Abstract
Current antimold agents that capitalize on broad spectrum biocidal toxicity for their efficacy can create potential hazards in the environment, as in many cases these biocides are not chemically bonded to the wood. They can therefore leach into the ecosystem to jeopardize off-target organisms. In this study, “bio-catalysis” of potassium iodide (KI) was used to impart non-diffusible antimold properties to 4-year-old bamboo, and the mechanisms of how this system works against Aspergillus niger and Trichoderma viride fungi were also studied. In addition to the bamboo block material tested, powdered samples of lignin, cellulose and hemicellulose were studied to better understand binding of iodide to the chemical subcomponents of the bamboo cell walls. Fourier transform infrared spectroscopy (FTIR) and X-ray photoelectron spectroscopy (XPS) analyses were used to investigate the sample chemistries and antimold mechanisms, respectively. The results revealed that a specific ratio of laccase enzyme with a laccase-mediator functioned best in binding KI to bamboo. Lignin samples showed the greatest changes in FTIR, especially relative to the methoxyl groups and aromatic skeleton. Additionally, enzyme activity on lignin moieties showed much more differences than on hemicellulose and cellulose after biocatalyzed KI modification. Moreover, the XPS binding energy readings of 621.1 and 632.7 eV (Aromatic region) for lignin were consistent with I–C binding associated with its phenolic structures. While the 620.7 eV (Aliphatic region) suggested I–C bonding that associated with residual lignin in hemicellulose, no peaks were observed for the iodine 3d XPS spectra of treated cellulose. XPS analyses of the O/C and C1/C2 ratios revealed that the formation of I–C bonds was likely due to the substitution of oxygen-contained groups which were formed from laccase-catalyzed oxidation. Thus, enzyme-catalyzed formation of I–C bonds with bamboo chemical constituents is a highly effectual, as well as an ecological method to protect bamboo against mold colonization in the present research.
Similar content being viewed by others
Introduction
The increasing rate of deforestation in many parts of the world combined with an ever-increasing global population has continued to stress existing wood resources. Alternative lignocellulose resources are therefore needed to help reduce the demand for wood (Jha and Bawa 2006). Bamboo, a woody grass, has been assisting to serve the purpose as a structural bio-based material due to its rapid growth rate and high density. With 1317 known species that are widely distributed throughout the world, it has the potential to be used as a global resource (Clark 2012; Hibbett et al. 2007). However, its products have low natural durability because of their relatively high moisture content in initial processing stages, culm sugar and starch contents in many species. For this reason, they are readily infected by both mold and decay fungi which cause the materials to lose value during storage, transport and even in final usage (Liese and Kumar 2003).
Many current fungicidal chemicals used in mold protection readily leach from treated materials in wet environments, and this is of particular concern for products that come in contact with food and eating utensils such as bamboo chopsticks, baskets, mats, chopping boards and toys (Sun and Duan 2004). Leaching of biocidal chemicals can potentially impact human health but is also a concern from the perspective of both abiotic and biotic contamination in the environment. Further, leaching of the chemical protectants from the bamboo structure leaves the material susceptible to mold attack. Current research has therefore focused on antifungal systems that can protect bamboo with low leaching potential, which also have low risk to human health and limited environmental impacts (Tang et al. 2012).
Being a widely known antiseptic, iodine (I2) is also approved by food and drug administrations around the world for low-dosage use by humans and in food contact materials (Lawrence 1998). More so, it is reported to be very effective against fungi even in minute quantities (Adimurthy et al. 2003; Choi et al. 2003). When higher concentrations of molecular I2 are added to water, excess of I− will form a brownish water-soluble triiodide (I3−) precipitate that will remain in equilibrium with dissolved I2.
