds (Gaugler and Grigsby 2009). The late degradation starts from 400 °C and occurs similarly for the three powders. Here, the weight loss is related to the oxidation of the residual carbon (Tondi et al. 2008b), while the major resistance of the mimosa is due to the easier arrangement in carbon networks due to the more compacted three-dimensional arrangement of the condensed tannin, which present already aggregates of rings (Konai et al. 2016).
Conclusion
In this study, a cherry (Prunus avium L.) extract was obtained and compared with two commercial wood extracts, namely one mostly composed of condensed tannin (Acacia mimosa De Wild.) and one typically constituted of hydrolysable tannins (Castanea sativa Mill.). The resulted cherry extract was constituted of condensed tannins with an important amount of carbohydrates strongly linked to the flavonoids. HRSEC analysis of the extract suggested the presence of probably covalent interactions between the phenolic and the saccharide moieties. Comparing the cherry extract with that of mimosa, the flavonoids of cherry are proportionally richer in hydroxy groups in the B-ring; hence, we suppose few − OH in the A-ring and a higher proportion of pyrogallic units. A lower cross-linking degree for cherry was also observed, possibly due to the higher proportion of carbohydrates in its structure. This abundance of carbohydrates was deemed responsible for the lower antioxidant capacity and thermal resistance of this extract. These findings shed light on the cherry extract that could be applied as milder antioxidant or as building block for bio-composites.
References
Amaral-Labat G, Szczurek A, Fierro V et al (2013) Systematic studies of tannin-formaldehyde aerogels: preparation and properties. Sci Technol Adv Mater 14:015001. https://doi.org/10.1088/1468-6996/14/1/015001
Arbenz A, Avérous L (2015) Chemical modification of tannins to elaborate aromatic biobased macromolecular architectures. Green Chem 17:2626–2646
Auad P, Spier F, Gutterres M (2020) Vegetable tannin composition and its association with the leather tanning effect. Chem Eng Commun 207:722–732. https://doi.org/10.1080/00986445.2019.1618843
Ayestarán B, Guadalupe Z, León D (2004) Quantification of major grape polysaccharides (Tempranillo v.) released by maceration enzymes during the fermentation process. Anal Chim Acta 513:29–39. https://doi.org/10.1016/j.aca.2003.12.012
Bianchi S, Kroslakova I, Janzon R, Mayer I, Saake B, Pichelin F (2015) Characterization of condensed tannins and carbohydrates in hot water bark extracts of European softwood species. Phytochemistry 120:53–61. https://doi.org/10.1016/j.phytochem.2015.10.006
Campo M, Pinelli P, Romani A (2016) Hydrolyzable tannins from sweet chestnut fractions obtained by a sustainable and eco-friendly industrial process. Nat Prod Commun 11:409–415. https://doi.org/10.1177/1934578x1601100323
Cesprini E, Šket P, Causin V, Zanetti M, Tondi G (2021) Development of quebracho (Schinopsis balansae) tannin-based thermoset resins. Polymers 13:4412. https://doi.org/10.3390/polym13244412
Cesprini E, Baccini R, Urso T, Zanetti M, Tondi G (2022) Quebracho-based wood preservatives : effect of concentration and hardener on timber properties. Coatings 12:568. https://doi.org/10.3390/coatings12050568
Cook NC, Samman S (1996) Flavonoids-chemistry, metabolism, cardioprotective effects, and dietary sources. J Nutr Biochem 7:66–76. https://doi.org/10.1016/0955-2863(95)00168-9
Davis AL, Cai Y, Davies AP, Lewis JR (1996) 1H and 13C NMR assignments of some green tea polyphenols. Magn Reson Chem 34:887–890. https://doi.org/10.1002/(SICI)1097-458X(199611)34:11%3c887::AID-OMR995%3e3.0.CO;2-U
De Iseppi A, Marangon M, Vincenzi S et al (2021) A novel approach for the valorization of wine lees as a source of compounds able to modify wine properties. Lwt 136:110274. https://doi.org/10.1016/j.lwt.2020.110274
De Rosso M, Marangon M, Flamini R (2020) Characterization of ellagitannins and oak lactone precursors in oak wood-aged grappa by high-resolution mass spectrometry. J Mass Spectrom 55:4472. https://doi.org/10.1002/jms.4472
