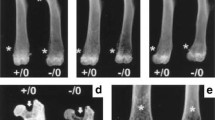
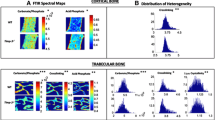
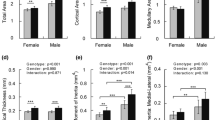
Abstract
Osteopontin (OPN) and Bone Sialoprotein (BSP), abundantly expressed by osteoblasts and osteoclasts, appear to have important, partly overlapping functions in bone. In gene-knockout (KO, -/-) models of either protein and their double (D)KO in the same CD1/129sv genetic background, we analyzed the morphology, matrix characteristics, and biomechanical properties of femur bone in 2 and 4 month old, male and female mice. OPN−/− mice display inconsistent, perhaps localized hypermineralization, while the BSP−/− are hypomineralized throughout ages and sexes, and the low mineralization of young DKO mice recovers with age. The higher contribution of primary bone remnants in OPN−/− shafts suggests a slow turnover, while their lower percentage in BSP−/− indicates rapid remodeling, despite FTIR-based evidence in this genotype of a high maturity of the mineralized matrix. In 3-point bending assays, OPN−/− bones consistently display higher Maximal Load, Work to Max. Load and in young mice Ultimate Stress, an intrinsic characteristic of the matrix. Young male and old female BSP−/− also display high Work to Max. Load along with low Ultimate Stress. Principal Component Analysis confirms the major role of morphological traits in mechanical competence, and evidences a grouping of the WT phenotype with the OPN−/− and of BSP−/− with DKO, driven by both structural and matrix parameters, suggesting that the presence or absence of BSP has the most profound effects on skeletal properties. Single or double gene KO of OPN and BSP thus have multiple distinct effects on skeletal phenotypes, confirming their importance in bone biology and their interplay in its regulation.






Similar content being viewed by others
References
Fisher LW, Fedarko NS (2003) Six genes expressed in bones and teeth encode the current members of the SIBLING family of proteins. Connect Tissue Res 44(Suppl 1):33–40
Bellahcène A, Castronovo V, Ogbureke KUE, Fisher LW, Fedarko NS (2008) Small integrin-binding ligand N-linked glycoproteins (SIBLINGs): multifunctional proteins in cancer. Nat Rev Cancer 8:212–226. https://doi.org/10.1038/nrc2345
Kawasaki K, Weiss KM (2006) Evolutionary genetics of vertebrate tissue mineralization: the origin and evolution of the secretory calcium-binding phosphoprotein family. J Exp Zoolog B Mol Dev Evol 306:295–316. https://doi.org/10.1002/jez.b.21088
Sodek J, Ganss B, McKee MD (2000) Osteopontin. Crit Rev Oral Biol Med 11:279–303
Ganss B, Kim RH, Sodek J (1999) Bone sialoprotein. Crit Rev Oral Biol Med 10:79–98
Rittling SR, Matsumoto HN, McKee MD, Nanci A, An XR, Novick KE, Kowalski AJ, Noda M, Denhardt DT (1998) Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res 13:1101–1111. https://doi.org/10.1359/jbmr.1998.13.7.1101
Malaval L, Wade-Gueye NM, Boudiffa M, Fei J, Zirngibl R, Chen F, Laroche N, Roux JP, Burt-Pichat B, Duboeuf F, Boivin G, Jurdic P, Lafage-Proust MH, Amedee J, Vico L, Rossant J, Aubin JE (2008) Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med 205:1145–1153
Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD (2002) Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int 71:145–154
Harmey D, Johnson KA, Zelken J, Camacho NP, Hoylaerts MF, Noda M, Terkeltaub R, Millan JL (2006) Elevated skeletal osteopontin levels contribute to the hypophosphatasia phenotype in Akp2(-/-) mice. J Bone Min Res 21:1377–1386
Yuan Q, Jiang Y, Zhao X, Sato T, Densmore M, Schüler C, Erben RG, McKee MD, Lanske B (2014) Increased osteopontin contributes to inhibition of bone mineralization in FGF23-deficient mice. J Bone Miner Res 29:693–704. https://doi.org/10.1002/jbmr.2079
Boudiffa M, Wade-Gueye NM, Guignandon A, Vanden-Bossche A, Sabido O, Aubin JE, Jurdic P, Vico L, Lafage-Proust MH, Malaval L (2010) Bone sialoprotein deficiency impairs osteoclastogenesis and mineral resorption in vitro. J Bone Min Res 25:2393–2403