Laccase is an enzyme that possesses high specific activity and stability and acts on a wide range of substrates, thus, it is of value in certain organic synthesis applications (Rodríguez and Toca Herrera 2006). Type III of this enzyme is known to oxidize various aromatic and non-aromatic compounds, including phenolic lignin, and the electrons generated are transferred to molecular oxygen which is then reduced to water (Alcalde and Bulter 2003). Additionally, the increased oxidation rates for lignin and certain xenobiotics have been achieved when a substrate like 2, 2′-azino-bis (3-ethylbenzthiazoline-6-sulfonic acid) (ABTS) is used as a redox mediator (Camarero et al. 2007; Cañas and Camarero 2010).
Furthermore, research over the past 10 years has disclosed that laccase isolated from the white-rot fungus (Trametes versicolor) in combination with ABTS has the ability to directly oxidize iodide to iodine (I2) in aqueous solution which is in equilibrium with iodine triiodide (I3−) (Couto and Toca-Herrera 2007). In an innovative and seminal research, Schubert et al. (2012) and Ihssen et al. (2014) demonstrated that KI could be bound to wood using the laccase-mediator system. In this way, KI would then provide antimicrobial surfaces even after leaching. The research of Schubert et al. (2012) and Ihssen et al. (2014) was some of the first to be conducted which demonstrated a new and bio-based technology to fix iodine residues onto Norway spruce wood for potential protection in a safe and environmentally acceptable manner. Their work was focused on wood rather than bamboo or grasses but demonstrated the effectiveness of such treatments against a wide variety of fungi including blue-stain, wood decay, yeasts, and gram-positive and gram-negative bacteria.
The present research extends the mediate KI binding concept to an exploration of whether the mediated KI system has potential for protecting bamboo. The purpose of this research was to test if bamboo, which is vulnerable to mold attack, can be protected using a laccase-catalyzed KI treatment. Since leaching resistance is essential to special bamboo products such as chopping boards, bamboo curtains and mats, this study also determined if the mold protection effects persisted after a water leaching procedure which would simulate natural weathering. Chemical analysis of iodine to assess binding to cellulose, hemicelluloses and lignin was conducted after treatment and leaching to find out which bamboo components played roles in the fixation of iodide to the substrate. Specifically, FTIR and XPS were employed to determine any chemical changes in the three major constituents of bamboo to assess the mechanism of KI fixation.
Materials and methods
Bamboo blocks
Bamboo samples 50 × 50 × 20 (radial) mm3 were produced from 4-year-old Phyllostachys pubescens culms collected from a plantation, in Lin’an, Hangzhou, Zhejiang province, China.
Reagents
Commercial 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulfonic acid) (ABTS) and laccase from Trametes versicolor were purchased from Sigma-Aldrich company; PBS buffer components, KH2PO4 solution and potassium iodide (KI), were purchased from Hangzhou East China Pharmaceutical Group Company limited.
Fungal species
Two species of mold fungi including Trichoderma viride (isolation number: GDMCC 3.140) and Aspergillus niger (isolation number: GDMCC 3.411) were purchased from Guangdong Institute of Microbiology (Guangdong Province, China) and archived by the National and Local Joint Engineering Laboratory for High Efficiency Pesticide Preparation Technology at Zhejiang Agriculture and Forestry University.
Culture conditions
Potato dextrose agar (PDA - final pH, 5.6 ± 2) was prepared as previously described (Sun et al. 2012) and under sterile conditions and the test fungi were activated on PDA medium at 25–28 °C and 85 ± 2% relative humidity for 7 days.
Impregnation and leaching of test blocks
To study the effect of enzyme on iodine fixation to bamboo, the blocks were treated with 2.5 wt% KI solution alone or catalyzed by laccase at activities which varied from 0.05 to 0.18 and 0.60 U/mL, in the presence of 18 mg/L ABTS with six replicates each. The test samples were submerged with weights to assure complete immersion in the treatment solutions and incubated in a 40 °C water bath for 20 h to permit enzymatic action. They were then removed from solutions and oven-dried (40 °C, 3 h). While the blocks numbered L-0, L-0.05, L-0.18 and L-0.60 (0, 0.05, 0.18 and 0.60 represent the laccase activity) were leached with distilled water prior to exposure to the fungi, UL-0, UL-0.05, UL-0.18 and UL-0.60 were unleached. The leaching test was conducted by immersing the treated blocks into 100 mL of distilled water and changing it after every 24 h for 2 weeks.