De Rosso M, Panighel A, Della Vedova A et al (2009) Changes in chemical composition of a red wine aged in acacia, cherry, chestnut, mulberry, and oak wood barrels. J Agric Food Chem 57:1915–1920. https://doi.org/10.1021/jf803161r
Drewes S, Roux D (1963) Condensed tannins. 15. interrelationships of flavonoid components in wattle-bark extract. Biochem J 87:167–172. https://doi.org/10.1042/bj0870167
Falcão L, Araújo MEM (2014) Application of ATR-FTIR spectroscopy to the analysis of tannins in historic leathers: the case study of the upholstery from the 19th century portuguese royal train. Vib Spectrosc 74:98–103. https://doi.org/10.1016/j.vibspec.2014.08.001
Falcão L, Araújo MEM (2018) Vegetable tannins used in the manufacture of historic leathers. Molecules 23:8–10. https://doi.org/10.3390/molecules23051081
Galiñanes C, Freire MS, González-Álvarez J (2015) Antioxidant activity of phenolic extracts from chestnut fruit and forest industries residues. Eur J Wood Prod 73:651–659. https://doi.org/10.1007/s00107-015-0936-7
Garro Galvez JM, Riedl B, Conner AH (1997) Analytical studies on tara tannins. Holzforschung 51:235–243
Gaugler M, Grigsby WJ (2009) Thermal degradation of condensed tannins from radiata pine bark. J Wood Chem Technol 29:305–321. https://doi.org/10.1080/02773810903165671
Giacosa S, Parpinello GP, Río Segade S et al (2021) Diversity of Italian red wines: a study by enological parameters, color, and phenolic indices. Food Res Int 143:110277. https://doi.org/10.1016/j.foodres.2021.110277
Guerrero ED, Chinnici F, Natali N et al (2008) Solid-phase extraction method for determination of volatile compounds in traditional balsamic vinegar. J Sep Sci 31:3030–3036. https://doi.org/10.1002/jssc.200800307
Hagerman AE, Zhao Y, Johnson S (1997) Methods for determination of condensed and hydrolyzable tannins. In: Shahidi F (ed) Antinutrients and phytochemicals in food. American Chemical Society, Washington, DC, pp 209–222. https://doi.org/10.1021/bk-1997-0662.ch012
Harbertson JF, Parpinello GP, Heymann H, Downey MO (2012) Impact of exogenous tannin additions on wine chemistry and wine sensory character. Food Chem 131:999–1008. https://doi.org/10.1016/j.foodchem.2011.09.101
Haslam E (1998) Practical polyphenolics: from structure to molecular recognition and physiological action, 1°. Cambridge University Press
Katalinić V, Možina SS, Skroza D et al (2010) Polyphenolic profile, antioxidant properties and antimicrobial activity of grape skin extracts of 14 Vitis vinifera varieties grown in Dalmatia (Croatia). Food Chem 119:715–723. https://doi.org/10.1016/j.foodchem.2009.07.019
Konai N, Raidandi D, Pizzi A et al (2016) Thermogravimetric analysis of anningre tannin resin. Maderas Cienc y Tecnol 18:245–252. https://doi.org/10.4067/S0718-221X2016005000022
Konai N, Raidandi D, Pizzi A, Meva’a L, (2017) Characterization of Ficus sycomorus tannin using ATR-FT MIR, MALDI-TOF MS and 13C NMR methods. Eur J Wood Prod 75:807–815. https://doi.org/10.1007/s00107-017-1177-8
Koopmann AK, Malfait WJ, Sepperer T, Huesing N (2021) A systematic study on bio-based hybrid aerogels made of tannin and silica. Materials 14(18):5231. https://doi.org/10.3390/ma14185231
Kotar A, Kotar M, Sket P, Plavec J (2014) Potential of solid-state NMR and SEM in characterization of tablets of ibuprofen. Curr Pharm Anal 11:124–130. https://doi.org/10.2174/1573412910666141111231325
Li X, Nicollin A, Pizzi A et al (2013) Natural tannin-furanic thermosetting moulding plastics. RSC Adv 3:17732–17740. https://doi.org/10.1039/c3ra43095b
Li L, Li Z, Wei Z et al (2020) Effect of tannin addition on chromatic characteristics, sensory qualities and antioxidant activities of red wines. RSC Adv 10:7108–7117. https://doi.org/10.1039/c9ra09846a
Maksimovíc Z, Maleňcíc D, Kovǎcevíc N (2005) Polyphenol contents and antioxidant activity of Maydis stigma extracts. Bioresour Technol 96:873–877. https://doi.org/10.1016/j.biortech.2004.09.006
