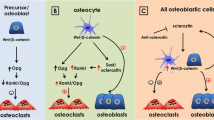
Abstract
As the most numerous and long-lived of all bone cells, osteocytes have essential functions in regulating skeletal health. Through the lacunar-canalicular system, secreted proteins from osteocytes can reach cells throughout the bone. Furthermore, the intimate connectivity between the lacunar-canalicular system and the bone vasculature allows for the transport of osteocyte-secreted factors into the circulation to reach the entire body. Local and endocrine osteocyte signaling regulates physiological processes such as bone remodeling, bone mechanoadaptation, and mineral homeostasis. However, these processes are disrupted by impaired osteocyte function induced by aging and disease. Dysfunctional osteocyte signaling is now associated with the pathogenesis of many disorders, including chronic kidney disease, cancer, diabetes mellitus, and periodontitis. In this review, we focus on the targeting of bone and extraskeletal tissues by the osteocyte secretome. In particular, we highlight the secreted osteocyte proteins, which are known to be dysregulated during aging and disease, and their roles during disease progression. We also discuss how therapeutic or genetic targeting of osteocyte-secreted proteins can improve both skeletal and systemic health.



Similar content being viewed by others
References
Prideaux M, Findlay DM, Atkins GJ (2016) Osteocytes: the master cells in bone remodelling. Curr Opin Pharmacol 28:24–30
Robling AG, Bonewald LF (2020) The osteocyte: new insights. Annu Rev Physiol 82:485–506
Delgado-Calle J, Bellido T (2022) The osteocyte as a signaling cell. Physiol Rev 102(1):379–410
Dallas SL, Prideaux M, Bonewald LF (2013) The osteocyte: an endocrine cell … and more. Endocr Rev 34(5):658–690
Plotkin LI, Speacht TL, Donahue HJ (2015) Cx43 and mechanotransduction in bone. Curr Osteoporos Rep 13(2):67–72
Fritton SP, Weinbaum S (2009) Fluid and solute transport in bone: flow-induced mechanotransduction. Annu Rev Fluid Mech 41:347–374
Tiede-Lewis LM, Xie Y, Hulbert MA, Campos R, Dallas MR, Dusevich V, Bonewald LF, Dallas SL (2017) Degeneration of the osteocyte network in the C57BL/6 mouse model of aging. Aging (Albany NY) 9(10):2190–2208
Tjalsma H, Antelmann H, Jongbloed JD, Braun PG, Darmon E, Dorenbos R, Dubois JY, Westers H, Zanen G, Quax WJ, Kuipers OP, Bron S, Hecker M, van Dijl JM (2004) Proteomics of protein secretion by Bacillus subtilis: separating the “secrets” of the secretome. Microbiol Mol Biol Rev 68(2):207–233
Benham AM (2012) Protein secretion and the endoplasmic reticulum. Cold Spring Harb Perspect Biol 4(8):a012872
Kim HJ, Kim G, Lee J, Lee Y, Kim JH (2022) Secretome of stem cells: roles of extracellular vesicles in diseases stemness, differentiation, and reprogramming. Tissue Eng Regen Med 19(1):19–33
Waqas MY, Javid MA, Nazir MM, Niaz N, Nisar MF, Manzoor Z, Bhatti SA, Hameed S, Khaliq MH (2022) Extracellular vesicles and exosome: insight from physiological regulatory perspectives. J Physiol Biochem 78(3):573–580
Jiang YL, Wang ZX, Liu XX, Wan MD, Liu YW, Jiao B, Liao XX, Luo ZW, Wang YY, Hong CG, Tan YJ, Weng L, Zhou YF, Rao SS, Cao J, Liu ZZ, Wan TF, Zhu Y, Xie H, Shen L (2022) The protective effects of osteocyte-derived extracellular vesicles against Alzheimer’s disease diminished with aging. Adv Sci (Weinh) 9(17):e2105316
Eichholz KF, Woods I, Riffault M, Johnson GP, Corrigan M, Lowry MC, Shen N, Labour MN, Wynne K, O’Driscoll L, Hoey DA (2020) Human bone marrow stem/stromal cell osteogenesis is regulated via mechanically activated osteocyte-derived extracellular vesicles. Stem Cells Transl Med 9(11):1431–1447
Elson A, Anuj A, Barnea-Zohar M, Reuven N (2022) The origins and formation of bone-resorbing osteoclasts. Bone 164:116538
Werner SL, Sharma R, Woodruff K, Horn D, Harris SE, Gorin Y, Lee DY, Hua R, Gu S, Fajardo RJ, Habib SL, Jiang JX (2020) CSF-1 in osteocytes inhibits Nox4-mediated oxidative stress and promotes normal bone homeostasis. JBMR Plus 4(7):e10080
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17(10):1235–1241
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17(10):1231–1234
Xiong J, Piemontese M, Onal M, Campbell J, Goellner JJ, Dusevich V, Bonewald L, Manolagas SC, O’Brien CA (2015) Osteocytes, not osteoblasts or lining cells, are the main source of the RANKL required for osteoclast formation in remodeling bone. PLoS ONE 10(9):e0138189
Xiong J, Cawley K, Piemontese M, Fujiwara Y, Zhao H, Goellner JJ, O’Brien CA (2018) Soluble RANKL contributes to osteoclast formation in adult mice but not ovariectomy-induced bone loss. Nat Commun 9(1):2909
Asano T, Okamoto K, Nakai Y, Tsutsumi M, Muro R, Suematsu A, Hashimoto K, Okamura T, Ehata S, Nitta T, Takayanagi H (2019) Soluble RANKL is physiologically dispensable but accelerates tumour metastasis to bone. Nat Metab 1(9):868–875
Delgado-Calle J, Hancock B, Likine EF, Sato AY, McAndrews K, Sanudo C, Bruzzaniti A, Riancho JA, Tonra JR, Bellido T (2018) MMP14 is a novel target of PTH signaling in osteocytes that controls resorption by regulating soluble RANKL production. FASEB J 32(5):2878–2890
Cawley KM, Bustamante-Gomez NC, Guha AG, MacLeod RS, Xiong J, Gubrij I, Liu Y, Mulkey R, Palmieri M, Thostenson JD, Goellner JJ, O’Brien CA (2020) Local production of osteoprotegerin by osteoblasts suppresses bone resorption. Cell Rep 32(10):108052
Choi RB, Robling AG (2021) The Wnt pathway: an important control mechanism in bone’s response to mechanical loading. Bone 153:116087
Du JH, Lin SX, Wu XL, Yang SM, Cao LY, Zheng A, Wu JN, Jiang XQ (2019) The function of Wnt ligands on osteocyte and bone remodeling. J Dent Res 98(8):930–938
Joeng KS, Lee YC, Lim J, Chen Y, Jiang MM, Munivez E, Ambrose C, Lee BH (2017) Osteocyte-specific WNT1 regulates osteoblast function during bone homeostasis. J Clin Invest 127(7):2678–2688
