Abstract
Hyperhomocysteinemia induces oxidative stress and chronic inflammation (both of which are catabolic to bone and muscle); thus, we examined the association between homocysteine and body composition and physical function in middle-aged and older adults. Data from the National Health and Nutrition Examination Survey was used to build regression models. Plasma homocysteine (fluorescence immunoassay) was used as the exposure and bone mineral density (BMD; dual-energy X-ray absorptiometry; DXA), lean mass (DXA), knee extensor strength (isokinetic dynamometer; newtons) and gait speed (m/s) were used as outcomes. Regression models were adjusted for confounders (age, sex, race/Hispanic origin, height, fat mass %, physical activity, smoking status, alcohol intakes, cardiovascular disease, diabetes, cancer and vitamin B12). All models accounted for complex survey design by using sampling weights provided by NHANES. 1480 adults (median age: 64 years [IQR: 56, 73]; 50.3% men) were included. In multivariable models, homocysteine was inversely associated with knee extensor strength (β = 0.98, 95% CI 0.96, 0.99, p = 0.012) and gait speed (β = 0.85, 95% CI 0.78, 0.94, p = 0.003) and borderline inversely associated with femur BMD (β = 0.84, 95% CI 0.69, 1.03, p = 0.086). In the sub-group analysis of older adults (≥ 65 years), homocysteine was inversely associated with gait speed and femur BMD (p < 0.05) and the slope for knee extensor strength and whole-body BMD were in the same direction. No significant associations were observed between homocysteine and total or appendicular lean mass in the full or sub-group analysis. We found inverse associations between plasma homocysteine and muscle strength/physical function, and borderline significant inverse associations for femur BMD.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Homocysteine, a non-essential amino acid biosynthesised from methionine during demethylation, is an important signalling molecule for gene expression of proteins and enzymes in the human body [1]. When maintained in normal ranges, homocysteine serves as an important cofactor in metabolic processes; however, abnormally high plasma/serum levels (termed hyperhomocysteinemia) are toxic to the body. More specifically, chronic hyperhomocysteinemia damages cells and tissues and induces oxidative stress and chronic inflammation in the body [2, 3], as well as disrupting the vasodilatory properties of nitric oxide in various tissues [4].
As a result, hyperhomocysteinemia has been identified as a risk factor for several age-related pathologies including cardiovascular diseases, diabetes and dementia [5,6,7]. Causes of elevated homocysteine levels include; but are not limited to, old age, sex (primarily men), smoking status, excess alcohol intakes, deficiencies in enzymes (i.e. Cystathionin/Methionine-β-synthase) and vitamins (folate, vitamin B6 and B12), genetic polymorphisms, and several pathologies (i.e. cancers, renal dysfunction, systemic lupus erythematosus) [8,9,10].
Given that hyperhomocysteinemia induces oxidative stress and chronic inflammation (both of which are catabolic to bone and muscle [11, 12]), it is not surprising that epidemiological studies have focussed on the link between homocysteine and musculoskeletal health. Some studies report positive relationships between homocysteine levels and the loss of muscle mass and function (sarcopenia) [13, 14] while others show no relationship [15]. These ambiguous findings are further supported by a 2021 narrative review which concluded that results between homocysteine levels and muscle function are inconclusive and warrant further research [16]. Mechanistically, hyperhomocysteinemia is suggested to adversely impact the structure and function of skeletal muscle by inducing oxidative stress which in turn leads to mitochondrial loss [2], or by decreasing the bioavailability of nitric oxide and blood flow to muscle cells [17].
Regarding low to very-low bone density (osteopenia/osteoporosis), findings are equally heterogeneous. A meta-analysis conducted in 2014 found that homocysteine levels were significantly higher in postmenopausal women with osteoporosis [18]; however, a later meta-analysis (2021) [19] found no such association. A recent (2022) cross-sectional study [20] examined the relationship between homocysteine and bone density (at femoral neck, spine and hip) in 760 postmenopausal women and also found no association. The suggested biological link between homocysteine and bone fragility stems from the role of this molecule in modulating osteoclastogenesis (bone breakdown) and decreasing blood flow in this tissue through its actions on nitric oxide [21].
Further research is still needed to examine the relationship between homocysteine and musculoskeletal health using large cohort studies among adults of different race/Hispanic origin. These studies should also include multiple measures of musculoskeletal health including bone density and muscle mass and function which are inherently linked [22]. Given this, we sought to build on current work and re-examine the relationship between plasma homocysteine and bone density, lean mass, muscle strength and physical function using data from middle-aged and older adults in the National Health and Nutrition Examination Survey (NHANES).