Bouet G, Bouleftour W, Juignet L, Linossier M-T, Thomas M, Vanden-Bossche A, Aubin JE, Vico L, Marchat D, Malaval L (2015) The impairment of osteogenesis in bone sialoprotein (BSP) knockout calvaria cell cultures is cell density dependent. PLoS ONE 10:e0117402. https://doi.org/10.1371/journal.pone.0117402
Bouleftour W, Juignet L, Bouet G, Granito RN, Vanden-Bossche A, Laroche N, Aubin JE, Lafage-Proust M-H, Vico L, Malaval L (2016) The role of the SIBLING, Bone Sialoprotein in skeletal biology—Contribution of mouse experimental genetics, Matrix Biol. J Int Soc Matrix Biol 52–54:60–77. https://doi.org/10.1016/j.matbio.2015.12.011
Granito RN, Bouleftour W, Sabido O, Lescale C, Thomas M, Aubin JE, Goodhardt M, Vico L, Malaval L (2015) Absence of bone sialoprotein (BSP) alters profoundly hematopoiesis and upregulates osteopontin. J Cell Physiol 230:1342–1351. https://doi.org/10.1002/jcp.24877
Bouleftour W, Bouet G, Granito RN, Thomas M, Linossier M-T, Vanden-Bossche A, Aubin JE, Lafage-Proust M-H, Vico L, Malaval L (2015) Blocking the expression of both bone sialoprotein (BSP) and osteopontin (OPN) impairs the anabolic action of PTH in mouse calvaria bone. J Cell Physiol 230:568–577. https://doi.org/10.1002/jcp.24772
Bouleftour W, Juignet L, Verdière L, Machuca-Gayet I, Thomas M, Laroche N, Vanden-Bossche A, Farlay D, Thomas C, Gineyts E, Concordet JP, Renaud JB, Aubert D, Teixeira M, Peyruchaud O, Vico L, Lafage-Proust MH, Follet H, Malaval L (2019) Deletion of OPN in BSP knockout mice does not correct bone hypomineralization but results in high bone turnover. Bone 120:411–422. https://doi.org/10.1016/j.bone.2018.12.001
Maalouf M, Çinar H, Bouleftour W, Thomas M, Vanden-Bossche A, Laroche N, Linossier MT, Peyroche S, Lafage-Proust MH, Vico L, Guignandon A, Malaval L (2022) Deletion of osteopontin or bone sialoprotein induces opposite bone responses to mechanical stimulation in mice. Bone Rep 17:101621. https://doi.org/10.1016/j.bonr.2022.101621
Montagner F, Kaftandjian V, Farlay D, Brau D, Boivin G, Follet H (2015) Validation of a novel microradiography device for characterization of bone mineralization. J X-Ray Sci Technol 23:201–211. https://doi.org/10.3233/XST-150481
Gardegaront M, Farlay D, Peyruchaud O, Follet H (2018) Automation of the peak fitting method in bone FTIR microspectroscopy spectrum analysis: human and mice bone study. J Spectrosc. https://doi.org/10.1155/2018/4131029
Farlay D, Panczer G, Rey C, Delmas PD, Boivin G (2010) Mineral maturity and crystallinity index are distinct characteristics of bone mineral. J Bone Miner Metab 28:433–445. https://doi.org/10.1007/s00774-009-0146-7
Farlay D, Duclos M-E, Gineyts E, Bertholon C, Viguet-Carrin S, Nallala J, Sockalingum GD, Bertrand D, Roger T, Hartmann DJ, Chapurlat R, Boivin G (2011) The ratio 1660/1690 cm(-1) measured by infrared microspectroscopy is not specific of enzymatic collagen cross-links in bone tissue. PLoS ONE 6:e28736. https://doi.org/10.1371/journal.pone.0028736
Gineyts E, Borel O, Chapurlat R, Garnero P (2010) Quantification of immature and mature collagen crosslinks by liquid chromatography-electrospray ionization mass spectrometry in connective tissues. J Chromatogr B Analyt Technol Biomed Life Sci 878:1449–1454. https://doi.org/10.1016/j.jchromb.2010.03.039
Vashishth D (2008) Small animal bone biomechanics. Bone 43:794–797. https://doi.org/10.1016/j.bone.2008.06.013
Jepsen KJ, Silva MJ, Vashishth D, Guo XE, van der Meulen MCH (2015) Establishing biomechanical mechanisms in mouse models: practical guidelines for systematically evaluating phenotypic changes in the diaphyses of long bones. J Bone Miner Res 30:951–966. https://doi.org/10.1002/jbmr.2539
Bailey S, Karsenty G, Gundberg C, Vashishth D (2017) Osteocalcin and osteopontin influence bone morphology and mechanical properties. Ann N Y Acad Sci 1409:79–84. https://doi.org/10.1111/nyas.13470
The jamovi project, jamovi. (Version 2.3) [Computer Software], (2022). https://www.jamovi.org.
R Core Team, R: A Language and environment for statistical computing. (Version 4.1) [Computer software], (2021). https://cran.r-project.org.