After air drying for 7 days, bamboo powder for iodine analyses was collected from the surfaces of six leached and unleached samples, by scraping with a razor. Then, 0.06 g of dried powder was digested with 10 mL concentrated nitric acid and 2 mL perchloric acid at 120 °C for 4–5 h until dissolved and transparent. The temperature of the solution was then raised to 160 °C to evaporate the solvent, and distilled water was added to 10 mL. The solutions were then assayed by inductively coupled plasma mass spectrometry (ICP-MS) to determine the level of iodine, and the leached blocks were also exposed to fungal incubation. The amount of iodine in bamboo was calculated as follows:
Iodine in bamboo (mg/g) = The amount of iodine determined by ICP-MS (mg)/the amount of bamboo powder collected (g).
Extraction of bamboo chemical constituents
To extract lignin, cellulose and hemicelluloses from the bamboo, the dried and sliced bamboo pieces were crushed, sieved (60 mesh) and then milled in a planetary ball milling machine (with 500 mL ZrO2) to 100 mesh. This milled bamboo powder was then used for extraction of the major chemical components of bamboo as follows:
Milled wood lignin (MWL) Milled powder (100 g) was shake-extracted twice in a p-dioxane–water solution (96% v/v) for 48 h before rotary evaporation to reduce the total volume to 40 mL. This extract concentrate was then added to 200 mL of deionized water, stirred and freeze-dried to produce a crude preparation. After that, it was then dissolved in 20 mL of 90% acetic acid, precipitated in 400 mL of deionized water and centrifuged to produce a crude lignin precipitate. This was then dissolved in 10 mL, 2:1 (v/v) of 1, 2-dichloroethane/ethanol and reprecipitated in 200 mL diethyl ether for frozen storage. Just before experimental use, this material was thawed, and then washed twice with 100 mL of petroleum ether, and then freeze-dried again to obtain the MWL (Bai et al. 2013).
Cellulose Toluene and ethanol (2:1, V/V) were used to soxhlet extract 10 g of the milled bamboo powder (2 h at 25 °C). This powder was dried overnight at 70 °C and then further processed at 130 °C, using a hydrogen peroxide/acetic acid/titanium oxide catalyst (molar ratio 1:2.08) for 2 h. The material was then rinsed with deionized water (pH = 7) before drying at 70 °C for 24 h and was further immersed in 6% NaOH (rt) and heated at 80 °C (150 rpm stirring, 8 h). This precipitate was filter rinsed in a Bucher funnel with deionized water until a pH of 7 was reached, and the final product was then freeze-dried (− 85 °C, 48 h) (Liew et al. 2015).
Hemicellulose The milled bamboo powder was Soxhlet extracted with toluene–ethanol (2:1, v/v) for 6 h to obtain a delignified sample. This was followed by treatment with sodium chlorite in an acidic solution (pH 4.2–4.7) for 6 h at 75 °C to obtain holocellulose that was then extracted in a 2% hot NaOH solution. The product was then neutralized to pH = 5.5 with acetic acid, concentrated at reduced pressure, and then precipitated three times with equal volumes of 95% ethanol. The final product was filtered and washed with 70% ethanol before freeze-drying (Luo et al. 2012).
Treatment of bamboo powder and chemical constitutes
Bamboo powder (100 mesh) and the extracted three chemical components were independently submerged in 2.5 wt% KI solution alone or catalyzed by laccase at activities of 0.60 U/mL, in the presence of 18 mg/L ABTS then reacted in a constant temperature water bath (40 °C, 12 h). The samples were removed from solution and vacuum-dried (60 °C, 2 h). 0.1 g of these samples was then individually leached with 6 mL distilled water that was changed every 24 h for 14 days, before a final vacuum drying at 60 °C for 30 min.