Materials and Methods
Population
We studied individuals participating in the 2001–2002 NHANES as it contained the outcomes of interest. The NHANES is a population-representative sample aimed at assessing the health and nutritional status of non-institutionalized civilians residing in the United States. From the 2001 to 2002 NHANES database, there were 11,039 participants in total. Plasma homocysteine data was missing in 2567 participants and DXA was not performed in 321 pregnant women. A further 966 participants had missing DXA data, leaving 7185 participants that had DXA scans. Exclusion criteria for DXA scan in NHANES included; pregnant women, or participants with a height above 196 cm (DXA table length) or weight above 136 kg (DXA table weight limit) [23]. Of note, even though DXA scan was performed in all ages, muscle strength and physical function were performed only in participants ≥ 50 years. In addition, participants with recent myocardial infarction (past 6 weeks), brain aneurysm/stroke, chest/abdominal surgery (past 3 weeks), knee surgery or severe back pain were excluded from strength testing. Since our research aimed at the associations on both muscle and bone health, out of 7185 participants, we included only n = 1480 participants that had full DXA scan (lean mass, bone density, fat mass) along with data on muscle strength and physical function in the final analytical sample. Given that homocysteine levels are higher and musculoskeletal health is deteriorating with age, we also performed sub-group analyses of adults aged ≥ 65 years comprising of n = 735 older adults. All participants provided written informed consent in accordance with the Centers for Disease Control and Prevention (CDC). Ethical approval for NHANES (1999–2004) was received from the NCHS Research Ethics Review Board (ERB): Protocol #98–12.
Plasma Homocysteine
As previously described [24], total plasma homocysteine was measured on the Abbott Homocysteine IMX in 2001 and Abbott Axsym in 2002, both a fully automated fluorescence polarization immunoassay. This method has shown to have excellent precision (coefficient of variation: ≤ 5%) when compared to high-performance liquid chromatography [25]. Age- and sex-specific reference ranges for homocysteine were reported following the 2001–2002 NHANES CDC data [24].
Anthropometry and Body Composition
Height (cm.) and weight (kg.) were measured using standardized procedures [26]. Whole-body DXA scans were performed on a QDR 4500A fan beam densitometer (Hologic, Inc., Bedford, MA) following manufactures guidelines. We reported bone mineral density (BMD) in g/cm2. Participants changed into gowns and removed any jewelry or metal objects which could interfere with the scan result. We used available data for total and regional bone mineral density (BMD) and lean mass (the sum of non-bone and non-fat masses in kg and %). Of note, the NHANES DXA lean mass and fat mass were adjusted based on the results of an analysis of QDR-4500A DXA data. The lean mass was decreased by 5% and an equivalent kg weight was added to the fat mass.
Muscle Strength and Physical Function
Muscle Strength
Average peak force of the knee extensors was measured using a Kin Com Isokinetic Dynamometer (Chattanooga group, Inc, Chattanooga, TN). Participants completed six repetitions (3 familiarization, 3 testing) with the highest value (in Newtons) from the final 3 tests used in the analysis. Where less than 4 trials were completed, the highest value from the remaining attempts was used in the analysis.
Physical Function
Participants completed a timed 20 feet (6.1 m) walk at usual speed. If needed, a cane or walker was permitted. Gait speed was then transformed into meters per second (m/s) and used in the analysis. The full protocol for these procedures is available elsewhere [27].
Demographics, Lifestyle, and Medical Conditions
Demographic, lifestyle, and medical conditions were recorded via self-reported questionnaires. We included data on demographics (age, sex, race/Hispanic origin), history of chronic illness (including diabetes, congestive heart failure, coronary heart disease, stroke, and cancer), physical activity levels, and lifestyle factors including smoking status and alcohol intakes. These methods have been described in previous NHANES articles [28, 29].
