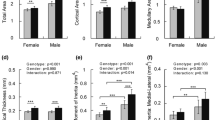
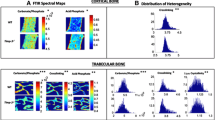
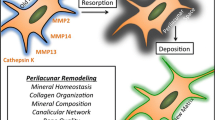
Abstract
The remarkable mechanical behavior of bone is attributed to its complex nanocomposite structure that, in addition to mineral and collagen, comprises a variety of non-collagenous matrix proteins or NCPs. Traditionally, NCPs have been studied as signaling molecules in biological processes including bone formation, resorption, and turnover. Limited attention has been given to their role in determining the mechanical properties of bone. Recent studies have highlighted that NCPs can indeed be lost or modified with aging, diseases, and drug therapies. Homozygous and heterozygous mice models of key NCP provide a useful approach to determine the impact of NCPs on bone morphology as well as matrix quality, and to carry out detailed mechanical analysis for elucidating the pathway by which NCPs can affect the mechanical properties of bone. In this article, we present a systematic analysis of a large cohort of NCPs on bone’s structural and material hierarchy, and identify three principal pathways by which they determine bone’s mechanical properties. These pathways include alterations of bone morphological parameters crucial for bone’s structural competency, bone quality changes in key matrix parameters (mineral and collagen), and a direct role as load-bearing structural proteins.





Similar content being viewed by others
References
Mccreadie BR, Goldstein SA (2000) Biomechanics of fracture: is bone mineral density sufficient to assess risk? J Bone Miner Res 15(12):2305–2308
Marshall D, Johnell O, Wedel H (1996) Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 312(7041):1254–1259
Bouxsein ML (2003) Bone quality: where do we go from here? Osteoporos Int 14(5):118–127
Paschalis EP, Shane E, Lyritis G, Skarantavos G, Mendelsohn R, Boskey AL (2004) Bone fragility and collagen cross-links. J Bone Miner Res 19(12):2000–2004
Sroga GE, Vashishth D (2012) Effects of bone matrix proteins on fracture and fragility in osteoporosis. Curr Osteoporos Rep 10(2):141–150
Ferris BD, Klenerman L, Dodds RA, Bitensky L, Chayen J (1987) Altered organization of non-collagenous bone matrix in osteoporosis. Bone 8(5):285–288
Grynpas MD, Tupy JH, Sodek J (1994) The distribution of soluble, mineral-bound, and matrix-bound proteins in osteoporotic and normal bones. Bone 15(5):505–513
Ducy P, Desbois C, Boyce B, Pinero G et al (1996) Increased bone formation in osteocalcin-deficient mice. Nature 382(6590):448–452
Gundberg CM (2003) Matrix proteins. Osteoporos Int 14(5):37–42
Glowacki J, Rey C, Glimcher MJ, Cox KA, Lian J (1991) A role for osteocalcin in osteoclast differentiation. J Cell Biochem 45(3):292–302
Rittling SR, Matsumoto HN, Mckee MD et al (1998) Mice lacking osteopontin show normal development and bone structure but display altered osteoclast formation in vitro. J Bone Miner Res 13(7):1101–1111
Romberg RW, Werness PG, Riggs BL, Mann KG (1986) Inhibition of hydroxyapatite-crystal growth by bone-specific and other calcium-binding proteins. Biochemistry 25(5):1176–1180
Price PA (1989) Gla-containing proteins of bone. Connect Tissue Res 21(1–4):51–60
Boskey AL (1989) Noncollagenous matrix proteins and their role in mineralization. Bone Miner. 6(2):111–123
Boskey AL, Gadaleta S, Gundberg C, Doty SB, Ducy P, Karsenty G (1998) Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 23(3):187–196
Ameye L, Young MF (2002) Mice deficient in small leucine-rich proteoglycans: novel in vivo models for osteoporosis, osteoarthritis, Ehlers-Danlos syndrome, muscular dystrophy, and corneal diseases. Glycobiology 12(9):107R–116R
Nikel O, Laurencin D, McCallum SA, Gundberg CM, Vashishth D (2013) NMR investigation of the role of osteocalcin and osteopontin at the organic-inorganic interface in bone. Langmuir 29(45):13873–13882