Alam I, Reilly AM, Alkhouli M, Gerard-O’Riley RL, Kasipathi C, Oakes DK, Wright WB, Acton D, McQueen AK, Patel B, Lim KE, Robling AG, Econs MJ (2017) Bone mass and strength are significantly improved in mice overexpressing human WNT16 in osteocytes. Calcif Tissue Int 100(4):361–373
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Lowik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. FASEB J 19(13):1842–1844
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280(20):19883–19887
Winkler DG, Sutherland MK, Geoghegan JC, Yu C, Hayes T, Skonier JE, Shpektor D, Jonas M, Kovacevich BR, Staehling-Hampton K, Appleby M, Brunkow ME, Latham JA (2003) Osteocyte control of bone formation via sclerostin, a novel BMP antagonist. EMBO J 22(23):6267–6276
Li X, Ominsky MS, Niu QT, Sun N, Daugherty B, D’Agostin D, Kurahara C, Gao Y, Cao J, Gong J, Asuncion F, Barrero M, Warmington K, Dwyer D, Stolina M, Morony S, Sarosi I, Kostenuik PJ, Lacey DL, Simonet WS, Ke HZ, Paszty C (2008) Targeted deletion of the sclerostin gene in mice results in increased bone formation and bone strength. J Bone Miner Res 23(6):860–869
Keller H, Kneissel M (2005) SOST is a target gene for PTH in bone. Bone 37(2):148–158
Bellido T, Ali AA, Gubrij I, Plotkin LI, Fu Q, O’Brien CA, Manolagas SC, Jilka RL (2005) Chronic elevation of parathyroid hormone in mice reduces expression of sclerostin by osteocytes: a novel mechanism for hormonal control of osteoblastogenesis. Endocrinology 146(11):4577–4583
Drake MT, Srinivasan B, Modder UI, Peterson JM, McCready LK, Riggs BL, Dwyer D, Stolina M, Kostenuik P, Khosla S (2010) Effects of parathyroid hormone treatment on circulating sclerostin levels in postmenopausal women. J Clin Endocrinol Metab 95(11):5056–5062
Delgado-Calle J, Tu X, Pacheco-Costa R, McAndrews K, Edwards R, Pellegrini GG, Kuhlenschmidt K, Olivos N, Robling A, Peacock M, Plotkin LI, Bellido T (2017) Control of bone anabolism in response to mechanical loading and PTH by distinct mechanisms downstream of the PTH receptor. J Bone Miner Res 32(3):522–535
Li J, Sarosi I, Cattley RC, Pretorius J, Asuncion F, Grisanti M, Morony S, Adamu S, Geng Z, Qiu W, Kostenuik P, Lacey DL, Simonet WS, Bolon B, Qian X, Shalhoub V, Ominsky MS, Ke HZ, Li X, Richards WG (2006) Dkk1-mediated inhibition of Wnt signaling in bone results in osteopenia. Bone 39(4):754–766
Witcher PC, Miner SE, Horan DJ, Bullock WA, Lim KE, Kang KS, Adaniya AL, Ross RD, Loots GG, Robling AG (2018) Sclerostin neutralization unleashes the osteoanabolic effects of Dkk1 inhibition. JCI Insight 3(11)
Guo YC, Yuan Q (2015) Fibroblast growth factor 23 and bone mineralisation. Int J Oral Sci 7(1):8–13
Clinkenbeard EL, White KE (2016) Systemic control of bone homeostasis by FGF23 signaling. Curr Mol Biol Rep 2(1):62–71
Sitara D, Kim S, Razzaque MS, Bergwitz C, Taguchi T, Schuler C, Erben RG, Lanske B (2008) Genetic evidence of serum phosphate-independent functions of FGF-23 on bone. PLoS Genet 4(8):e1000154
Lara-Castillo N, Kim-Weroha NA, Kamel MA, Javaheri B, Ellies DL, Krumlauf RE, Thiagarajan G, Johnson ML (2015) In vivo mechanical loading rapidly activates beta-catenin signaling in osteocytes through a prostaglandin mediated mechanism. Bone 76:58–66
Cherian PP, Cheng B, Gu S, Sprague E, Bonewald LF, Jiang JX (2003) Effects of mechanical strain on the function of Gap junctions in osteocytes are mediated through the prostaglandin EP2 receptor. J Biol Chem 278(44):43146–43156
Pitsillides AA, Rawlinson SC, Suswillo RF, Bourrin S, Zaman G, Lanyon LE (1995) Mechanical strain-induced NO production by bone cells: a possible role in adaptive bone (re)modeling? FASEB J 9(15):1614–1622
Genetos DC, Kephart CJ, Zhang Y, Yellowley CE, Donahue HJ (2007) Oscillating fluid flow activation of gap junction hemichannels induces ATP release from MLO-Y4 osteocytes. J Cell Physiol 212(1):207–214
Sawakami K, Robling AG, Ai M, Pitner ND, Liu D, Warden SJ, Li J, Maye P, Rowe DW, Duncan RL, Warman ML, Turner CH (2006) The Wnt co-receptor LRP5 is essential for skeletal mechanotransduction but not for the anabolic bone response to parathyroid hormone treatment. J Biol Chem 281(33):23698–23711
Robling AG, Niziolek PJ, Baldridge LA, Condon KW, Allen MR, Alam I, Mantila SM, Gluhak-Heinrich J, Bellido TM, Harris SE, Turner CH (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283(9):5866–5875
Tu X, Rhee Y, Condon KW, Bivi N, Allen MR, Dwyer D, Stolina M, Turner CH, Robling AG, Plotkin LI, Bellido T (2012) Sost downregulation and local Wnt signaling are required for the osteogenic response to mechanical loading. Bone 50(1):209–217
Pflanz D, Birkhold AI, Albiol L, Thiele T, Julien C, Seliger A, Thomson E, Kramer I, Kneissel M, Duda GN, Kornak U, Checa S, Willie BM (2017) Sost deficiency led to a greater cortical bone formation response to mechanical loading and altered gene expression. Sci Rep 7(1):9435
Holguin N, Brodt MD, Silva MJ (2016) Activation of Wnt signaling by mechanical loading is impaired in the bone of old mice. J Bone Miner Res 31(12):2215–2226
Yan Z, Wang P, Wu J, Feng X, Cai J, Zhai M, Li J, Liu X, Jiang M, Luo E, Jing D (2018) Fluid shear stress improves morphology, cytoskeleton architecture, viability, and regulates cytokine expression in a time-dependent manner in MLO-Y4 cells. Cell Biol Int 42(10):1410–1422
Lau KH, Baylink DJ, Zhou XD, Rodriguez D, Bonewald LF, Li Z, Ruffoni D, Muller R, Kesavan C, Sheng MH (2013) Osteocyte-derived insulin-like growth factor I is essential for determining bone mechanosensitivity. Am J Physiol Endocrinol Metab 305(2):E271–E281
Reijnders CM, Bravenboer N, Holzmann PJ, Bhoelan F, Blankenstein MA, Lips P (2007) In vivo mechanical loading modulates insulin-like growth factor binding protein-2 gene expression in rat osteocytes. Calcif Tissue Int 80(2):137–143
Li X, Han L, Nookaew I, Mannen E, Silva MJ, Almeida M, Xiong J (2019) Stimulation of Piezo1 by mechanical signals promotes bone anabolism. Elife 8.