Mold tests and characterization
Mold resistance tests were conducted according to AWPA E 24-12 (2013) standard in which the treated samples were put into an environmental chamber with both Trichoderma viride and Aspergillus niger at 28 ± 2 °C and 85% RH (Fig. 1). Surface mold growth observations on the specimens were recorded on a weekly basis for 3 months using a 5-point ranking criteria listed in Table 1.
The efficiency of mold resistance (E) was calculated as follows;
where D t was the average infection value of the treated samples and D0 was the average infection value of the untreated control samples.
Fourier transform infrared spectroscopy (FTIR) analysis and X-ray photoelectron spectroscopy (XPS)
FTIR was used to study the chemical changes of the bamboo before and after laccase-catalyzed iodination. Finely ground 1 mg samples were crushed and dispersed in a matrix of 100 mg of KBr, followed by compression at 16 MPa to form pellets. The spectra were measured using a Spectrum GX spectrometer (PerkinElmer, USA) from 4000 to 500 cm−1 at a spectral resolution of 8 cm−1, 32 scans/min, and a 0.36 mm2 spot size.
For XPS analysis, the samples (100 mesh) were dried and etched for 30 s in argon gas before the analysis to exclude the effect of impurities present on the sample surfaces. The XPS data were collected using a K-Alpha XPS system (Thermo Fisher Scientific, USA) with a Magnesium Kα X-ray source. The base pressure in the analytical chamber was less than 5 × 10−7 Pa, and the survey scans spanned from 1100 to 0 eV binding energy, which were collected using a constant pass energy of 50 eV and a step interval of 1.00 eV.
Results and discussion
Fixation of iodine in bamboo blocks
This fixation was conducted on the treatment of 2.5 wt% KI with a laccase activity of 0.60 (UL-0.60 and L-0.60), in the presence of 18 mg/L ABTS as compared with the treatment of 2.5 wt% KI solution alone (UL-0 and L-0) and the untreated bamboo (UL-Control and L-Control). The untreated bamboo contained a small amount of iodine (Table 2). In treated samples, the amount of iodine absorbed in bamboo was similar in both the KI alone treatments and laccase-catalyzed KI treatments. After leaching with water for 14 days, however, more iodine leached from the bamboo samples treated with KI alone and it decreased from 10.86 to 0.40 mg/g (Table 2). That of laccase-catalyzed KI samples reduced from 10.82 to 5.41 mg/g, indicating that the presence of laccase accelerated the fixation of iodine onto bamboo.
Mold resistances of bamboo treated with laccase-catalyzed potassium iodide
To better understand the influence of iodine leaching on the protection of bamboo from mold attack, mold tests were conducted on KI-treated bamboo at different levels of laccase-catalyzed KI treatments. KI alone treatment is effective to some extent against mold attack (similar to L-0.05; see Fig. 2a), but the biocidal efficacy is greatly decreased after leaching (Fig. 2b) as would be expected from the leaching data provided in Table 2.
Besides, the biocidal effect of the treated samples increased with the activity of laccase in the order from 0.05 to 0.18 and 0.60 U/mL, for both leached and unleached samples which was attributed to the increased levels of KI bound to the bamboo during the laccase-mediator treatment with KI. In the unleached blocks, the KI alone treated blocks lost most of their biocidal capacity against mold, while the UL-0.60 samples decreased in biocidal efficacy after 2 months only to 48.1%, and to 22.5% in the third month. The leached blocks (Fig. 2b) also showed much better biocidal effects than the reference KI-only with the results similar to those of the unleached blocks except that the unleached blocks were able to control mold activity better toward the end of the test period.
Furthermore, the biocidal effect displayed by the leached blocks was considerably less than that for the unleached samples, with the 0.05 laccase activity treatment being covered with mold after about 50 days in the leached samples compared to 70 days for the unleached samples. At the end of the test period, the UL-0.60 samples still retained some biocidal effect with a rating of 22.5% biocidal efficacy (Fig. 2). The data of leached sample showed that increasing activity of laccase treatment enhanced fixation of iodine to the bamboo; however, the effectiveness was not so good as the unleached laccase-fixed material suggesting even at the highest level of laccase-mediator treatment, fixation of the entire 2.5 wt% KI amount was not achieved. This suggests that treatment might also be effective with the same laccase-ABTS treatment used with lower KI concentrations.