Boskey AL, Spevak L, Paschalis E, Doty SB, McKee MD (2002) Osteopontin deficiency increases mineral content and mineral crystallinity in mouse bone. Calcif Tissue Int 71(2):145–154
Kavukcuoglu NB, Denhardt DT, Guzelsu N, Mann AB (2007) Osteopontin deficiency and aging on nanomechanics of mouse bone. J Biomed Mater Res Part A 83(1):136–144
Hansma PK, Fantner GE, Kindt JH et al (2005) Sacrificial bonds in the interfibrillar matrix of bone. J Musculoskelet Neuronal Interact 5(4):313
Gupta HS, Wagermaier W, Zickler GA et al (2005) Nanoscale deformation mechanisms in bone. Nano Lett 5(10):2108–2111
Poundarik A, Diab T, Sroga GE et al (2012) Dilatational band formation in bone. Proc Natl Acad Sci 109(47):19178–19183
Garnero P, Delmas PD (2004) Contribution of bone mineral density and bone turnover markers to the estimation of risk of osteoporotic fracture in postmenopausal women. J Musculoskelet Neuronal Interact 4(1):50
Seibel MJ (2005) Biochemical markers of bone turnover part I: biochemistry and variability. Clin Biochemist 26(4):97
Ammann P, Rizzoli R, Meyer JM, Bonjour JP (1996) Bone density and shape as determinants of bone strength in IGF-I and/or pamidronate-treated ovariectomized rats. Osteoporos Int 6(3):219–227
Crabtree N, Loveridge N, Parker M, Rushton N et al (2001) Intracapsular hip fracture and the region-specific loss of cortical bone: analysis by peripheral quantitative computed tomography. J Bone Miner Res 16(7):1318–1328
Cole JH, van der Meulen MC (2011) Whole bone mechanics and bone quality. Clin Orthop Relat Res 469(8):2139–2149
Van der Meulen MCH, Jepsen KJ, Mikić B (2001) Understanding bone strength: size isn’t everything. Bone 29(2):101–104
Wolf G (1996) Function of the bone protein osteocalcin: definitive evidence. Nutr Rev 54(10):332–333
Ingram RT, Clarke BL, Fisher LW, Fitzpatrick LA (1993) Distribution of noncollagenous proteins in the matrix of adult human bone: evidence of anatomic and functional heterogeneity. J Bone Miner Res 8(9):1019–1029
Xu T, Bianco P, Fisher LW, Longenecker G et al (1998) Targeted disruption of the biglycan gene leads to an osteoporosis-like phenotype in mice. Nat Genet 20(1):78–82
Corsi A, Xu T, Chen XD, Boyde A et al (2002) Phenotypic effects of biglycan deficiency are linked to collagen fibril abnormalities, are synergized by decorin deficiency, and mimic Ehlers-Danlos-like changes in bone and other connective tissues. J Bone Miner Res 17(7):1180–1189
Arteaga-Solis E, Sui-Arteaga L, Kim M, Schaffler MB et al (2011) Material and mechanical properties of bones deficient for fibrillin-1 or fibrillin-2 microfibrils. Matrix Biol 30(3):188–194
Termine JD, Kleinman HK, Whitson SW, Conn KM et al (1981) Osteonectin, a bone-specific protein linking mineral to collagen. Cell 26(1):99–105
Delany AM, Amling M, Priemel M et al (2000) Osteopenia and decreased bone formation in osteonectin-deficient mice. J Clin Investig 105(7):915
Rios H, Koushik SV, Wang H, Wang J et al (2005) Periostin null mice exhibit dwarfism, incisor enamel defects, and an early-onset periodontal disease-like phenotype. Mol Cell Biol 25(24):11131–11144
Bonnet N, Standley KN, Bianchi EN, Stadelmann V et al (2009) The matricellular protein periostin is required for sost inhibition and the anabolic response to mechanical loading and physical activity. J Biol Chem 284(51):35939–35950
Gowen LC, Petersen DN, Mansolf AL, Qi H et al (2003) Targeted disruption of the osteoblast/osteocyte factor 45 gene (OF45) results in increased bone formation and bone mass. J Biol Chem 278(3):1998–2007
Malaval L, Wade-Guéye NM, Boudiffa M, Fei J et al (2008) Bone sialoprotein plays a functional role in bone formation and osteoclastogenesis. J Exp Med 205(5):1145–1153