Statistical Methods
Statistical analyses were performed using Stata 16.1 (StataCorp. 2019. Stata Statistical Software: Release 16. College Station, TX: StataCorp LLC.). Data were presented as frequency (percentage [%]) for categorical variables or median (interquartile range [IQR]) for continuous variables. Scatter plots were used to visualize the relationship between the exposure and outcomes of interest. Outcomes of interest were: total BMD (in g/cm2 and T score), lumbar spine and femur BMD (both in g/cm2), total and appendicular lean mass (both in kg), knee extensor strength (in Newtons) and gait speed (in m/s). In these data, fit of linear regression (assessed by visual inspection of residuals) was better when homocysteine was log transformed. As such, exponentiated beta (\(\beta\)) coefficients with 95% CI represent fold difference in homocysteine as the variable increases for 1 unit. Univariable linear regression was first used to assess associations between homocysteine and other variables. Regardless of univariable results, all multivariable models were adjusted for all pre-specified confounders, which were chosen based on current literature. These are: age, sex, race/Hispanic origin, smoking status, alcohol intakes, height, DXA fat mass (%), physical activity, cardiovascular disease, diabetes, cancer and vitamin B12. Missing DXA data were imputed using sequential regression imputation method (further description available in NHANES documentation) [30]. Coefficients and standard errors for all analyses were adjusted for the variability between imputations according to the combination rules by Rubin [31]. All regression results also took into account complex survey design by using sampling weights (medical examination clinic weights [32] provided by NHANES) and Taylor linearized variance estimation. All analyses were repeated in a sub-group of older adults (≥ 65 years old). A p-value < 0.05 was considered statistically significant and a p-value between 0.05 and 0.10 was considered borderline significant.
Results
Study Population
Median age of participants was 64 years (IQR: 56, 73) with a relatively equal proportion of men (50.3%) and women (49.7%) in the sample. Median plasma concentration of homocysteine was 9.15 µmol/L (IQR: 7.58, 11.24). According to age- and sex-specific reference ranges for homocysteine, over two-thirds (70.9%) of participants had normal levels, 26.3% had mild to severe hyperhomocysteinemia, and 2.8% had hypohomocysteinemia (Table 1). Tables 1 and 2 showed the participants characteristics in the full population (≥ 50 years) and the sub-group of older adults (≥ 65 years), respectively. Compared to the full population, the prevalence of moderate to severe hyperhomocysteinemia tended to be higher in older adults (8.7% vs 5.5%, p < 0.001; Tables 1 and 2). Compared to the full population, older adults have significantly lower BMD, appendicular lean mass and knee extensor strength as well as slower gait speed (p < 0.001 for all).
Univariable Associations Between Homocysteine and Demographic, Lifestyle, and Medical Conditions
In univariable analyses of the full population (Table 1), homocysteine was associated with age, sex, height, smoking status, alcohol intakes, physical activity and total fat mass (%), as well as certain diseases including coronary heart disease, congestive heart failure, and diabetes.
Univariable- and Multivariable-Associations Between Homocysteine and Outcome Measures
Muscle Outcome Measures
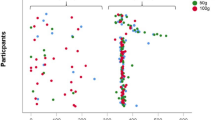
In univariable analyses of the full population, homocysteine was positively associated with total lean mass and appendicular lean mass and inversely associated with knee extensor strength and gait speed (p < 0.05; Fig. 1 and Table 3). In the fully adjusted model, homocysteine was inversely associated with knee extensor strength (β = 0.98, 95% CI 0.96, 0.99, p = 0.012) and gait speed (β = 0.85, 95% CI 0.78, 0.94, p = 0.003). No significant associations (p ≥ 0.290 to 0.316) were observed between homocysteine and total/appendicular lean mass in the full population.
In the sub-group analysis of older adults, the inverse associations between homocysteine and gait speed remained (β = 0.80, 95% CI 0.70, 0.91, p = 0.003) in the fully adjusted model. Associations for knee extensor strength were attenuated to non-significance (β = 0.97, 95% CI 0.94, 1.01, p = 0.112) in older adults, although the slope was in the same direction.
Bone Outcome Measures
In the fully adjusted model, homocysteine was borderline inversely associated with femur BMD (β = 0.84, 95% CI: 0.69, 1.03, p = 0.086). No significant associations (p ≥ 0.361 to 0.448) were observed between homocysteine and total/lumbar-spine BMD in the full population.
In the sub-group of older adults, in the fully adjusted models, homocysteine was significantly, and inversely associated with femoral BMD (β = 0.71, 95% CI 0.56, 0.90, p = 0.008) and was borderline inversely associated with total body BMD (β = 0.78, 95% CI 0.59, 1.03, p = 0.080). (Table 4).