Sasaki F, Hayashi M, Mouri Y, Nakamura S, Adachi T, Nakashima T (2020) Mechanotransduction via the Piezo1-Akt pathway underlies Sost suppression in osteocytes. Biochem Biophys Res Commun 521(3):806–813
Lin C, Jiang X, Dai Z, Guo X, Weng T, Wang J, Li Y, Feng G, Gao X, He L (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/beta-catenin signaling. J Bone Miner Res 24(10):1651–1661
Agoro R, White KE (2023) Regulation of FGF23 production and phosphate metabolism by bone-kidney interactions. Nat Rev Nephrol
Yamamoto H, Ramos-Molina B, Lick AN, Prideaux M, Albornoz V, Bonewald L, Lindberg I (2016) Posttranslational processing of FGF23 in osteocytes during the osteoblast to osteocyte transition. Bone 84:120–130
Ichikawa S, Sorenson AH, Austin AM, Mackenzie DS, Fritz TA, Moh A, Hui SL, Econs MJ (2009) Ablation of the Galnt3 gene leads to low-circulating intact fibroblast growth factor 23 (Fgf23) concentrations and hyperphosphatemia despite increased Fgf23 expression. Endocrinology 150(6):2543–2550
Kuro-o M (2009) Klotho and aging. Biochim Biophys Acta 1790(10):1049–1058
Dalton GD, Xie J, An SW, Huang CL (2017) New insights into the mechanism of action of soluble klotho. Front Endocrinol (Lausanne) 8:323
Minamizaki T, Konishi Y, Sakurai K, Yoshioka H, Aubin JE, Kozai K, Yoshiko Y (2018) Soluble Klotho causes hypomineralization in Klotho-deficient mice. J Endocrinol 237(3):285–300
Komaba H, Kaludjerovic J, Hu DZ, Nagano K, Amano K, Ide N, Sato T, Densmore MJ, Hanai JI, Olauson H, Bellido T, Larsson TE, Baron R, Lanske B (2017) Klotho expression in osteocytes regulates bone metabolism and controls bone formation. Kidney Int 92(3):599–611
Rhee Y, Bivi N, Farrow E, Lezcano V, Plotkin LI, White KE, Bellido T (2011) Parathyroid hormone receptor signaling in osteocytes increases the expression of fibroblast growth factor-23 in vitro and in vivo. Bone 49(4):636–643
Ben-Dov IZ, Galitzer H, Lavi-Moshayoff V, Goetz R, Kuro-o M, Mohammadi M, Sirkis R, Naveh-Many T, Silver J (2007) The parathyroid is a target organ for FGF23 in rats. J Clin Invest 117(12):4003–4008
Bar L, Stournaras C, Lang F, Foller M (2019) Regulation of fibroblast growth factor 23 (FGF23) in health and disease. FEBS Lett 593(15):1879–1900
Ito N, Prideaux M, Wijenayaka AR, Yang D, Ormsby RT, Bonewald LF, Atkins GJ (2021) Sclerostin directly stimulates osteocyte synthesis of fibroblast growth factor-23. Calcif Tissue Int 109(1):66–76
Bonewald L (2019) Use it or lose it to age: a review of bone and muscle communication. Bone 120:212–218
Huang J, Romero-Suarez S, Lara N, Mo C, Kaja S, Brotto L, Dallas SL, Johnson ML, Jahn K, Bonewald LF, Brotto M (2017) Crosstalk between MLO-Y4 osteocytes and C2C12 muscle cells is mediated by the Wnt/beta-catenin pathway. JBMR Plus 1(2):86–100
Li G, Zhang L, Ning K, Yang B, Acosta FM, Shang P, Jiang JX, Xu H (2021) Osteocytic Connexin43 channels regulate bone-muscle crosstalk. Cells 10(2)
Wood CL, Pajevic PD, Gooi JH (2017) Osteocyte secreted factors inhibit skeletal muscle differentiation. Bone Rep 6:74–80
Huang J, Wang K, Shiflett LA, Brotto L, Bonewald LF, Wacker MJ, Dallas SL, Brotto M (2019) Fibroblast growth factor 9 (FGF9) inhibits myogenic differentiation of C2C12 and human muscle cells. Cell Cycle 18(24):3562–3580
Essex AL, Deosthale P, Huot JR, Davis HM, Momeni N, Bonetto A, Plotkin LI (2022) miR21 deletion in osteocytes has direct and indirect effects on skeletal muscle in a sex-dimorphic manner in mice. Biol Sex Differ 13(1):56
Sheng Z, Tong D, Ou Y, Zhang H, Zhang Z, Li S, Zhou J, Zhang J, Liao E (2012) Serum sclerostin levels were positively correlated with fat mass and bone mineral density in central south Chinese postmenopausal women. Clin Endocrinol (Oxf) 76(6):797–801
Kim SP, Frey JL, Li Z, Kushwaha P, Zoch ML, Tomlinson RE, Da H, Aja S, Noh HL, Kim JK, Hussain MA, Thorek DLJ, Wolfgang MJ, Riddle RC (2017) Sclerostin influences body composition by regulating catabolic and anabolic metabolism in adipocytes. Proc Natl Acad Sci U S A 114(52):E11238–E11247
Kim SP, Da H, Wang L, Taketo MM, Wan M, Riddle RC (2021) Bone-derived sclerostin and Wnt/beta-catenin signaling regulate PDGFRalpha(+) adipoprogenitor cell differentiation. FASEB J 35(11):e21957
