Abstract
Analyzing the development of cohesive strength of polymeric diphenylmethane diisocyanate (pMDI) with the help of the small-scale test method proposed in the standard (ASTM-D7998-15) is rarely used, as resulting lap-shear strength values are lacking in informative value. Up to now, the gained strength values have been dramatically below the ones typically achieved with most standard wood adhesives, while the performance in a panel product is at least of equal quality. In order to address this discrepancy and to fill the gap of the lacking method to properly investigate curing behavior of pMDI adhesives, a modification of the specimen geometry for pMDI adhesive analysis is proposed, resulting in meaningful tensile shear strength values also when using pMDI as adhesive. Applying this modified specimen geometry enables investigating relevant processing parameters, such as the effect of press time, press temperature and wood moisture content on the tensile shear strength development using pMDI. The strength development was found to be positively affected by all three factors significantly. Furthermore, the calculated reactivity index showed a decrease in activation energy of 20% with increasing wood moisture content. Based on the results gained, it can be concluded that both, wood moisture and temperature, are crucial factors to accelerate curing of pMDI. Even so the principle influence of the exemplary selected parameters has already been shown earlier, the new methodology opens up new possibilities in investigating the curing behavior of pMDI.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
The development of cohesive strength of a thermosetting adhesive usually takes place during the curing process under the influence of temperature, pressure and time, whereby the pressing time strongly depends on the properties of the binder (reactivity of the system, molar ratio, amount of hardener, solid content, additives, degree of condensation, etc.). Additionally, the wood substrate (thickness of the composite, wood species, extractives, moisture content, etc.) (Dunky 2002; Humphrey and Shan 1989; Jost and Sernek 2008) influences the curing behavior. To examine the mechanical strength development as a result of the chemical curing of an adhesive, the automated bonding evaluation system (ABES) proposed by Humphrey (1990) has been shown to be a powerful and versatile method. The method has been standardized and is defined in detail in ASTM-D7998-15 (2015). The testing principle is based on hot pressing and mechanically testing of a single lap-shear assembly using thin veneer stripes (~ 0.6 mm). The tenuity enables a nearly isothermal evaluation of the bonding strength development. Derived from the measured values, Humphrey and Shan (1989) defined the reactivity index (Ri), which shows the relationship between the strength development rate to the press temperature and can be further used for simulation models.
Polymeric diphenylmethane diisocyanate (pMDI) is a widely used adhesive in the wood-based panel industry. PMDI possesses good wetting behavior, good penetration capacity and good mechanical bonding. Furthermore, it shows high reactivity, good water stability and no formaldehyde emission in the cured state (Deppe 1977; Deppe and Ernst 1971; Dunky 2002; Pizzi 2014). Isocyanates are capable of reacting with numerous nucleophiles (Frazier 2003). The isocyanate groups R–N=C=O in pMDI are highly reactive to all compounds containing active hydrogen. A possible reaction is the reaction of the isocyanate group with a hydroxyl group, which leads to the formation of urethane. Such a reaction can theoretically take place between isocyanate groups and OH groups of cellulose or lignin, while forming covalent bonds. Such reactions only occur under very specific conditions, i.e., high temperature and almost the absence of moisture (Yelle et al. 2011a, b). Wendler and Frazier (1996) and Bao et al. (2003) showed with nuclear magnetic resonance spectroscopy (NMR) that the major components from the curing of pMDI in wood are structures arising from the reaction with water, while follow-up reaction products were formed at higher temperature and low moisture content. Yelle et al. (2011b) also figured out, that upon rising moisture content and decreasing ratio of isocyanate and OH groups, the occurrence of urethane structures rapidly decreases. Another study by Li et al. (2017) investigated the influence of recycled polyol on the curing rate and bonding strength of pMDI with different NCO/OH ratios and found that adhesives with slightly excessive isocyanate groups showed better bonding performance between the wood substrate and adhesive materials. The essential curing reaction relevant for actual wood bonding takes place via water by forming amine bonds with simultaneous elimination of carbon dioxide (CO2). The amine intermediate further reacts with isocyanate groups forming polyurea structures. The reaction with water is preferential over any of the other hydroxyl-containing compounds in wood, which was demonstrated in various studies using infrared spectroscopy and differential scanning calorimetry (Daniel-da-Silva et al. 2007, 2008; He and Yan 2005). With regard to the effect of moisture content on mechanical properties of bonds, Smith (2007) found that a high amount of moisture is necessary to obtain relevant lap-shear strength. In his study, adherends had to be conditioned to 23% moisture content, to develop a comparably low strength value. The need of such high moisture values does not reflect the conditions needed to cure pMDI in an industrial process at all. On the contrary, Papadopoulos (2007) could not find any significant effect of wood moisture content in the range of 7–13% on the internal bond strength of fully cured particle boards in his study.