FTIR analyses
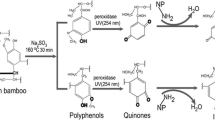
To better understand the chemical changes which occurred in the bamboo after laccase iodination, FTIR was used to assess changes in lignin, hemicelluloses and cellulose after treatment. The portions of the FTIR spectra displayed notable changes (Fig. 3) with the aromatic skeleton of lignin peaks at 1600, 1510 and 1421 cm−1, all decreasing, and the 1510 cm−1 peak also splitting, indicating changes in the skeleton of lignin (Xu et al. 2013; Zhou et al. 2011). The peak at 1460 cm−1 is the bending vibration of C–H bond connected on benzene ring, which decreased after treatment, indicating a change in C–H bonds connected on benzene ring.
Moreover, the new bands at 1087 and 880 might be caused by the I-substituted ring, which in most cases shifted to higher frequency than the aliphatic C–I bonds (500–600 cm−1) (Coates 2000). Although no well-defined I-substituted aromatic compounds are observed, the presence of a halogen on an aromatic ring can be detected indirectly from its electronic impact on the C–H bands. The bands at 2972 cm−1 for C–H stretches of side chain CH3 and CH2 increased after treatment, and 2928 cm−1 and a new band at 1380 cm−1 further suggested changes in C–H bonds. The laccase-mediated formation of phenoxyl radicals might also result in cleavage of carbon–carbon and aromatic rings in lignin (Bourbonnais et al. 1995; Kawai et al. 1999), and the changes in C–H bonds were probably caused by the variation of the benzene structure.
Additionally, iodine substitution on the benzene ring is also potentially supported by the relatively good resistance of the treated samples against mold fungi after leaching. The formation of aromatic organo-iodine complexes which can impart antifungal properties to wood has been previously reported (Ihssen et al. 2014; Schubert et al. 2012). In addition, the XPS analyses in the Section XPS analysis results of the samples demonstrated possible formation of C–I covalent bonds.
Conversely, hemicellulose (Fig. 4) and cellulose (Fig. 5) treated samples displayed fewer differences from the controls except for C–H stretches in hemicellulose at 2922 and 2854 cm−1, which were probably caused by the cleavage of xylan backbone (U.S. DOE 2006). In comparison with control samples in Figs. 3, 4, 5 to 6, the spectra for bamboo powder were basically a combination of lignin, hemicellulose and cellulose.
In Fig. 6, the lignin-related peaks including 1600, 1510 and 1420 cm−1 changed greatly, as well as peaks at 1087 and 882 cm−1, suggesting laccase reacted with lignin and the iodine grafted onto bamboo.
Hence, the overall FTIR analysis indicates that enzymatic treatment of bamboo in the presence of potassium iodide resulted in structural changes for lignin and hemicellulose, but that bonding of iodine was associated primarily with lignin.
XPS analysis results of the samples
In an effort to reveal the reasons for the better protection of bamboo from mold after in situ treatment with increasing laccase-catalyzed potassium iodide (Fig. 2b), XPS analyses were conducted to determine specifically where iodide bonds to bamboo surfaces. With XPS, surface information of a material can be revealed, especially with regard to elemental identity, chemical state and quantity of an element of interest.
Accordingly, the binding energies of 618.8 and 630.2 eV (Fig. 7) in the iodine 3d XPS spectra of lignin are consistent with that of KI. In the cellulose and hemicellulose samples, these peaks are not readily observable above the baseline. Peaks at 621.1 and 632.7 eV in Fig. 7 represent I–C (aromatic) binding on the phenolic structures of lignin as has previously been demonstrated using GC–MS analysis (Wang et al. 2004). Other studies have also shown that I− can be oxidized to I· by laccase (Xu 1996), as well as phenolic compounds undergo this process to produce benzyl and phenoxy radicals (Sjöström 1993) which in turn can react with I· to form aromatic-I covalent bonds to impart fungal resistance (Ihssen et al. 2014).