Kim SP, Da H, Li Z, Kushwaha P, Beil C, Mei L, Xiong WC, Wolfgang MJ, Clemens TL, Riddle RC (2019) Lrp4 expression by adipocytes and osteoblasts differentially impacts sclerostin’s endocrine effects on body composition and glucose metabolism. J Biol Chem 294(17):6899–6911
Fulzele K, Lai F, Dedic C, Saini V, Uda Y, Shi C, Tuck P, Aronson JL, Liu X, Spatz JM, Wein MN, Pajevic PD (2017) Osteocyte-secreted wnt signaling inhibitor sclerostin contributes to beige adipogenesis in peripheral fat depots. J Bone Miner Res 32(2):373–384
Brun J, Berthou F, Trajkovski M, Maechler P, Foti M, Bonnet N (2017) Bone regulates browning and energy metabolism through mature osteoblast/osteocyte PPARgamma expression. Diabetes 66(10):2541–2554
Baroi S, Czernik PJ, Chougule A, Griffin PR, Lecka-Czernik B (2021) PPARG in osteocytes controls sclerostin expression, bone mass, marrow adiposity and mediates TZD-induced bone loss. Bone 147:115913
Dong B, Hiasa M, Higa Y, Ohnishi Y, Endo I, Kondo T, Takashi Y, Tsoumpra M, Kainuma R, Sawatsubashi S, Kiyonari H, Shioi G, Sakaue H, Nakashima T, Kato S, Abe M, Fukumoto S, Matsumoto T (2022) Osteoblast/osteocyte-derived interleukin-11 regulates osteogenesis and systemic adipogenesis. Nat Commun 13(1):7194
Jansson JO, Palsdottir V, Hagg DA, Schele E, Dickson SL, Anesten F, Bake T, Montelius M, Bellman J, Johansson ME, Cone RD, Drucker DJ, Wu J, Aleksic B, Tornqvist AE, Sjogren K, Gustafsson JA, Windahl SH, Ohlsson C (2018) Body weight homeostat that regulates fat mass independently of leptin in rats and mice. Proc Natl Acad Sci USA 115(2):427–432
Busse B, Djonic D, Milovanovic P, Hahn M, Puschel K, Ritchie RO, Djuric M, Amling M (2010) Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 9(6):1065–1075
Roforth MM, Fujita K, McGregor UI, Kirmani S, McCready LK, Peterson JM, Drake MT, Monroe DG, Khosla S (2014) Effects of age on bone mRNA levels of sclerostin and other genes relevant to bone metabolism in humans. Bone 59:1–6
Zhang C, Xu S, Zhang S, Liu M, Du H, Sun R, Jing B, Sun Y (2019) Ageing characteristics of bone indicated by transcriptomic and exosomal proteomic analysis of cortical bone cells. J Orthop Surg Res 14(1):129
Negishi-Koga T, Takayanagi H (2012) Bone cell communication factors and Semaphorins. Bonekey Rep 1:183
Hayashi M, Nakashima T, Yoshimura N, Okamoto K, Tanaka S, Takayanagi H (2019) Autoregulation of osteocyte Sema3A orchestrates estrogen action and counteracts bone aging. Cell Metab 29(3):627–637 e5.
Niimura M, Sato T, Enoki Y, Okubo M, Kokabu S, Takeda S, Yoda T (2016) Semaphorin 3A promotes dendrite elongation of osteocytes in association with down-regulation of CDK6. In Vivo 30(3):231–236
Farr JN, Fraser DG, Wang H, Jaehn K, Ogrodnik MB, Weivoda MM, Drake MT, Tchkonia T, LeBrasseur NK, Kirkland JL, Bonewald LF, Pignolo RJ, Monroe DG, Khosla S (2016) Identification of senescent cells in the bone microenvironment. J Bone Miner Res 31(11):1920–1929
Farr JN, Xu M, Weivoda MM, Monroe DG, Fraser DG, Onken JL, Negley BA, Sfeir JG, Ogrodnik MB, Hachfeld CM, LeBrasseur NK, Drake MT, Pignolo RJ, Pirtskhalava T, Tchkonia T, Oursler MJ, Kirkland JL, Khosla S (2017) Targeting cellular senescence prevents age-related bone loss in mice. Nat Med 23(9):1072–1079
Farr JN, Saul D, Doolittle ML, Kaur J, Rowsey JL, Vos SJ, Froemming MN, Lagnado AB, Zhu Y, Weivoda MM, Ikeno Y, Pignolo RJ, Niedernhofer LJ, Robbins PD, Jurk D, Passos JF, LeBrasseur NK, Tchkonia T, Kirkland JL, Monroe DG, Khosla S (2023) Local senolysis in aged mice only partially replicates the benefits of systemic senolysis. J Clin Invest (2023).
Farr JN, Kaur J, Doolittle ML, Khosla S (2020) Osteocyte cellular senescence. Curr Osteoporos Rep 18(5):559–567
Piemontese M, Almeida M, Robling AG, Kim HN, Xiong J, Thostenson JD, Weinstein RS, Manolagas SC, O'Brien CA, Jilka RL (2017) Old age causes de novo intracortical bone remodeling and porosity in mice. JCI Insight 2(17)
Kim HN, Xiong J, MacLeod RS, Iyer S, Fujiwara Y, Cawley KM, Han L, He Y, Thostenson JD, Ferreira E, Jilka RL, Zhou D, Almeida M, O'Brien CA (2020) Osteocyte RANKL is required for cortical bone loss with age and is induced by senescence. JCI Insight 5(19).