Besides the positive effect of increasing moisture content on the bonding strength, Smith (2007) also found an increase in bonding strength with increasing press temperature and time, but a strength decrease with higher pMDI resin loads. From the mechanical performance of pMDI bonded particle boards and oriented strand boards (OSB), high internal bond strength levels have been reported even for very low resin amounts (2–6 wt%) (Papadopoulos 2007). When investigating the bonding strength development of pMDI using ABES, the measured tensile shear strength reaches a maximum value of 1.5 N/mm2 (Smith 2005, 2007) have been reported, which is significantly below the values of urea formaldehyde and phenol formaldehyde ranging from 5 to 8 N/mm2 (Ferra et al. 2011; Jost and Sernek 2008; Rohumaa et al. 2014; Stöckel et al. 2010). The low tensile shear strength value does not reflect experience of final product performance in wood composite industry, where pMDI performs similar to urea formaldehyde and phenol formaldehyde resins, while applying significantly lower resin loads (Pizzi 2014).
Based on the above-described findings on accelerated curing of pMDI by water, higher achievable strength values by increasing the sample geometry are hypothesized. With the approach of increased specimen volume a higher amount of moisture should be available for more complete pMDI curing. As a consequence, it is aimed for comparable results of pMDI investigations to those typically achieved when investigating formaldehyde-based binders. The primary focus of the present work is to investigate the applicability of a modified specimen geometry to analyze the cohesive strength development of pMDI. Using the example of moisture content and press temperature, the tensile shear strength development was investigated. To obtain further information on pMDI curing kinetics, the reactivity index and the activation energy were calculated and analyzed to underline the dependence of bonding rate on the moisture content of the wood substrate.
Materials and methods
Materials
For this study, a solvent-free 4,4′-diphenylmethane diisocyanate (pMDI; Lupranate M20FB) with average functionality of 2.7 and a NCO content of 31.8 g/100 g from BASF AG (Ludwigshafen, Germany) was used. The viscosity of 220 ± 5 mPa*s was determined with a cone plate rheometer (Bohlin CVO; Malvern Institute Limited, Malvern, UK) at 20 °C with a shear rate of 200 1/s. The adhesive density was 1.23 g/cm3.
Birch veneers (Betula L.) with a thickness of 1.5 mm were acquired from J. u. A. Frischeis GmbH (Stockerau, Austria). The veneers were selected by focusing on homogenous and straight fiber alignment and had an average density of 664 ± 10 kg/m3.
Development of bonding strength
To evaluate the bonding strength as a function of press time and press temperature, a self-constructed hot-press was mounted in a Zwick/Roell Z100 universal testing machine (Zwick GmbH & Co KG, Ulm, Germany). This specific setup was selected in order to comply with the testing method described in ASTM-D7998-15 (2015) as originally proposed by Humphrey (1990), but allowing for higher loads and bigger specimen geometries and overlapping dimensions.