Discussion
In this population-based study of middle-aged and older adults, we found inverse associations between plasma homocysteine and muscle strength/physical function, and borderline significant inverse associations for BMD at the femur (similar patterns were observed with these outcomes in our sub-group analysis of older adults). However, we observed no association between plasma homocysteine and total or appendicular lean mass in the full population or sub-group analysis. Of note, we are the first to report on the relationship between homocysteine and bone and muscle health in the same cohort.
Various mechanisms may explain the relationship between homocysteine and muscle strength/physical function. First, hyperhomocysteinemia may increase the release of reactive oxygen species that lead to mitochondrial damage and resulting inflammation [33]. Second, hyperhomocysteinemia decreases bioavailability of nitric oxide and decreases blood flow to muscle cells [17] which may lead to lower muscle strength and physical function. Indeed, in the Baltimore Longitudinal Study of Aging, there was an inverse association between homocysteine and grip strength in healthy women \(\ge\)50 years over a period of 4.7 years follow up [34]. In the same study, there was inverse relationship between homocysteine and gait speed [35]. Our data strengthen these findings and support the possible role of homocysteine influencing lower-limb muscle strength and physical function, the latter of which are both crucial for healthy ageing and preventing falls and fractures [36].
Several other studies have investigated the association between homocysteine and sarcopenia definitions using muscle mass, strength and/or physical function. The Maastricht Sarcopenia Study [14] found that homocysteine levels in participants with sarcopenia (defined by the European Working Group on Sarcopenia in Older People (EWGSOP) [37]) were 27% higher compared to participants without sarcopenia. Another observational study [13] in 1582 Asian participants demonstrated that elevated homocysteine was associated with sarcopenia (defined by Asian Working Group for Sarcopenia (AWGS 2019) in community dwelling adults [38]. However, one Japanese small study [15] assessing 47 women with sarcopenia and 23 age- and sex-matched controls found no association of homocysteine levels with sarcopenia. Another European study showed that sarcopenia (defined according to EWGSOP) may be related to vitamin B12 deficiency in 403 older adults [39], even though homocysteine was not directly assessed. This is supported by another study in 66 older adults that showed that vitamin B12 was 15% lower in the sarcopenic group compared to controls [40]. A more recent study [41] conducted in type 2 diabetes mellitus patients > 60 years also found a positive correlation between homocysteine and sarcopenia (defined by the updated criteria from the EWGSOP2 [42]) independent of HbA1c levels. From these studies, it is not clear if homocysteine is linked to specific components of sarcopenia (i.e. lean mass, strength or function), but our data does not support any association between this biomarker and lean mass which includes all non-fat and non-bone body masses. Further studies should examine the association between homocysteine and direct measures of muscle mass/volume using magnetic resonance imaging, creatine dilution or high-resolution computed tomography. This will allow a better understanding of the relationship between homocysteine and muscle morphology.
In the full population, we found borderline significant and inverse associations between homocysteine and femur BMD but not whole-body or lumbar-spine BMD. In our sub-group of older adults, we found inverse associations between homocysteine and femur BMD and borderline significant inverse associations with whole-body BMD. The associations were stronger than in full population. Differences in sample sizes, bone density (which is lower in older versus younger adults), and region of interest (lumbar-spine less reliable due to stenosis/kyphosis with ageing) may account for the observed patterns. Irrespective of this, our findings add to the literature on this topic. Indeed, a meta-analysis [18] found that homocysteine levels were significantly higher in postmenopausal women with osteoporosis compared to those without osteoporosis. Another meta-analysis considering oxidative stress-related biomarkers in postmenopausal women with osteoporosis [43] showed higher levels of homocysteine in postmenopausal women with osteoporosis. However, a later meta-analysis [19] did not find higher homocysteine in postmenopausal women with osteoporosis compared to healthy controls. A recent cross-sectional study in 760 postmenopausal women with a mean age of 56 [20] also found no association between homocysteine and BMD in the lumbar spine, femoral neck and total hip. Given our findings, future research studies should re-examine this relationship using a longitudinal design to determine if any cause-and-effect exists. These studies should also include mechanisms of bone biology to confirm whether the mechanistic links were through bone formation and/or bone resorption.