Kim BJ, Bae SJ, Lee SY, Lee YS, Baek JE, Park SY, Lee SH, Koh JM, Kim GS (2012) TNF-alpha mediates the stimulation of sclerostin expression in an estrogen-deficient condition. Biochem Biophys Res Commun 424(1):170–175
Li X, Ominsky MS, Warmington KS, Morony S, Gong J, Cao J, Gao Y, Shalhoub V, Tipton B, Haldankar R, Chen Q, Winters A, Boone T, Geng Z, Niu QT, Ke HZ, Kostenuik PJ, Simonet WS, Lacey DL, Paszty C (2009) Sclerostin antibody treatment increases bone formation, bone mass, and bone strength in a rat model of postmenopausal osteoporosis. J Bone Miner Res 24(4):578–588
Garnero P (2017) The utility of biomarkers in osteoporosis management. Mol Diagn Ther 21(4):401–418
Peng J, Dong Z, Hui Z, Aifei W, Lianfu D, Youjia X (2021) Bone Sclerostin and Dickkopf-related protein-1 are positively correlated with bone mineral density, bone microarchitecture, and bone strength in postmenopausal osteoporosis. BMC Musculoskelet Disord 22(1):480
Pacicca DM, Brown T, Watkins D, Kover K, Yan Y, Prideaux M, Bonewald L (2019) Elevated glucose acts directly on osteocytes to increase sclerostin expression in diabetes. Sci Rep 9(1):17353
Boltenstal H, Qureshi AR, Behets GJ, Lindholm B, Stenvinkel P, D’Haese PC, Haarhaus M (2019) Association of serum sclerostin with bone sclerostin in chronic kidney disease is lost in glucocorticoid treated patients. Calcif Tissue Int 104(2):214–223
Rachner TD, Hofbauer LC, Gobel A, Tsourdi E (2019) Novel therapies in osteoporosis: PTH-related peptide analogs and inhibitors of sclerostin. J Mol Endocrinol 62(2):R145–R154
Fujiwara Y, Piemontese M, Liu Y, Thostenson JD, Xiong J, O’Brien CA (2016) RANKL (Receptor Activator of NFkappaB Ligand) produced by osteocytes is required for the increase in B cells and bone loss caused by estrogen deficiency in mice. J Biol Chem 291(48):24838–24850
Zhang Y, Chen CY, Liu YW, Rao SS, Tan YJ, Qian YX, Xia K, Huang J, Liu XX, Hong CG, Yin H, Cao J, Feng SK, He ZH, Li YY, Luo ZW, Wu B, Yan ZQ, Chen TH, Chen ML, Wang YY, Wang ZX, Liu ZZ, Luo MJ, Hu XK, Jin L, Wan TF, Yue T, Tang SY, Xie H (2021) Neuronal induction of bone-fat imbalance through osteocyte neuropeptide Y. Adv Sci (Weinh) 8(24):e2100808
Tomlinson RE, Christiansen BA, Giannone AA, Genetos DC (2020) The role of nerves in skeletal development, adaptation, and aging. Front Endocrinol (Lausanne) 11:646
de Vries TJ, Huesa C (2019) The osteocyte as a novel key player in understanding periodontitis through its expression of RANKL and sclerostin: a review. Curr Osteoporos Rep 17(3):116–121
Yoshimoto T, Kittaka M, Doan AAP, Urata R, Prideaux M, Rojas RE, Harding CV, Boom WH, Bonewald LF, Greenfield EM, Ueki Y (2022) Osteocytes directly regulate osteolysis via MYD88 signaling in bacterial bone infection. Nat Commun 13(1):6648
Graves DT, Alshabab A, Albiero ML, Mattos M, Correa JD, Chen S, Yang Y (2018) Osteocytes play an important role in experimental periodontitis in healthy and diabetic mice through expression of RANKL. J Clin Periodontol 45(3):285–292
Kanzaki H, Makihira S, Suzuki M, Ishii T, Movila A, Hirschfeld J, Mawardi H, Lin X, Han X, Taubman MA, Kawai T (2016) Soluble RANKL cleaved from activated lymphocytes by TNF-alpha-converting enzyme contributes to osteoclastogenesis in periodontitis. J Immunol 197(10):3871–3883
Balli U, Aydogdu A, Dede FO, Turer CC, Guven B (2015) Gingival crevicular fluid levels of sclerostin, osteoprotegerin, and receptor activator of nuclear factor-kappaB ligand in periodontitis. J Periodontol 86(12):1396–1404
Taut AD, Jin Q, Chung JH, Galindo-Moreno P, Yi ES, Sugai JV, Ke HZ, Liu M, Giannobile WV (2013) Sclerostin antibody stimulates bone regeneration after experimental periodontitis. J Bone Miner Res 28(11):2347–2356
Goes P, Dutra C, Losser L, Hofbauer LC, Rauner M, Thiele S (2019) Loss of Dkk-1 in osteocytes mitigates alveolar bone loss in mice with periodontitis. Front Immunol 10:2924
Yang D, Wijenayaka AR, Solomon LB, Pederson SM, Findlay DM, Kidd SP, Atkins GJ (2018) Novel insights into staphylococcus aureus deep bone infections: the involvement of osteocytes. mBio 9(2)
Masters EA, Muthukrishnan G, Ho L, Gill AL, de Mesy Bentley KL, Galloway CA, McGrath JL, Awad HA, Gill SR, Schwarz EM (2021) Staphylococcus aureus cell wall biosynthesis modulates bone invasion and osteomyelitis pathogenesis. Front Microbiol 12:723498
Ormsby RT, Zelmer AR, Yang D, Gunn NJ, Starczak Y, Kidd SP, Nelson R, Solomon LB, Atkins GJ (2021) Evidence for osteocyte-mediated bone-matrix degradation associated with periprosthetic joint infection (PJI). Eur Cell Mater 42:264–280