Prior to starting the investigation, preliminary test series were done to create a test design with limited parameters in order to keep the number of samples rejected during the main test low and to generate reliable data. Therefore, veneers of different wood species (spruce, beech and birch) were tested, whereby the wood moisture content (12, 6 and 3%), press temperature (100 to 160 °C) and spread rate (100 to 200 g/m2) were varied. The sample geometry of the lap joint specimens for the preliminary tests was based on standard ASTM D7998-15 specimens (thickness: 0.6 mm, overlap: 5 to 10 mm). This preliminary test has demonstrated that a range of material, thickness, (high) spread rate and humidity combination does not result in proper curing of pMDI. Significantly higher tensile shear strength values have been achieved by increasing the sample geometry compared to standard specifications (veneer thickness: 0.6–0.8 mm; veneer width: 20 mm) to a width of 35 mm with a thickness of 1.5 mm. Furthermore, birch was used contrarily to the standard specification (wood species: beech (Fagus sylvatica) or maple (Acer sacharum)). In addition, the test speed was reduced from approx. 50 to 10 mm/min, which again enabled higher overall strength values and a better differentiation between parameters.
To determine the influence of moisture content (8, 12 and 16%) on the strength development, the following parameters shown in Table 1 were defined. Thereto, veneer stripes were stored in a climate chamber (type: 37240099310, WTB-Binder GmbH, Tuttlingen, Germany) for one week. Considering the Keylwerth diagram (Keylwerth and Noack 1963), which is based on spruce wood, the climate conditions were adjusted to 35 °C and 47% rh to obtain 8% moisture content and to 35 °C and 84% rh to adjust to 16% moisture content. For 12% moisture content, samples were stored under standardized climate conditions (20 °C, 65% rh). The actual wood moisture content was then verified with the kiln-dry method according to ISO-3130 (1994).
For measuring the cohesive strength development, one veneer stripe was coated with 100 g/m2 of pMDI and glued together with a second one considering an overlap of 10 mm. The samples were hot pressed at three different temperatures (110, 120, 130 °C) for 75, 150, 300 and 600 s, respectively, at a specific pressure of 2.7 N/mm2. With a very short transition from press opening to the onset of pulling force, the tensile shear strength of the sample was determined at a testing speed of 10 mm/min. As the standard test method does not include cooling before bond strength testing, consequently, the joint is still in a hot state. Each parameter combination was examined using five repetitions. The results were analyzed by performing a univariate analysis of variances (ANOVA) with an α-value of 0.05 using SPSS® software.
Results and discussion
Development of bonding strength
The modified specimen geometry for analyzing the cohesive strength development of pMDI under the influence of temperature and moisture content of the samples was applied. Figure 1 illustrates the development of lap-shear strengths of the tested samples as a function of press time. As an overall trend, a high influence of press time, moisture content as well as of press temperature is noticeable. The displayed results show that for all temperature levels higher strength values are achieved with increasing wood moisture content. Furthermore, a steeper slope in the development of bonding strength can be observed with increasing press temperature. While strength develops relatively slowly at 8% humidity and 110 °C press temperature, an acceleration occurs at the same humidity using 130 °C. The maximum strength values observed are in the range of 6 to 7 N/mm2, representing an apparent plateau (e.g., at 16% and 130 °C, Fig. 1). This flattening is attributed to the limited strength of the birch veneer adherends, since the wood failure amount reached almost 100% at the corresponding strength levels. In order to reach the maximum observable tensile shear strength level of approximately 6–7 N/mm2 of the configuration used, 10-min press time was necessary at a wood moisture content of 16% and a press temperature of 110 °C, while only half of the time (5 min) was needed when press temperature was increased to 130 °C at the same moisture content level. The same difference in pressing time necessary to reach ultimate performance was noticed when increasing the moisture content from 12 to 16% using the same pressing temperature of 130 °C.
Further measurements (not displayed) showed that by extending the press time to 20 min, configurations possessing a low moisture content of 8% and hot pressed at 110 °C, exhibit a continuation of the almost linear, weak strength increase. Thereafter, it cannot be excluded that with sufficient press time, pMDI can also develop substantial strength levels within the lower humidity and temperature conditions investigated here.