A major strength of our study is the inclusion of a diverse range of men and women (and older adults) which are representative of the US population. As shown in previous studies, bone strength is different between races (BMD is higher in blacks [44]) and we found there were significant differences between the races on homocysteine levels in older adults (Table 2). The inclusion of different races in the analyses makes our results more applicable to a wider range of population. Our analysis also accounted for various demographic, lifestyle and medical factors known to impact the exposure (homocysteine) or outcome measures (bone density, lean mass, muscle strength, physical function). However, the cross-sectional nature of the study limits cause-and-effect interpretation. We also acknowledge that population-based studies are open to residual confounding. Lastly, including more accurate measures of muscle mass/size and bone structure would have strengthen our ability to examine association with homocysteine levels. Future population-based studies should consider these factors.
To conclude, in this population-based study of middle-aged and older adults, we found inverse associations between plasma homocysteine and muscle strength/physical function, and borderline inverse associations for BMD at the femur (similar patterns for these outcomes were observed in our sub-group of older adults). Longitudinal studies should now investigate the link between homocysteine and bone density, muscle strength and physical function, and these studies should consider accurate measures of muscle mass instead of lean mass.
References
De Martinis M, Sirufo MM, Nocelli C, Fontanella L, Ginaldi L (2020) Hyperhomocysteinemia is associated with inflammation, bone resorption, vitamin B12 and folate deficiency and MTHFR C677T polymorphism in postmenopausal women with decreased bone mineral density. Int J Environ Res Public Health 17(12):4260
Tyagi N, Sedoris KC, Steed M, Ovechkin AV, Moshal KS, Tyagi SC (2005) Mechanisms of homocysteine-induced oxidative stress. Am J Physiol Heart Circ Physiol 289:H2649-2656
Elsherbiny NM, Sharma I, Kira D, Alhusban S, Samra YA, Jadeja R, Martin P, Al-Shabrawey M, Tawfik A (2020) Homocysteine induces inflammation in retina and brain. Biomolecules 10(3):393
Zhang X, Li H, Jin H, Ebin Z, Brodsky S, Goligorsky MS (2000) Effects of homocysteine on endothelial nitric oxide production. Am J Physiol Renal Physiol 279:F671-678
Boushey CJ, Beresford SA, Omenn GS, Motulsky AG (1995) A quantitative assessment of plasma homocysteine as a risk factor for vascular disease. Probable benefits of increasing folic acid intakes. JAMA 274:1049–1057
Diaz-Arrastia R (2000) Homocysteine and neurologic disease. Arch Neurol 57:1422–1427
Elias MF (2021) Reclaiming the importance of homocysteine as a marker of cardiovascular and neurologic disease. J Intern Med 290:1098–1099
He Q, Yang Z, Sun Y, Qu Z, Jia X, Li J, Lin Y, Luo Y (2021) The impact of homocysteine on the risk of hormone-related cancers: a Mendelian randomization study. Front Nutr 8:645371
Sam NB, Zhang Q, Li BZ, Li XM, Wang DG, Pan HF, Ye DQ (2020) Serum/plasma homocysteine levels in patients with systemic lupus erythematosus: a systematic review and meta-analysis. Clin Rheumatol 39:1725–1736
van Guldener C, Robinson K (2000) Homocysteine and renal disease. Semin Thromb Hemost 26:313–324
Kirk B, Feehan J, Lombardi G, Duque G (2020) Muscle, bone, and fat crosstalk: the biological role of Myokines, Osteokines, and Adipokines. Curr Osteoporos Rep 18:388–400
Kositsawat J, Duque G, Kirk B (2021) Nutrients with anabolic/anticatabolic, antioxidant, and anti-inflammatory properties: targeting the biological mechanisms of aging to support musculoskeletal health. Exp Gerontol 154:111521
Lee WJ, Peng LN, Loh CH, Chen LK (2020) Sex-different associations between serum homocysteine, high-sensitivity C-reactive protein and sarcopenia: results from I-Lan longitudinal aging study. Exp Gerontol 132:110832