The binding energies of 620.7 and 632.2 eV in Fig. 8 were likely I–C bonding associated with residual lignin which remained in the treated hemicellulose samples (Mercier et al. 2002). In future research, other purified hemicellulose fractions should be tested to determine if iodine can be bound to hemicellulose directly or indirectly through the laccase-mediator system. No peak could be observed for iodine in 3d XPS spectra of the treated cellulose (Fig. 9) indicating that iodine had very limited or no binding to cellulose.
In bamboo powder (Fig. 10), no observable peaks in the 610–640 eV region were apparent in the untreated sample, while in the treated sample the binding energies of 620.7, 632.2, 621.1 and 632.8 eV were very strong, indicating the formation of I–C bonds. However, the specific binding energy peaks for unbound KI or I2 were not obvious because the treated samples were leached with water for 14 days before XPS tests and any soluble KI or I2 would have been lost.
The atomic percent, oxygen to carbon (O/C) and carbon1 to carbon2 (C1/C2) ratios are related to the chemical composition of bamboo, which allow for the identification of the principal components and changes on its surface (Sernek 2002). To assess the extent of I–C bond formation in bamboo after treatment, the O/C and I/C ratios of treated cellulose, hemicellulose, lignin and bamboo powder were calculated (Table 3). Cellulose samples were observed to contain higher O/C ratio, but no I–C bonds were detected, thus it is hypothesized that the oxidation of cellulose occurred indirectly from the laccase-mediator treatment, even though iodine did not bind to the oxidized cellulose. The O/C ratio for lignin before and after treatment was the same even though lignin is more sensitive to laccase oxidation, and the current data indicate that after oxidation, some oxygen groups on lignin did bind iodine. Hemicellulose oxidation also showed very little change, and it is believed that the small increase in iodine content in those samples (Table 3) may be due to the binding of iodine to lignin that may have been associated with the hemicellulose fraction.
The curve fit for carbon peaks included the four main carbon components: C1, C2, C3 and C4 (Fig. 11 and Table 4). The C1 peak can provide additional evidence in support of the O/C interpretation of the wood surface chemistry, especially the C1/C2 component ratio (Sernek 2002). Each of the three component samples, lignin, cellulose and hemicellulose had reduced C1/C2 ratios after treatment compared to the untreated samples, with hemicellulose and cellulose changing the most (Table 4). The reduced C1 peak compared to the C2 peak resulted from the oxidation of carbon, which should have increased the O/C ratio. However, the treated lignin sample displayed reduced O/C ratios, which were probably caused by substitution of oxygen-contained groups by iodine. This reduced O/C ratio was also observed in the hemicelluloses sample, with residual lignin in the hemicellulose possibly binding some iodine. The C3 peak in treated lignin was also distinctly reduced hence, providing more information about the mechanisms. Bamboo powder that contained cellulose, hemicellulose and lignin had reduced O/C and increased C1/C2 ratios (Fig. 11 and Table 4), which demonstrated the balance between carbon oxidization and iodine substitution.
Conclusion
-
1.
The covalent coupling of iodine to phenolic structures in bamboo provided a leach resistant as well as an antimicrobial surface to help protect against the growth of mold fungi on bamboo surfaces. The best combination for the biotransformation of bamboo to impart antimold, biocidal properties was 18 mg/L ABTS + 0.60 U/mL laccase + 2.5 wt% KI at pH 5 and 40 °C. However, leaching of some unfixed KI suggests that a lower percentage of KI with the same level of enzyme and mediator may be effective. Leaching of the samples reduced the biocidal capacity of the laccase-fixed KI treatments but to a much lesser degree than in samples treated with KI alone.
-
2.