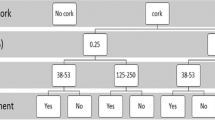
The strength values were further analyzed by performing an ANOVA considering each factor. The statistical analysis confirms a highly significant influence (p value < 0.001) of all factors (press time, press temperature and moisture content) on tensile shear strength. The relative impact of the three factors on the response strength value was positive. Likewise, all two-factorial interactions have also been proven to be highly significant influencing factors (Fig. 2).
Since the results in Fig. 1 show that the curing reaction of pMDI is accelerated by temperature, the equation of Arrhenius can be used to describe the regressed bonding rate. To investigate this dependency on moisture content of the samples, the natural logarithm of the inverse strength development rate (N/mm2s)—focusing only on the constant, nearly linear strength increase in the curves—was plotted against the reciprocal absolute temperature (K) for each moisture content. The curves show an almost parallel decrease in the regressed bonding strength and thus the same slope, or rate, but with difference in the y-intercept. As a result, an almost linear correlation for each individual moisture content level (R2 ≥ 0.98) was found. Since the measurements were taken at different press temperatures, the temperature dependence of the mechanical curing rate is represented in the form of the Arrhenius equation, where Φ is the rate of bonding strength development (MN/m2 s), A is the pre-exponential factor tentatively set at 1 (MN/m2 s), Ea is the activation energy (J/mol), R is the universal gas constant (8.314 J/K mol), and T is the absolute temperature (K).
Humphrey and Shan (1989) proposed to calculate the reactivity index (Ri = Ea/R), which describes the responsiveness of a physical system or parameter to temperature.
For the pMDI system examined here, Ri was calculated for each moisture content level and resulted in 5.3 × 103 (K) for both, 12% and 16%, and 6.5 × 103 (K) for 8% moisture content, respectively. Multiplying Ri with the gas constant, an activation energy of 44 kJ/mol for both, 12% and 18%, and 54 kJ/mol for 8% was obtained.
The present investigation supports the findings by Smith (2007), who concluded that the bonding strength increases with press temperature, press time and moisture content. Considering the tensile lap-shear strengths, however, his best combination achieved values in the range of 1.4 N/mm2, with the need of an extremely high moisture content of 23%. Similar results were achieved during preliminary tests from these authors, when using veneers with a thickness of only approx. 0.7 mm. Only when specimen dimensions were increased to 1.5 mm by 35 mm, and the overlapping area to 10 mm, similar strength values to the typically observed ones for urea formaldehyde (UF) and phenol formaldehyde (PF)-based adhesives could be achieved (Humphrey and Shan 1989; Stöckel et al. 2010).
According to the standard test method, thin veneers are used to achieve pure lap-shear. As known from numerical predictions, for example simplified Volkersen equation (Stöckel et al. 2013), peak stresses occur depending on substrate thickness and joint length. Furthermore, with increasing substrate thickness, the induced bending moment increases, leading to additional stresses, which additionally reduce joint strength. Taking into account the adapted sample dimensions used in the present study, this would result in an increased stress concentration in the lap-shear assembly of about 30%. The consequence of the change in specimen geometry should therefore result in reduced strength values. However, due to a dominating improved curing behavior of the pMDI adhesive clearly the opposite was observed in the present study. It is therefore assumed that a substantial improvement in the bonding strength of pMDI bonded veneer lap joints originates from a different mechanism involved in pMDI curing that can be achieved by using the enlarged sample geometry only. It is proposed that the increased volume in combination with the typically applied high press temperature allows for retaining wood moisture comparably longer in the adherend and consequently close to the bond line compared to the smaller dimensions proposed in the standard. At temperatures above 110 °C, the moisture in thin veneer strips is assumed to evaporate within an extremely short time, leaving too little time for the pMDI to react with the water and resulting in incomplete formation of a polymer network. The higher water-holding capacity of the veneers with bigger dimension may have an impact on the availability of water for the curing reaction, which could further justify differences between species. As moisture is known to be crucial for pMDI curing, its longer availability compared to standard test setups may be the key factor allowing for proper cure of pMDI using veneer-based tensile shear test setup.