Ter Borg S, de Groot LC, Mijnarends DM, de Vries JH, Verlaan S, Meijboom S, Luiking YC, Schols JM (2016) Differences in nutrient intake and biochemical nutrient status between sarcopenic and nonsarcopenic older adults-results from the Maastricht sarcopenia study. J Am Med Dir Assoc 17:393–401
Eguchi Y, Toyoguchi T, Inage K, Fujimoto K, Orita S, Suzuki M, Kanamoto H, Abe K, Norimoto M, Umimura T, Koda M, Furuya T, Aoki Y, Nakamura J, Akazawa T, Takahashi K, Ohtori S (2021) Advanced glycation end products are associated with sarcopenia in older women: aging marker dynamics. J Women Aging 33:328–340
De Giuseppe R, Tomasinelli CE, Vincenti A, Di Napoli I, Negro M, Cena H (2021) Sarcopenia and homocysteine: is there a possible association in the elderly? A narrative review. Nutr Res Rev 35:98–111
Veeranki S, Tyagi SC (2013) Defective homocysteine metabolism: potential implications for skeletal muscle malfunction. Int J Mol Sci 14:15074–15091
Zhang H, Tao X, Wu J (2014) Association of homocysteine, vitamin B12, and folate with bone mineral density in postmenopausal women: a meta-analysis. Arch Gynecol Obstet 289:1003–1009
Zhao F, Guo L, Wang X, Zhang Y (2021) Correlation of oxidative stress-related biomarkers with postmenopausal osteoporosis: a systematic review and meta-analysis. Arch Osteoporos 16:4
Garcia-Alfaro P, Rodriguez I, Pascual MA (2022) Evaluation of the relationship between homocysteine levels and bone mineral density in postmenopausal women. Climacteric 25:179–185
Behera J, Bala J, Nuru M, Tyagi SC, Tyagi N (2017) Homocysteine as a pathological biomarker for bone disease. J Cell Physiol 232:2704–2709
Kirk B, Duque G (2022) Muscle and bone: an indissoluble union. J Bone Miner Res 37:1211–1212
Kelly TL, Wilson KE, Heymsfield SB (2009) Dual energy X-Ray absorptiometry body composition reference values from NHANES. PLoS ONE 4:e7038
Total homocysteine in plasma by abbott Axsym- NHANES 2001-2002 https://wwwn.cdc.gov/nchs/data/nhanes/2001-2002/labmethods/l06_b_met_homocysteine_axsym.pdf
Lonati S, Novembrino C, Ippolito S, Accinni R, Galli C, Troonen H, Campolo J, Della Noce C, Lunghi G, Catena FB (2004) Analytical performance and method comparison study of the total homocysteine fluorescence polarization immunoassay (FPIA) on the AxSYM analyzer. Clin Chem Lab Med 42:228–234
Anthropometry procedures manual. https://wwwn.cdc.gov/nchs/data/nhanes/2001-2002/manuals/body_measures_year_3.pdf
Muscle strength https://wwwn.cdc.gov/Nchs/Nhanes/2001-2002/MSX_B.htm
Curtin LR, Mohadjer LK, Dohrmann SM, Kruszon-Moran D, Mirel LB, Carroll MD, Hirsch R, Burt VL, Johnson CL (2013) National health and nutrition examination survey: sample design, 2007–2010. Vital Health Stat 2:1–23
Johnson CL, Paulose-Ram R, Ogden CL, Carroll MD, Kruszon-Moran D, Dohrmann SM, Curtin LR (2013) National health and nutrition examination survey: analytic guidelines, 1999–2010. Vital Health Stat 2:1–24
Schenker N, Borrud LG, Burt VL, Curtin LR, Flegal KM, Hughes J, Johnson CL, Looker AC, Mirel L (2011) Multiple imputation of missing dual-energy X-ray absorptiometry data in the national health and nutrition examination survey. Stat Med 30:260–276
Rubin DB (1987) Multiple imputation for nonresponse in surveys. Wiley, New York
Weighting https://wwwn.cdc.gov/nchs/nhanes/tutorials/module3.aspx
Kaplan P, Tatarkova Z, Sivonova MK, Racay P, Lehotsky J (2020) Homocysteine and mitochondria in cardiovascular and cerebrovascular systems. Int J Mol Sci 21(20):7698
Vidoni ML, Pettee Gabriel K, Luo ST, Simonsick EM, Day RS (2018) Relationship between homocysteine and muscle strength decline: the Baltimore longitudinal study of aging. J Gerontol A Biol Sci Med Sci 73:546–551
Vidoni ML, Pettee Gabriel K, Luo ST, Simonsick EM, Day RS (2017) Vitamin B12 and homocysteine associations with gait speed in older adults: the Baltimore longitudinal study of aging. J Nutr Health Aging 21:1321–1328
Kirk B, Zanker J, Bani Hassan E, Bird S, Brennan-Olsen S, Duque G (2021) Sarcopenia definitions and outcomes consortium (SDOC) criteria are strongly associated with malnutrition, depression, falls, and fractures in high-risk older persons. J Am Med Dir Assoc 22:741–745