Bailey KN, Nguyen J, Yee CS, Dole NS, Dang A, Alliston T (2021) Mechanosensitive control of articular cartilage and subchondral bone homeostasis in mice requires osteocytic transforming growth factor beta signaling. Arthritis Rheumatol 73(3):414–425
Muratovic D, Findlay DM, Quarrington RD, Cao X, Solomon LB, Atkins GJ, Kuliwaba JS (2022) Elevated levels of active transforming growth factor beta1 in the subchondral bone relate spatially to cartilage loss and impaired bone quality in human knee osteoarthritis. Osteoarthritis Cartil 30(6):896–907
Tsourdi E, Jahn K, Rauner M, Busse B, Bonewald LF (2018) Physiological and pathological osteocytic osteolysis. J Musculoskelet Neuronal Interact 18(3):292–303
Mazur CM, Woo JJ, Yee CS, Fields AJ, Acevedo C, Bailey KN, Kaya S, Fowler TW, Lotz JC, Dang A, Kuo AC, Vail TP, Alliston T (2019) Osteocyte dysfunction promotes osteoarthritis through MMP13-dependent suppression of subchondral bone homeostasis. Bone Res 7:34
Dole NS, Mazur CM, Acevedo C, Lopez JP, Monteiro DA, Fowler TW, Gludovatz B, Walsh F, Regan JN, Messina S, Evans DS, Lang TF, Zhang B, Ritchie RO, Mohammad KS, Alliston T (2017) Osteocyte-intrinsic TGF-beta signaling regulates bone quality through perilacunar/canalicular remodeling. Cell Rep 21(9):2585–2596
Yamazaki M, Michigami T (2022) Osteocytes and the pathogenesis of hypophosphatemic rickets. Front Endocrinol (Lausanne) 13:1005189
Clinkenbeard EL, Cass TA, Ni P, Hum JM, Bellido T, Allen MR, White KE (2016) Conditional deletion of murine Fgf 23: interruption of the normal skeletal responses to phosphate challenge and rescue of genetic hypophosphatemia. J Bone Miner Res 31(6):1247–1257
Aono Y, Yamazaki Y, Yasutake J, Kawata T, Hasegawa H, Urakawa I, Fujita T, Wada M, Yamashita T, Fukumoto S, Shimada T (2009) Therapeutic effects of anti-FGF23 antibodies in hypophosphatemic rickets/osteomalacia. J Bone Miner Res 24(11):1879–1888
Imel EA, Glorieux FH, Whyte MP, Munns CF, Ward LM, Nilsson O, Simmons JH, Padidela R, Namba N, Cheong HI, Pitukcheewanont P, Sochett E, Hogler W, Muroya K, Tanaka H, Gottesman GS, Biggin A, Perwad F, Mao M, Chen CY, Skrinar A, Martin JS, Portale AA (2019) Burosumab versus conventional therapy in children with X-linked hypophosphataemia: a randomised, active-controlled, open-label, phase 3 trial. Lancet 393(10189):2416–2427
Carpenter KA, Ross RD (2020) Sclerostin antibody treatment increases bone mass and normalizes circulating phosphate levels in growing hyp mice. J Bone Miner Res 35(3):596–607
Sarathchandra P, Pope FM, Kayser MV, Ali SY (2000) A light and electron microscopic study of osteogenesis imperfecta bone samples, with reference to collagen chemistry and clinical phenotype. J Pathol 192(3):385–395
Zimmerman SM, Heard-Lipsmeyer ME, Dimori M, Thostenson JD, Mannen EM, O’Brien CA, Morello R (2018) Loss of RANKL in osteocytes dramatically increases cancellous bone mass in the osteogenesis imperfecta mouse (oim). Bone Rep 9:61–73
Zimmerman SM, Dimori M, Heard-Lipsmeyer ME, Morello R (2019) The osteocyte transcriptome is extensively dysregulated in mouse models of osteogenesis imperfecta. JBMR Plus 3(7):e10171
Balemans W, Patel N, Ebeling M, Van Hul E, Wuyts W, Lacza C, Dioszegi M, Dikkers FG, Hildering P, Willems PJ, Verheij JB, Lindpaintner K, Vickery B, Foernzler D, Van Hul W (2002) Identification of a 52 kb deletion downstream of the SOST gene in patients with van Buchem disease. J Med Genet 39(2):91–97
Brunkow ME, Gardner JC, Van Ness J, Paeper BW, Kovacevich BR, Proll S, Skonier JE, Zhao L, Sabo PJ, Fu Y, Alisch RS, Gillett L, Colbert T, Tacconi P, Galas D, Hamersma H, Beighton P, Mulligan J (2001) Bone dysplasia sclerosteosis results from loss of the SOST gene product, a novel cystine knot-containing protein. Am J Hum Genet 68(3):577–589
van Lierop AH, Moester MJ, Hamdy NA, Papapoulos SE (2014) Serum Dickkopf 1 levels in sclerostin deficiency. J Clin Endocrinol Metab 99(2):E252–E256
Bovijn J, Krebs K, Chen CY, Boxall R, Censin JC, Ferreira T, Pulit SL, Glastonbury CA, Laber S, Millwood IY, Lin K, Li L, Chen Z, Milani L, Smith GD, Walters RG, Magi R, Neale BM, Lindgren CM, Holmes MV (2020) Evaluating the cardiovascular safety of sclerostin inhibition using evidence from meta-analysis of clinical trials and human genetics. Sci Transl Med 12(549)
Golledge J, Thanigaimani S (2022) Role of sclerostin in cardiovascular disease. Arterioscler Thromb Vasc Biol 42(7):e187–e202
Joll JE 2nd, Riley LA, Bersi MR, Nyman JS, Merryman WD (2022) Sclerostin ablation prevents aortic valve stenosis in mice. Am J Physiol Heart Circ Physiol 323(5):H1037–H1047
Langdahl BL, Hofbauer LC, Forfar JC (2021) Cardiovascular safety and sclerostin inhibition. J Clin Endocrinol Metab 106(7):1845–1853