One disadvantage caused by the enlarged veneer thickness is a slower temperature increase in the bond line compared to the thin veneer adherends. Figure 3 shows the measured temperature development in the glued joint of 0.6-mm beech veneer compared to the glued joint temperature of the birch veneer used in this study. Both veneers were previously conditioned in the standard climate (20 °C, 65% rh) in order to achieve an equilibrium moisture content of 12%. The curves show that with thin veneers the desired (press) temperature of 120 °C deviates by 2 °C, whereas the 1.5-mm-thicker wood joints achieve only 90% of the required press temperature. The same applies to the pressing temperatures of 110 °C and 130 °C, at which the 1.5-mm-thick veneer resulted in a 10% lower bond line temperature (not shown). In addition, it can be seen that the beech reference already reached its temperature plateau (118 °C) after 10 s, while the thicker birch veneer needed twice as long. Therefore, the temperature within the bond line of the thicker veneer adherends is more distant to isothermal testing conditions as the thin ones, which must be taken into account when evaluating the results. With reference to the temperature in the adhesive joint, it should be noted that all samples were tested hot. A study by Solt et al. (2019) investigating bonding strength of lignin-based phenol formaldehyde as a function of press time, has shown that the testing temperature can have a significant influence on the measured bond strength of adhesives. Likewise, it is known from the literature that strength properties of wood depend significantly on temperature. However, in this study joints were tested according to the standard method ASTM-D7998-15 (2015) and the influence of temperature on the tensile shear strength of the joint was not further evaluated.
Temperature development in the adhesive bond line between 0.6-mm beech veneers—according to ASTM-D7998-15 (2015)—in comparison with 1.5-mm birch veneers at 120 °C press temperature
The present results further support the strong interaction between moisture content and curing rate, which have already been identified in earlier studies by Smith (2005, 2007) using lap joints or Gruver and Brown (2007) using compression shear blocks. Both of them also examined the adhesive strength of samples at almost 0% moisture and found that the resin does not form a distinct polymer network under these dry conditions, resulting in poor shear strength. This finding corresponds to the results of preliminary tests in the present study, where almost all thin veneer samples failed to bond even using long press times. It is assumed that the adhesive is only partially cured and no complete network could be formed. Due to the absence of moisture, pMDI has the possibility to react mainly with OH groups of wood and has limited access to bonded water, which slows down the reaction (He and Yan 2005; Weaver and Owen 1995; Yelle et al. 2011a). Furthermore, He and Yan (2005) showed that with increasing wood moisture, the activation energy—measured by DSC—increased to a value of Ea 63 kJ/mol at a wood moisture content of 12%. This finding is well in line with the present results of the calculated activation energy regarding bond rate, whereas He and Yan (2005) reached slightly higher values. As moisture increases, the preferred reaction between NCO groups and water to form amines and further react to polyurea structures can take place and results in a better network in the wood substrate and higher adhesive and consequently bond strengths. Interestingly, this positive effect of moisture could only be determined with solid wood samples but not in particle boards (Papadopoulos 2007). A possible explanation for this observation could be that particle boards are hot pressed in a closed system where a loss of humidity is limited. This stands in contrast to short diffusion pathways for single veneer samples where a loss of humidity may easily occur. Therefore, it is assumed that sufficient humidity was available for all moisture content levels in particleboard production leading to complete cure for all cases, whereas a lack of humidity can lead to incomplete cure of pMDI in veneer-based setups. Furthermore, the positive effect of temperature was already observed by Smith (2007), who concluded that at higher temperatures, more thermal energy is available to accelerate the evaporation of the absorbed moisture in the wood substrate, while simultaneously more polyurea can be formed. Bao et al. (2003) also noted that isocyanate reacts more rapidly at 160 °C than at lower temperatures, whereby the available water molecules already reacted with NCO groups, so the urea groups are the only available reactive group. Therefore, the isocyanate can react to form biuret.