Thurner PJ, Chen CG, Ionova-Martin S et al (2010) Osteopontin deficiency increases bone fragility but preserves bone mass. Bone 46(6):1564–1573
Duvall CL, Taylor WR, Weiss D, Wojtowicz AM, Guldberg RE (2007) Impaired angiogenesis, early callus formation, and late stage remodeling in fracture healing of osteopontin-deficient mice. J Bone Miner Res 22(2):286–297
Sodek J, Ganss B, McKee MD (2000) Osteopontin. Crit Rev Oral Biol Med 11(3):279–303
Delany AM, Hankenson KD (2009) Thrombospondin-2 and SPARC/osteonectin are critical regulators of bone remodeling. J Cell Commun Signal 3(3–4):227–238
Bonnet N, Gineyts E, Ammann P, Conway SJ, Garnero P, Ferrari S (2013) Periostin deficiency increases bone damage and impairs injury response to fatigue loading in adult mice. PLoS One 8(10):e78347
Currey JD (1984) Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B 304(1121):509–518
Schaffler MB, Burr DB (1988) Stiffness of compact bone: effects of porosity and density. J Biomech 21(1):13–16
Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporos Int 17(3):319–336
Vashishth D (2007) The role of the collagen matrix in skeletal fragility. Current Osteoporos Rep 5(2):62–66
Yoshitake H, Rittling SR, Denhardt DT, Noda M (1999) Osteopontin-deficient mice are resistant to ovariectomy-induced bone resorption. Proc Natl Acad Sci 96(14):8156–8160
Rodriguez DE, Thula-Mata T, Toro EJ, Yeh YW, Holt C, Holliday LS, Gower LB (2014) Multifunctional role of osteopontin in directing intrafibrillar mineralization of collagen and activation of osteoclasts. Acta Biomater 10(1):494–507
Boskey A (2003) Bone mineral crystal size. Osteoporosis international 14(5):16–21
Augat P, Schorlemmer S (2006) The role of cortical bone and its microstructure in bone strength. Age Ageing 35(suppl 2):ii27–ii31
Price PA, Otsuka AA, Poser JW, Kristaponis J, Raman N (1976) Characterization of a gamma-carboxyglutamic acid-containing protein from bone. Proc Natl Acad Sci 73(5):1447–1451
Kavukcuoglu NB, Patterson-Buckendahl P, Mann AB (2009) Effect of osteocalcin deficiency on the nanomechanics and chemistry of mouse bones. J Mech Behav Biomed Mater 2(4):348–354
Jepsen KJ, Davy DT, Krzypow DJ (1999) The role of the lamellar interface during torsional yielding of human cortical bone. J Biomech 32(3):303–310
Vashishth D (2004) Rising crack-growth-resistance behavior in cortical bone: implications for toughness measurements. J Biomech 37(6):943–946
Burstein AH, Zika JM, Heiple KG, Klein L (1975) Contribution of collagen and mineral to the elastic-plastic properties of bone. J Bone Joint Surg 57(7):956–961
Vashishth D, Gibson GJ, Khoury JI, Schaffler MB, Kimura J, Fyhrie DP (2001) Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 28(2):195–201
Kavukcuoglu NB, Arteaga-Solis E, Lee-Arteaga S, Ramirez F, Mann AB (2007) Nanomechanics and Raman spectroscopy of fibrillin 2 knock-out mouse bones. J Mater Sci 42(21):8788–8794
Vogel KG, Paulsson M, Heinegard D (1984) Specific inhibition of type I and type II collagen fibrillogenesis by the small proteoglycan of tendon. Biochem J 223:587–597
Danielson KG, Baribault H, Holmes DF, Graham H, Kadler KE, Iozzo RV (1997) Targeted disruption of decorin leads to abnormal collagen fibril morphology and skin fragility. J Cell Biol 136(3):729–743
Hoshi K, Kemmotsu S, Takeuchi Y, Amizuka N, Ozawa H (1999) The primary calcification in bones follows removal of decorin and fusion of collagen fibrils. J Bone Miner Res 14(2):273–280
Sugars RV, Milan AM, Brown JO, Waddington RJ et al (2002) Molecular interaction of recombinant decorin and biglycan with type I collagen influences crystal growth. Connect Tissue Res 44:189–195
Boskey AL, Spevak L, Doty SB, Rosenberg L (1997) Effects of bone CS-proteoglycans, DS-decorin, and DS-biglycan on hydroxyapatite formation in a gelatin gel. Calcif Tissue Int 61(4):298–305