FTIR and XPS analyses showed that the treatment of bamboo with the KI-laccase-mediator systems allowed iodine to covalently bond specifically to lignin. The results showed that cellulose was not greatly modified and did not bind iodine. As laccase-mediator systems are well documented relative to their modification of lignin, this study clearly demonstrated the effect of the treatment on the bonding of iodine to lignin (dominant).
-
3.
Analyses of iodine with 3d XPS spectra demonstrated characteristic peaks for lignin-iodine bonds. The formation of I–C bonds to lignin permitted the protection of bamboo from mold after leaching to a much greater extent than KI treatment only. The O/C ratios of lignin, hemicelluloses and bamboo powder were reduced after modification, while the O/C ratio for cellulose increased. In addition, lower C1/C2 ratios in treated lignin, cellulose and hemicellulose were observed compared to the untreated samples. Based on the above phenomena, a possible mechanism for laccase-catalyzed iodination of bamboo and its components is proposed where substitution of iodine for some of the oxygen-containing groups in lignin, and in the lignin of bamboo powder, occurred when sample materials were treated using the laccase-mediator system with iodine.
References
Adimurthy S, Ramachandraiah G, Ghosh PK, Bedekar AV (2003) A new, environment friendly protocol for iodination of electron-rich aromatic compounds. J Tetrahedron Lett 34:5099–5101
Alcalde M, Bulter T (2003) Colorimetric assays for screening laccases. J Methods Mol Biol 230:193–201
AWPA E 24-12 (2013) Standard method of evaluating the resistance of wood product surfaces to mold growth
Bai YY, Xiao LP, Shi ZJ, Run CS (2013) Structural variation of bamboo lignin before and after ethanol organosolv pretreatment. Int J Mol Sci 14(11):21394–21413
Bourbonnais R, Paice MG, Reid ID, Lantheir P, Yaguchi M (1995) Lignin oxidation by laccase isozymes from Trametes versicolor and role of the mediator 2,2′-azino-bis(3-ethylbenzothiazoline-6-sulphonic acid) in kraft lignin depolymerization. Appl Environ Microbiol 61:1876–1880
Camarero S, Ibara D, Martinez AT, Romero J, Gutiérrez A, Río JD (2007) Paper pulp delignification using laccase and natural mediators. Enzyme Microb Technol 40:1264–1271
Cañas AI, Camarero S (2010) Laccases and their natural mediators: biotechnological tools for sustainable eco-friendly processes. Biotechnol Adv 28:694–705
Choi S, McComb JG, Levy ML, Gonzalez-Gomez I, Bayston R (2003) Use of elemental iodine for shunt infection prophylaxis. Neurosurgery 52:908–913
Clark L (2012) An updated tribal and subtribal classification for the Bambusoideae (Poaceae). In: Gielis J, Potters G (eds) Proceedings of the 9th world bamboo congress. World Bamboo Organization, Antwerp, pp 3–27
Coates J (2000) Interpretation of infrared spectra, a practical approach. In: Meyers RA (ed) Encyclopedia of analytical chemistry. Wiley, Chichester, pp 10815–10837
Couto SR, Toca-Herrera JL (2007) Laccase production at reactor scale by filamentous fungi. Biotechnol Adv 25:558–569
Hibbett DS, Binder M, Bischoff JF et al (2007) A higher-level phylogenetic classification of the Fungi. Mycol Res 111:509–547
Ihssen J, Schubert M, Li Thöny-Meyer, Richter M (2014) Laccase catalyzed synthesis of iodinated phenolic compounds with antifungal activity. PLoS ONE 9(3):e89924
Jha S, Bawa KS (2006) Population growth, human development, and deforestation in biodiversity hotspots. Conserv Biol 20(3):906–912
Kawai S, Asukai M, Ohya N, Okita K, Ito T, Ohashi H (1999) Degradation of a non-phenolic β-O-4 substructure and of polymeric lignin model compounds by laccase of Coriolus versicolor in the presence of 1-hydroxybenzotriazole. FEMS Microbiol Lett 170(1):51–57