Conclusion
The increase in specimen size compared to conventional ASTM-D7998-15 (2015) specimens led for the first time to similar lap joint strength values when using pMDI to the ones typically observed with UF and PF adhesives. This modification enables an effective investigation of parameters involved in the bonding development of pMDI lap joints. It is assumed that the increased sample volume is contributing to a more complete curing of pMDI adhesive, as more water is available for the curing reaction. The development of the bonding strength showed that the curing rate of pMDI is significantly accelerated by the factors press temperature and wood moisture content. A lack in moisture is resulting in incomplete curing and low tensile shear strength values. In addition, it could be shown that by increasing the sample moisture content from 8 to 16%, a lower activation energy is needed for curing, while between 8 and 12%, no change in activation energy could be observed.
References
ASTM-D7998-15 (2015) Standard test method for measuring the effect of temperature on the cohesive strength development of adhesives using lap shear bonds under tensile loading. ASTM International, West Conshohocken
Bao S, Daunch WA, Sun Y, Rinaldi PL, Marcinko JJ, Phanopoulos C (2003) Solid state two-dimensional NMR studies of polymeric diphenylmethane diisocyanate (PMDI) reaction in wood. Forest Prod J 53:63–71
Daniel-da-Silva AL, Bordado JCM, Martín-Martínez JM (2007) Use of isoconversional methods to analyze the cure kinetics of isocyanate-ended quasi-prepolymers with water. J Appl Polym Sci 104:1049–1057. https://doi.org/10.1002/app.24914
Daniel-da-Silva AL, Bordado JCM, Martín-Martínez JM (2008) Moisture curing kinetics of isocyanate ended urethane quasi-prepolymers monitored by IR spectroscopy and DSC. J Appl Polym Sci 107:700–709. https://doi.org/10.1002/app.26453
Deppe HJ (1977) Technical advances in isocyanate gluing of particleboard. Holz Roh- Werkst 35:295–299. https://doi.org/10.1007/bf02608992
Deppe HJ, Ernst K (1971) Isocyanates as adhesives for particle board. Holz Roh- Werkst 29:45–50. https://doi.org/10.1007/bf02615003
Dunky M (2002) Bindemittel und Verleimung. (Binding agent and gluing). In: Dunky M, Niemz P (eds) Holzwerkstoffe und Leime—Technologie und Einflussfaktoren. Springer, Berlin (in German)
Ferra JMM, Ohlmeyer M, Mendes AM, Costa MRN, Carvalho LH, Magalhães FD (2011) Evaluation of urea-formaldehyde adhesives performance by recently developed mechanical tests. Int J Adhes Adhes 31:127–134. https://doi.org/10.1016/j.ijadhadh.2010.11.013
Frazier CE (2003) Isocyanate wood binders. In: Pizzi A, Mittal KL (eds) Handbook of adhesive technology, revised and expanded. CRC Press, New York
Gruver TM, Brown NR (2007) Penetration and performance of isocyanate wood binders on selected wood species. BioResources 1:233–247
He G, Yan N (2005) Effect of moisture content on curing kinetics of pMDI resin and wood mixtures. Int J Adhes Adhes 25:450–455. https://doi.org/10.1016/j.ijadhadh.2004.12.002
Humphrey PE (1990) Device for testing adhesive bonds. US Patent US 5176028 A, 5. Jan. 1993
Humphrey PE, Shan R (1989) Bonding kinetics of thermosetting adhesive systems used in wood-based composites: the combined effect of temperature and moisture content. J Adhes Sci Technol 3:397–413. https://doi.org/10.1163/156856189X00290
ISO-3130 (1994) Wood—determination of moisture content for physical and mechanical tests. ISO- International Organization for Standardization