Ip V, Toth Z, Chibnall J, McBride-Gagyi S (2016) Remnant woven bone and calcified cartilage in mouse bone: differences between ages/sex and effects on bone strength. PLoS ONE 11:e0166476. https://doi.org/10.1371/journal.pone.0166476
Bentolila V, Boyce TM, Fyhrie DP, Drumb R, Skerry TM, Schaffler MB (1998) Intracortical remodeling in adult rat long bones after fatigue loading. Bone 23:275–281. https://doi.org/10.1016/s8756-3282(98)00104-5
Blumer MJF, Longato S, Fritsch H (2004) Cartilage canals in the chicken embryo are involved in the process of endochondral bone formation within the epiphyseal growth plate. Anat Rec A Discov Mol Cell Evol Biol 279:692–700. https://doi.org/10.1002/ar.a.20058
Bach-Gansmo FL, Irvine SC, Brüel A, Thomsen JS, Birkedal H (2013) Calcified cartilage islands in rat cortical bone. Calcif Tissue Int 92:330–338. https://doi.org/10.1007/s00223-012-9682-6
Shipov A, Zaslansky P, Riesemeier H, Segev G, Atkins A, Shahar R (2013) Unremodeled endochondral bone is a major architectural component of the cortical bone of the rat (Rattus norvegicus). J Struct Biol 183:132–140. https://doi.org/10.1016/j.jsb.2013.04.010
Thurner PJ, Chen CG, Ionova-Martin S, Sun L, Harman A, Porter A, Ager JW, Ritchie RO, Alliston T (2010) Osteopontin deficiency increases bone fragility but preserves bone mass. Bone 46:1564–1573. https://doi.org/10.1016/j.bone.2010.02.014
Kavukcuoglu NB, Denhardt DT, Guzelsu N, Mann AB (2007) Osteopontin deficiency and aging on nanomechanics of mouse bone. J Biomed Mater Res A 83:136–144. https://doi.org/10.1002/jbm.a.31081
van Lenthe GH, Voide R, Boyd SK, Müller R (2008) Tissue modulus calculated from beam theory is biased by bone size and geometry: implications for the use of three-point bending tests to determine bone tissue modulus. Bone 43:717–723. https://doi.org/10.1016/j.bone.2008.06.008
Schaffler MB, Burr DB (1988) Stiffness of compact bone: effects of porosity and density. J Biomech 21:13–16. https://doi.org/10.1016/0021-9290(88)90186-8
Acknowledgements
The work was funded by the “Agence Nationale de la Recherche” for the “Mouse_Kosto” project (Grants ANR-13-BSV1-0010-01 to Luc Malaval and ANR-13-BSV1-0010-02 to Hélène Follet), the “Institut National de la Santé et de la Recherche Médicale” (INSERM), the “Université Jean Monnet” of Saint-Etienne, and the “Ministère de l’Enseignement supérieur, de la Recherche et de l’Innovation” (PhD scholarship for Mathieu Maalouf). The authors thank the staff of PLEXAN for skillful technical assistance.
Funding
This article was funded by Agence Nationale de la Recherche, ANR-13-BSV1-0010-01, Luc Malaval, ANR-13-BSV1-0010-02, Hélène Follet, Institut National de la Santé et de la Recherche Médicale (FR), Université Jean Monnet, Saint-Etienne (FR), Ministère de l’Enseignement supérieur, de la Recherche et de l’Innovation (FR).
Author information
Authors and Affiliations
Contributions
Luc Malaval, Hélène Follet and Delphine Farlay designed the study. Luc Malaval wrote the paper; he is the guarantor. Hélène Follet, Delphine Farlay, Evelyne Gineyts, Sebastien Rizzo, Charlene Thomas, Mathieu Maalouf, Brigitte Burt-Pichat, Wafa Bouleftour-Esquis, Arnaud Vanden-Boscche, and Norbert Laroche contributed to the experimental work. Myriam Normand supervised the statistical analysis of the data. Luc Malaval and Hélène Follet procured funding, along with Laurence Vico who also provided resources and advice for experimental work. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflict of interest
Luc Malaval, Hélène Follet, Delphine Farlay, Evelyne Gineyts, Sebastien Rizzo, Charlene Thomas, Mathieu Maalouf, Myriam Normand, Brigitte Burt-Pichat, Wafa Bouleftour-Esquis, Arnaud Vanden-Boscche, Norbert Laroche, Laurence Vico have no conflict of interest.
Human and Animal Rights and Informed Consent
All procedures dealing with animals were in compliance with the European Community Standards on the care and use of laboratory animals (Ministère de l’Agriculture, France, Authorization D18-0801) and approved by the local ethical committee (Comité d’Ethique en Expérimentation Animale de la Loire CEEAL-UJM, agreement n°98) and the Animal Welfare Committee of the PLEXAN facility (Platform for Experiments and Analysis, Faculty of Medicine, University of Saint-Etienne, Saint-Etienne, France).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Malaval, L., Follet, H., Farlay, D. et al. OPN, BSP, and Bone Quality—Structural, Biochemical, and Biomechanical Assessment in OPN−/−, BSP−/−, and DKO Mice. Calcif Tissue Int (2024). https://doi.org/10.1007/s00223-024-01217-0
Received:
Accepted:
Published:
DOI: https://doi.org/10.1007/s00223-024-01217-0