Garnero P (2012) The contribution of collagen crosslinks to bone strength. Bonekey Rep 1:182
Termine JD, Kleinman HK, Whitson SW et al (1981) Osteonectin, a bone-specific protein linking mineral to collagen. Cell 26(1):99–105
Boskey AL, Moore DJ, Amling M, Canalis E, Delany AM (2003) Infrared analysis of the mineral and matrix in bones of osteonectin-null mice and their wildtype controls. J Bone Miner Res 18(6):1005–1011
Oxlund H, Barckman M, Ortoft G, Andreassen TT (1995) Reduced concentrations of collagen cross-links are associated with reduced strength of bone. Bone 17(4):S365–S371
Vashishth D (2005) Collagen glycation and its role in fracture properties of bone. J Musculoskelet Neuronal Interact 5(4):316
Landis WJ, Hodgens KJ, Song MJ, Arena J et al (1996) Mineralization of collagen may occur on fibril surfaces: evidence from conventional and high-voltage electron microscopy and three-dimensional imaging. J Struct Biol 117(1):24–35
Sasaki N, Nakayama Y, Yoshikawa M, Enyo A (1993) Stress relaxation function of bone and bone collagen. J Biomech 26(12):1369–1376
Tai K, Dao M, Suresh S, Palazoglu A, Ortiz C (2007) Nanoscale heterogeneity promotes energy dissipation in bone. Nat Mater 6(6):454–462
Gao H, Ji B, Jäger IL, Arzt E, Fratzl P (2003) Materials become insensitive to flaws at nanoscale: lessons from nature. Proc Natl Acad Sci 100(10):5597–5600
Jackson AP, Vincent JFV, Turner RM (1988) The mechanical design of nacre. Proc R Soc Lond B Biol Sci 234(1277):415–440
Barthelat F, Rabiei R (2011) Toughness amplification in natural composites. J Mech Phys Solids 59(4):829–840
Maruyama N, Shibata Y, Mochizuki A, Yamada A et al (2015) Bone micro-fragility caused by the mimetic aging processes in α-klotho deficient mice: in situ nanoindentation assessment of dilatational bands. Biomaterials 47:62–71
Ritter NM, Farach-Carson MC, Butler WT (1992) Evidence for the formation of a complex between osteopontin and osteocalcin. J Bone Miner Res 7(8):877–885
Thompson JB, Kindt JH, Drake B, Hansma HG et al (2001) Bone indentation recovery time correlates with bond reforming time. Nature 414(6865):773–776
Hassenkam T, Fantner GE, Cutroni JA, Weaver JC et al (2004) High-resolution AFM imaging of intact and fractured trabecular bone. Bone 35(1):4–10
Fantner GE, Hassenkam T, Kindt JH et al (2005) Sacrificial bonds and hidden length dissipate energy as mineralized fibrils separate during bone fracture. Nat Mater 4(8):612–616
Fantner GE, Adams J, Turner P, Thurner PJ et al (2007) Nanoscale ion mediated networks in bone: osteopontin can repeatedly dissipate large amounts of energy. Nano Lett 7(8):2491–2498
Diab T, Vashishth D (2005) Effects of damage morphology on cortical bone fragility. Bone 37(1):96–102
Diab T, Vashishth D (2007) Morphology, localization and accumulation of in vivo microdamage in human cortical bone. Bone 40(3):612–618
Burr DB, Turner CH, Naick P, Forwood MR et al (1998) Does microdamage accumulation affect the mechanical properties of bone? J Biomech 31(4):337–345
Acknowledgments
This work has been funded by National Institutes of Health/National Institute of Arthritis and Musculoskeletal and Skin Diseases AR49635.
Conflict of interest
Ms. Stacyann Morgan, Dr. Atharva Poundarik, and Dr. Deepak Vashishth report no biomedical financial interest or potential conflict of interest.
Human and Animal Rights and Informed Consent
All studies were carried out in compliance with NIH and IACUC guidelines for animal use.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Morgan, S., Poundarik, A.A. & Vashishth, D. Do Non-collagenous Proteins Affect Skeletal Mechanical Properties?. Calcif Tissue Int 97, 281–291 (2015). https://doi.org/10.1007/s00223-015-0016-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-015-0016-3