Jost M, Sernek M (2008) Shear strength development of the phenol–formaldehyde adhesive bond during cure. Wood Sci Technol 43:153–166. https://doi.org/10.1007/s00226-008-0217-2
Keylwerth R, Noack D (1963) Die Kammertrocknung von Schnittholz (Kiln-drying of sawn timber). Holz Roh-Werkst 22:29–36 (in German)
Li Q, Li M, Chen C, Cao G, Mao A, Wan H (2017) Adhesives from polymeric methylene diphenyl diisocyanate resin and recycled polyols for plywood. Forest Prod J 67:275–282. https://doi.org/10.13073/fpj-d-16-00054
Papadopoulos A (2007) Property comparisons and bonding efficiency of UF and PMDI bonded particleboards as affected by key process variables. BioResources 1:201–208
Pizzi A (2014) Synthetic adhesives for wood panels: chemistry and technology—a critical review. Rev Adhes Adhes 2:85–126. https://doi.org/10.7569/RAA.2013.097317
Rohumaa A, Hunt CG, Frihart CR, Saranpää P, Ohlmeyer M, Hughes M (2014) The influence of felling season and log-soaking temperature on the wetting and phenol formaldehyde adhesive bonding characteristics of birch veneer. Holzforschung 68:965–970
Smith GD (2005) The lap-shear strength of bonds between Oriented Strand Board (OSB) like strands coated with pMDI resin. Holz Roh- Werkst 63:311–312. https://doi.org/10.1007/s00107-004-0527-5
Smith GD (2007) The effect of some process variables on the lap-shear strength of aspen strands uniformly coated with pMDI-resin Wood and fiber science 36:228–238
Solt P, van Herwijnen HWG, Konnerth J (2019) Thermoplastic and moisture-dependent behavior of lignin phenol formaldehyde resins. J Appl Polym Sci 136:48011. https://doi.org/10.1002/app.48011
Stöckel F, Konnerth J, Kantner W, Moser J, Gindl W (2010) Tensile shear strength of UF- and MUF-bonded veneer related to data of adhesives and cell walls measured by nanoindentation. Holzforschung 64:337–342. https://doi.org/10.1515/hf.2010.046
Stöckel F, Konnerth J, Gindl-Altmutter W (2013) Mechanical properties of adhesives for bonding wood—a review. Int J Adhes Adhes 45:32–41. https://doi.org/10.1016/j.ijadhadh.2013.03.013
Weaver FW, Owen NL (1995) Isocyanate-wood adhesive bond. Appl Spectrosc 49:171–176. https://doi.org/10.1366/0003702953963751
Wendler SL, Frazier CE (1996) Effect of moisture content on the isocyanate/wood adhesive bondline by 15N CP/MAS NMR. J Appl Polym Sci 61:775–782
Yelle DJ, Ralph J, Frihart CR (2011a) Delineating pMDI model reactions with loblolly pine via solution-state NMR spectroscopy. Part 1. Catalyzed reactions with wood models and wood polymers. Holzforschung 65:131–143. https://doi.org/10.1515/hf.2011.028
Yelle DJ, Ralph J, Frihart CR (2011b) Delineating pMDI model reactions with loblolly pine via solution-state NMR spectroscopy. Part 2. Non-catalyzed reactions with the wood cell wall. Holzforschung 65:145. https://doi.org/10.1515/hf.2011.029
Acknowledgements
Open access funding provided by University of Natural Resources and Life Sciences Vienna (BOKU).
Author information
Authors and Affiliations
Corresponding author
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit https://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Solt, P., Libowitzky, S., van Herwijnen, H.W.G. et al. Improved method for analyzing cohesive strength development of pMDI. Wood Sci Technol 54, 7–17 (2020). https://doi.org/10.1007/s00226-019-01143-7
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-019-01143-7