Batra J, Buttar RS, Kaur P, Kreimerman J, Melamed ML (2016) FGF-23 and cardiovascular disease: review of literature. Curr Opin Endocrinol Diabetes Obes 23(6):423–429
Vazquez-Sanchez S, Poveda J, Navarro-Garcia JA, Gonzalez-Lafuente L, Rodriguez-Sanchez E, Ruilope LM, Ruiz-Hurtado G (2021) An overview of FGF-23 as a novel candidate biomarker of cardiovascular risk. Front Physiol 12:632260
Rausch S, Foller M (2022) The regulation of FGF23 under physiological and pathophysiological conditions. Pflugers Arch 474(3):281–292
Pazianas M, Miller PD (2021) Osteoporosis and chronic kidney disease-mineral and bone disorder (CKD-MBD): back to basics. Am J Kidney Dis 78(4):582–589
Faul C, Amaral AP, Oskouei B, Hu MC, Sloan A, Isakova T, Gutierrez OM, Aguillon-Prada R, Lincoln J, Hare JM, Mundel P, Morales A, Scialla J, Fischer M, Soliman EZ, Chen J, Go AS, Rosas SE, Nessel L, Townsend RR, Feldman HI, St John Sutton M, Ojo A, Gadegbeku C, Di Marco GS, Reuter S, Kentrup D, Tiemann K, Brand M, Hill JA, Moe OW, Kuro OM, Kusek JW, Keane MG, Wolf M (2011) FGF23 induces left ventricular hypertrophy. J Clin Invest 121(11):4393–4408
Touchberry CD, Green TM, Tchikrizov V, Mannix JE, Mao TF, Carney BW, Girgis M, Vincent RJ, Wetmore LA, Dawn B, Bonewald LF, Stubbs JR, Wacker MJ (2013) FGF23 is a novel regulator of intracellular calcium and cardiac contractility in addition to cardiac hypertrophy. Am J Physiol Endocrinol Metab 304(8):E863–E873
Clinkenbeard EL, Noonan ML, Thomas JC, Ni P, Hum JM, Aref M, Swallow EA, Moe SN, Allen MR, White KE (2019) Increased FGF23 protects against detrimental cardio-renal consequences during elevated blood phosphate in CKD. JCI Insight 4(4)
Shalhoub V, Shatzen EM, Ward SC, Davis J, Stevens J, Bi V, Renshaw L, Hawkins N, Wang W, Chen C, Tsai MM, Cattley RC, Wronski TJ, Xia X, Li X, Henley C, Eschenberg M, Richards WG (2012) FGF23 neutralization improves chronic kidney disease-associated hyperparathyroidism yet increases mortality. J Clin Invest 122(7):2543–2553
Avin KG, Vallejo JA, Chen NX, Wang K, Touchberry CD, Brotto M, Dallas SL, Moe SM, Wacker MJ (2018) Fibroblast growth factor 23 does not directly influence skeletal muscle cell proliferation and differentiation or ex vivo muscle contractility. Am J Physiol Endocrinol Metab 315(4):E594–E604
Drechsler C, Evenepoel P, Vervloet MG, Wanner C, Ketteler M, Marx N, Floege J, Dekker FW, Brandenburg VM, N.S. Group (2015) High levels of circulating sclerostin are associated with better cardiovascular survival in incident dialysis patients: results from the NECOSAD study. Nephrol Dial Transpl 30(2):288–293
Gong L, Zheng D, Yuan J, Cao L, Ni Z, Fang W (2018) Elevated levels of serum sclerostin are linked to adverse cardiovascular outcomes in peritoneal dialysis patients. Int Urol Nephrol 50(5):955–961
Moe SM, Chen NX, Newman CL, Organ JM, Kneissel M, Kramer I, Gattone VH 2nd, Allen MR (2015) Anti-sclerostin antibody treatment in a rat model of progressive renal osteodystrophy. J Bone Miner Res 30(3):499–509
Schacter GI, Leslie WD (2021) Diabetes and osteoporosis: part i, epidemiology and pathophysiology. Endocrinol Metab Clin North Am 50(2):275–285
Murray CE, Coleman CM (2019) Impact of diabetes mellitus on bone health. Int J Mol Sci 20(19)
Maycas M, McAndrews KA, Sato AY, Pellegrini GG, Brown DM, Allen MR, Plotkin LI, Gortazar AR, Esbrit P, Bellido T (2017) PTHrP-derived peptides restore bone mass and strength in diabetic mice: additive effect of mechanical loading. J Bone Miner Res 32(3):486–497
Hildebrandt N, Colditz J, Dutra C, Goes P, Salbach-Hirsch J, Thiele S, Hofbauer LC, Rauner M (2021) Role of osteogenic Dickkopf-1 in bone remodeling and bone healing in mice with type I diabetes mellitus. Sci Rep 11(1):1920
Piccoli A, Cannata F, Strollo R, Pedone C, Leanza G, Russo F, Greto V, Isgro C, Quattrocchi CC, Massaroni C, Silvestri S, Vadala G, Bisogno T, Denaro V, Pozzilli P, Tang SY, Silva MJ, Conte C, Papalia R, Maccarrone M, Napoli N (2020) Sclerostin regulation, microarchitecture, and advanced glycation end-products in the bone of elderly women with type 2 diabetes. J Bone Miner Res 35(12):2415–2422
Tanaka K, Yamaguchi T, Kanazawa I, Sugimoto T (2015) Effects of high glucose and advanced glycation end products on the expressions of sclerostin and RANKL as well as apoptosis in osteocyte-like MLO-Y4-A2 cells. Biochem Biophys Res Commun 461(2):193–199
Eckhardt BA, Rowsey JL, Thicke BD, Fraser DG, O'Grady KL, Bondar OP, Hines JM, Singh RJ, Thoreson AR, Rakshit K, Lagnado AB, Passos JF, Vella A, Matveyenko AV, Khosla S, Monroe DG, Farr JN (2020) Accelerated osteocyte senescence and skeletal fragility in mice with type 2 diabetes. JCI Insight 5(9) (2020).