Michel J, Jourdes M, Silva MA et al (2011) Impact of concentration of ellagitannins in oak wood on their levels and organoleptic influence in red wine. J Agric Food Chem 59:5677–5683. https://doi.org/10.1021/jf200275w
Missio AL, Tischer B, dos Santos PSB, Codevilla C, de Menezes CR, Barin JS, Haselein CR, Labidi J, Gatto DA, Petutschnigg A, Tondi G (2017) Analytical characterization of purified mimosa (Acacia mearnsii) industrial tannin extract: single and sequential fractionation. Sep Purif Technol 186:218–225. https://doi.org/10.1016/j.seppur.2017.06.010
Molino S, Fernández-Miyakawa M, Giovando S, Rufián-Henares JÁ (2018) Study of antioxidant capacity and metabolization of quebracho and chestnut tannins through in vitro gastrointestinal digestion-fermentation. J Funct Foods 49:188–195. https://doi.org/10.1016/j.jff.2018.07.056
Muller-Harvey I (2001) Analysis of hydrolizable tannins. Anim Feed Sci Technol 91:3–20
Navarrete P, Pizzi A, Pasch H et al (2010) MALDI-TOF and 13C NMR characterization of maritime pine industrial tannin extract. Ind Crops Prod 32:105–110. https://doi.org/10.1016/j.indcrop.2010.03.010
Panzella L, Moccia F, Toscanesi M et al (2019) Exhausted woods from tannin extraction as an unexplored waste biomass: evaluation of the antioxidant and pollutant adsorption properties and activating effects of hydrolytic treatments. Antioxidants 8:10–14. https://doi.org/10.3390/antiox8040084
Pizzi A (1980) Tannin-based adhesives. J Macromol Sci Part C C18:247–315
Pizzi A (1994) Advanced wood adhesives technology, 1st edn. CRC Press, Florida
Pizzi A (2019) Tannins: Prospectives and actual industrial applications. Biomolecules 9(8):344. https://doi.org/10.3390/biom9080344
Ricci A, Olejar KJ, Parpinello GP et al (2015) Application of fourier transform infrared (FTIR) spectroscopy in the characterization of tannins. Appl Spectrosc Rev 50:407–442. https://doi.org/10.1080/05704928.2014.1000461
Rinaldi A, Moio L (2018) Effect of enological tannin addition on astringency subqualities and phenolic content of red wines. J Sens Stud 33:1–11. https://doi.org/10.1111/joss.12325
Santos J, Antorrena G, Freire MS et al (2017) Environmentally friendly wood adhesives based on chestnut (Castanea sativa) shell tannins. Eur J Wood Prod 75:89–100. https://doi.org/10.1007/s00107-016-1054-x
Schwanninger M, Rodrigues JC, Pereira H, Hinterstoisser B (2004) Effects of short-time vibratory ball milling on the shape of FT-IR spectra of wood and cellulose. Vib Spectrosc 36:23–40. https://doi.org/10.1016/j.vibspec.2004.02.003
Sepperer T, Hernandez-Ramos F, Labidi J et al (2019a) Purification of industrial tannin extract through simple solid-liquid extractions. Ind Crops Prod 139:111502. https://doi.org/10.1016/j.indcrop.2019.111502
Sepperer T, Neubauer J, Eckardt J et al (2019b) Pollutant absorption as a possible end-of-life solution for polyphenolic polymers. Polymers 11:911. https://doi.org/10.3390/polym11050911
Shirmohammadli Y, Efhamisisi D, Pizzi A (2018) Tannins as a sustainable raw material for green chemistry: a review. Ind Crops Prod 126:316–332. https://doi.org/10.1016/j.indcrop.2018.10.034
Singleton VL, Orthofer R, Lamuela-Raventos RM (1999) Analysis of total phenols and other oxidation substrates and antioxidants by means of folin-ciocalteu reagent. Methods Enzymol 299:152–178. https://doi.org/10.1016/j.scienta.2016.11.004
Sommerauer L, Thevenon MF, Petutschnigg A, Tondi G (2019) Effect of hardening parameters of wood preservatives based on tannin copolymers. Holzforschung 73:457–467. https://doi.org/10.1515/hf-2018-0130
Tiam Kapen P, Nandou Tenkeu M, Yadjie E, Tchuen G (2021) Production and characterization of environmentally friendly charcoal briquettes obtained from agriculture waste: case of Cameroon. Int J Environ Sci Technol 19(6):5253–5260. https://doi.org/10.1007/s13762-021-03497-7
Tondi G (2017) Tannin-based copolymer resins: synthesis and characterization by solid state 13C NMR and FT-IR spectroscopy. Polymers 9(6):223. https://doi.org/10.3390/polym9060223