Cruz-Jentoft AJ, Baeyens JP, Bauer JM, Boirie Y, Cederholm T, Landi F, Martin FC, Michel JP, Rolland Y, Schneider SM, Topinkova E, Vandewoude M, Zamboni M, European Working Group on Sarcopenia in Older P (2010) Sarcopenia: European consensus on definition and diagnosis: report of the European working group on sarcopenia in older people. Age Ageing 39:412–423
Chen LK, Liu LK, Woo J, Assantachai P, Auyeung TW, Bahyah KS, Chou MY, Chen LY, Hsu PS, Krairit O, Lee JS, Lee WJ, Lee Y, Liang CK, Limpawattana P, Lin CS, Peng LN, Satake S, Suzuki T, Won CW, Wu CH, Wu SN, Zhang T, Zeng P, Akishita M, Arai H (2014) Sarcopenia in Asia: consensus report of the Asian working group for sarcopenia. J Am Med Dir Assoc 15:95–101
Ates Bulut E, Soysal P, Aydin AE, Dokuzlar O, Kocyigit SE, Isik AT (2017) Vitamin B12 deficiency might be related to sarcopenia in older adults. Exp Gerontol 95:136–140
Verlaan S, Aspray TJ, Bauer JM, Cederholm T, Hemsworth J, Hill TR, McPhee JS, Piasecki M, Seal C, Sieber CC, Ter Borg S, Wijers SL, Brandt K (2017) Nutritional status, body composition, and quality of life in community-dwelling sarcopenic and non-sarcopenic older adults: a case-control study. Clin Nutr 36:267–274
Mu ZJ, Fu JL, Sun LN, Chan P, Xiu SL (2021) Associations between homocysteine, inflammatory cytokines and sarcopenia in Chinese older adults with type 2 diabetes. BMC Geriatr 21:692
Cruz-Jentoft AJ, Bahat G, Bauer J, Boirie Y, Bruyere O, Cederholm T, Cooper C, Landi F, Rolland Y, Sayer AA, Schneider SM, Sieber CC, Topinkova E, Vandewoude M, Visser M, Zamboni M, Writing Group for the European Working Group on Sarcopenia in Older P, the Extended Group for E (2019) Sarcopenia: revised European consensus on definition and diagnosis. Age Ageing 48:16–31
Zhou Q, Zhu L, Zhang D, Li N, Li Q, Dai P, Mao Y, Li X, Ma J, Huang S (2016) Oxidative stress-related biomarkers in postmenopausal osteoporosis: a systematic review and meta-analyses. Dis Mark 2016:7067984
Ettinger B, Sidney S, Cummings SR, Libanati C, Bikle DD, Tekawa IS, Tolan K, Steiger P (1997) Racial differences in bone density between young adult black and white subjects persist after adjustment for anthropometric, lifestyle, and biochemical differences. J Clin Endocrinol Metab 82:429–434
Funding
Open Access funding enabled and organized by CAUL and its Member Institutions. No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Conceptualization, JK, BK and GD; statistical analysis, SV; Literature search, JK, CF and MG; writing—original draft preparation, JK, CF and BK; writing—review and editing; BK, SV, DGC and GD. All authors have read and agreed the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
GD serves as a member of the Advisory Board for Abbott Australia and TSI Pharmaceuticals both of which manufacture/sell nutritional products. BK has received speaker fees from Abbott UK (over 36 months ago) and is currently supported by a research fellowship from TSI Pharmaceuticals. DGC serves on the Scientific Advisory Board for Alzchem, a company that manufactures a nutritional product called creatine. GD, BK and DC declare they have no direct conflicts of interest in relation to the current study. JK, SV, CF and MG have no conflicts of interest to declare.
Human and Animal Rights
Ethical approval for NHANES (1999–2004) was received from the NCHS Research Ethics Review Board (ERB): Protocol #98–12. All study procedures followed the ethical standards outlined by the NCHS Research ERB for human participants and were in line with the Declaration of Helsinki.
Informed Consent
All participants provided written informed consent in accordance with the Centers for Disease Control and Prevention (CDC).
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Kositsawat, J., Vogrin, S., French, C. et al. Relationship Between Plasma Homocysteine and Bone Density, Lean Mass, Muscle Strength and Physical Function in 1480 Middle-Aged and Older Adults: Data from NHANES. Calcif Tissue Int 112, 45–54 (2023). https://doi.org/10.1007/s00223-022-01037-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01037-0