Pin F, Prideaux M, Bonewald LF, Bonetto A (2021) Osteocytes and cancer. Curr Osteoporos Rep 19(6):616–625
Atkinson EG, Delgado-Calle J (2019) The emerging role of osteocytes in cancer in bone. JBMR Plus 3(3):e10186
Ideta H, Yoshida K, Okamoto M, Sasaki J, Kito M, Aoki K, Yoshimura Y, Suzuki S, Tanaka A, Takazawa A, Haniu H, Uemura T, Takizawa T, Sobajima A, Kamanaka T, Takahashi J, Kato H, Saito N (2021) Antitumor effect of sclerostin against osteosarcoma. Cancers (Basel) 13(23)
Hesse E, Schroder S, Brandt D, Pamperin J, Saito H, Taipaleenmaki H (2019) Sclerostin inhibition alleviates breast cancer-induced bone metastases and muscle weakness. JCI Insight 5(9)
Zhu M, Liu C, Li S, Zhang S, Yao Q, Song Q (2017) Sclerostin induced tumor growth, bone metastasis and osteolysis in breast cancer. Sci Rep 7(1):11399
Hiraga T, Horibe K, Koide M, Yamashita T, Kobayashi Y (2023) Sclerostin blockade promotes bone metastases of Wnt-responsive breast cancer cells. Cancer Sci
Liu S, Wu D, Sun X, Fan Y, Zha R, Jalali A, Feng Y, Li K, Sano T, Vike N, Li F, Rispoli J, Sudo A, Liu J, Robling A, Nakshatri H, Li BY, Yokota H (2021) Overexpression of Lrp5 enhanced the anti-breast cancer effects of osteocytes in bone. Bone Res 9(1):32
Dwivedi A, Kiely PA, Hoey DA (2021) Mechanically stimulated osteocytes promote the proliferation and migration of breast cancer cells via a potential CXCL1/2 mechanism. Biochem Biophys Res Commun 534:14–20
Sottnik JL, Dai J, Zhang H, Campbell B, Keller ET (2015) Tumor-induced pressure in the bone microenvironment causes osteocytes to promote the growth of prostate cancer bone metastases. Cancer Res 75(11):2151–2158
McDonald MM, Reagan MR, Youlten SE, Mohanty ST, Seckinger A, Terry RL, Pettitt JA, Simic MK, Cheng TL, Morse A, Le LMT, Abi-Hanna D, Kramer I, Falank C, Fairfield H, Ghobrial IM, Baldock PA, Little DG, Kneissel M, Vanderkerken K, Bassett JHD, Williams GR, Oyajobi BO, Hose D, Phan TG, Croucher PI (2017) Inhibiting the osteocyte-specific protein sclerostin increases bone mass and fracture resistance in multiple myeloma. Blood 129(26):3452–3464
Delgado-Calle J, Anderson J, Cregor MD, Condon KW, Kuhstoss SA, Plotkin LI, Bellido T, Roodman GD (2017) Genetic deletion of Sost or pharmacological inhibition of sclerostin prevent multiple myeloma-induced bone disease without affecting tumor growth. Leukemia 31(12):2686–2694
Liu H, He J, Bagheri-Yarmand R, Li Z, Liu R, Wang Z, Bach DH, Huang YH, Lin P, Guise TA, Gagel RF, Yang J (2022) Osteocyte CIITA aggravates osteolytic bone lesions in myeloma. Nat Commun 13(1):3684
Delgado-Calle J, Anderson J, Cregor MD, Hiasa M, Chirgwin JM, Carlesso N, Yoneda T, Mohammad KS, Plotkin LI, Roodman GD, Bellido T (2016) Bidirectional notch signaling and osteocyte-derived factors in the bone marrow microenvironment promote tumor cell proliferation and bone destruction in multiple myeloma. Cancer Res 76(5):1089–1100
Giuliani N, Ferretti M, Bolzoni M, Storti P, Lazzaretti M, Palma BD, Bonomini S, Martella E, Agnelli L, Neri A, Ceccarelli F, Palumbo C (2012) Increased osteocyte death in multiple myeloma patients: role in myeloma-induced osteoclast formation. Leukemia 26(6):1391–1401
Wang W, Yang X, Dai J, Lu Y, Zhang J, Keller ET (2019) Prostate cancer promotes a vicious cycle of bone metastasis progression through inducing osteocytes to secrete GDF15 that stimulates prostate cancer growth and invasion. Oncogene 38(23):4540–4559
Bonetto A, Kays JK, Parker VA, Matthews RR, Barreto R, Puppa MJ, Kang KS, Carson JA, Guise TA, Mohammad KS, Robling AG, Couch ME, Koniaris LG, Zimmers TA (2016) Differential bone loss in mouse models of colon cancer cachexia. Front Physiol 7:679
Pin F, Prideaux M, Huot JR, Essex AL, Plotkin LI, Bonetto A, Bonewald LF (2021) Non-bone metastatic cancers promote osteocyte-induced bone destruction. Cancer Lett 520:80–90
Meng Q, Lin MS, Tzeng IS (2020) Relationship between exercise and Alzheimer’s disease: a narrative literature review. Front Neurosci 14:131
Sabol HM, Ferrari AJ, Adhikari M, Amorim T, McAndrews K, Anderson J, Vigolo M, Lehal R, Cregor M, Khan S, Cuevas PL, Helms JA, Kurihara N, Srinivasan V, Ebetino FH, Boeckman RK Jr, Roodman GD, Bellido T, Delgado-Calle J (2021) Targeting notch inhibitors to the myeloma bone marrow niche decreases tumor growth and bone destruction without gut toxicity. Cancer Res 81(19):5102–5114
Sabol HM, Amorim T, Ashby C, Halladay D, Anderson J, Cregor M, Sweet M, Nookaew I, Kurihara N, Roodman GD, Bellido T, Delgado-Calle J (2022) Notch3 signaling between myeloma cells and osteocytes in the tumor niche promotes tumor growth and bone destruction. Neoplasia 28:100785
Kitase Y, Prideaux M (2023) Targeting osteocytes vs osteoblasts. Bone 170:116724
Yang R, Meyer AS, Droujinine IA, Udeshi ND, Hu Y, Guo J, McMahon JA, Carey DK, Xu C, Fang Q, Sha J, Qin S, Rocco D, Wohlschlegel J, Ting AY, Carr SA, Perrimon N, McMahon AP (2022) A genetic model for in vivo proximity labelling of the mammalian secretome. Open Biol 12(8):220149
Liu J, Jang JY, Pirooznia M, Liu S, Finkel T (2021) The secretome mouse provides a genetic platform to delineate tissue-specific in vivo secretion. Proc Natl Acad Sci USA 118(3)
Evans HT, Bodea LG, Gotz J (2020) Cell-specific non-canonical amino acid labelling identifies changes in the de novo proteome during memory formation. Elife 9
Acknowledgements
This work was supported by NIH (NIA) Award R01 AG076569 (YK and MP).
Funding
Funding was provided by National Institute on Aging (R01 AG076569).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Yukiko Kitase and Matthew Prideaux declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Kitase, Y., Prideaux, M. Regulation of the Osteocyte Secretome with Aging and Disease. Calcif Tissue Int 113, 48–67 (2023). https://doi.org/10.1007/s00223-023-01089-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-023-01089-w