Tondi G, Petutschnigg A (2015) Middle infrared (ATR FT-MIR) characterization of industrial tannin extracts. Ind Crops Prod 65:422–428. https://doi.org/10.1016/j.indcrop.2014.11.005
Tondi G, Pizzi A, Olives R (2008a) Natural tannin-based rigid foams as insulation for doors and wall panels. Maderas Cienc y Tecnol 10:219–228. https://doi.org/10.4067/S0718-221X2008000300005
Tondi G, Pizzi A, Pasch H, Celzard A (2008b) Structure degradation, conservation and rearrangement in the carbonisation of polyflavonoid tannin/furanic rigid foams-A MALDI-TOF investigation. Polym Degrad Stab 93:968–975. https://doi.org/10.1016/j.polymdegradstab.2008.01.024
Tondi G, Wieland S, Wimmer T et al (2012) Tannin-boron preservatives for wood buildings: mechanical and fire properties. Eur J Wood Prod 70:689–696. https://doi.org/10.1007/s00107-012-0603-1
Tondi G, Thevenon MF, Mies B et al (2013) Impregnation of Scots pine and beech with tannin solutions: effect of viscosity and wood anatomy in wood infiltration. Wood Sci Technol 47:615–626. https://doi.org/10.1007/s00226-012-0524-5
Tondi G, Link M, Kolbitsch C et al (2016) Pilot plant up-scaling of tannin foams. Ind Crops Prod 79:211–218. https://doi.org/10.1016/j.indcrop.2015.11.013
Vázquez G, Fontenla E, Santos J et al (2008) Antioxidant activity and phenolic content of chestnut (Castanea sativa) shell and eucalyptus (Eucalyptus globulus) bark extracts. Ind Crops Prod 28:279–285. https://doi.org/10.1016/j.indcrop.2008.03.003
Vázquez G, Pizzi A, Freire MS et al (2013) MALDI-TOF, HPLC-ESI-TOF and 13C-NMR characterization of chestnut (Castanea sativa) shell tannins for wood adhesives. Wood Sci Technol 47:523–535. https://doi.org/10.1007/s00226-012-0513-8
Versari A, Du Toit W, Parpinello GP (2013) Oenological tannins: a review. Aust J Grape Wine Res 19:1–10. https://doi.org/10.1111/ajgw.12002
Vignault A, González-Centeno MR, Pascual O et al (2018) Chemical characterization, antioxidant properties and oxygen consumption rate of 36 commercial oenological tannins in a model wine solution. Food Chem 268:210–219. https://doi.org/10.1016/j.foodchem.2018.06.031
Wawer I, Wolniak M, Paradowska K (2006) Solid state NMR study of dietary fiber powders from aronia, bilberry, black currant and apple. Solid State Nucl Magn Reson 30:106–113. https://doi.org/10.1016/j.ssnmr.2006.05.001
Yu B, Zhang Y, Shukla A et al (2001) The removal of heavy metals from aqueous solutions by sawdust adsorption-removal of lead and comparison of its adsorption with copper. J Hazard Mater 84:83–94. https://doi.org/10.1016/S0304-3894(01)00198-4
Zanetti M, Causin V, Saini R et al (2014) Effect of tannin on increasing UF adhesive performance at high temperature investigated by TMA and TGA analysis. Eur J Wood Prod 72:385–392. https://doi.org/10.1007/s00107-014-0795-7
Zanetti M, Cesprini E, Marangon M et al (2021) Thermal valorization and elemental composition of industrial tannin extracts. Fuel 289:119907. https://doi.org/10.1016/j.fuel.2020.119907
Zhang LL, Lin YM, Zhou HC et al (2010) Condensed tannins from mangrove species Kandelia candel and Rhizophora mangle and their antioxidant activity. Molecules 15:420–431. https://doi.org/10.3390/molecules15010420
Acknowledgements
The authors gratefully acknowledge the support of the project BIRD 2021 funds, TESAF department, University of Padua—Italy, as well as the CERIC-ERIC Consortium for access to solid state 13C-NMR experimental facilities.
Funding
Open access funding provided by Università degli Studi di Padova within the CRUI-CARE Agreement.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that in this article there are no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Cesprini, E., De Iseppi, A., Giovando, S. et al. Chemical characterization of cherry (Prunus avium) extract in comparison with commercial mimosa and chestnut tannins. Wood Sci Technol 56, 1455–1473 (2022). https://doi.org/10.1007/s00226-022-01401-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-022-01401-1