Lawrence JC (1998) The use of iodine as an antiseptic. J Wound Care 7(8):421–425
Liese W, Kumar, S (2003) Bamboo preservation compendium. In: International network for bamboo and rattan, Beijing, China, Technical report 22, pp 231
Liew FK, Hamdan S, Rusop M, Lai JCH, Hossen MF, Rahman MM (2015) Synthesis and characterization of cellulose from green bamboo by chemical treatment with mechanical process. J Chem 212158:1–6
Luo Q, Peng H, Zhou MY, Lin D, Ruan R, Wam YQ, Zhang QS, Liu YH (2012) Alkali extraction and physical-chemical characterization of hemicelluloses from young bamboo (Phyllostachys pubescens mazeli). BioResources 7(4):5815–5828
Mercier F, Moulin V, Guittet MJ, Barré N, Gautier-Soyer M, Trocellier P, Toulhoat P (2002) Applications of new surface analysis techniques (NMA and XPS) to humic substances. Org Geochem 33:247–255
Rodríguez CS, Toca Herrera JL (2006) Industrial and biotechnological applications of laccases: a review. Biotechnol Adv 24:500–513
Schubert M, Engel J, Thöny-Meyer L, Schwarze FWMR, Ihssen J (2012) Protection of wood from microorganisms by laccase-catalyzed iodination. Appl Environ Microbiol 78(20):7267–7275
Sernek M (2002) Comparative analysis of inactivated wood surfaces Dissertation submitted to the Faculty of the Virginia Polytechnic Institute and State University. Blacksburg
Sjöström E (1993) Wood chemistry. Fundamentals and applications, 2nd edn. Academic Press, San Diego, p 78
Sun FL, Duan XF (2004) Situation and overview of bamboo mold-preservation. Word Bamboo Rattan 2(4):1–4
Sun FL, Bao BF, Ma LF, Chen AL, Duan XF (2012) Mould-resistance of bamboo treated with the compound of chitosan-copper complex and organic fungicides. J Wood Sci 58:51–56
Tang TKH, Schmidt O, Liese W (2012) Protection of bamboo against mould using environment-friendly chemicals. J Trop For Sci 24(2):285–290
U.S. DOE (2006) Breaking the biological barriers to cellulosic ethanol. A Joint Research Agenda, DOE/SC-0095, US. Department of Energy Office of Science and Office of Energy Efficiency and Renewable Energy (www.doegenomestolife.org/biofuel/)
Wang HF, Deng XY, Wang J, Gao XF, Shi ZJ, Gu ZN, Liu YF, Zhao YL (2004) XPS study of C–I covalent bond on single-walled carbon nanotubes (SWNTS). Acta Phys Chim Sin 20(7):673–675
Xu F (1996) Catalysis of novel enzymatic iodide oxidation by fungal laccase. Appl Biochem Biotechnol 59(3):221–230
Xu GQ, Wang LH, Liu JL, Wu JZ (2013) FTIR and XPS analysis of the changes in bamboo chemical structure decayed by white-rot and brown–rot fungi. Appl Surf Sci 280(8):799–805
Zhou HD, Liu YH, Ruan RS, Peng H, Zhang JS (2011) Extraction of lignin from bamboo (Phyllostachys edulis) by atmospheric acetic acid process. Sci Silvae Sin 47(10):153–159
Acknowledgements
We are grateful to the Natural Science Foundation of China No. 31470587 and the Zhejiang Provincial Natural Science Foundation No. Z14C160009. This research was also supported by the National Institute of Food and Agriculture, US Department of Agriculture, the Center for Agriculture, Food and the Environment, and the Microbiology department at University of Massachusetts Amherst, under Project No. MAS00511. The contents are solely the responsibility of the authors and do not necessarily represent the official views of the USDA or NIFA.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Prosper, N.K., Zhang, S., Wu, H. et al. Enzymatic biocatalysis of bamboo chemical constituents to impart antimold properties. Wood Sci Technol 52, 619–635 (2018). https://doi.org/10.1007/s00226-018-0987-0
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00226-018-0987-0