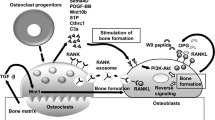
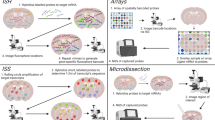
Abstract
Static and dynamic bone histomorphometry and identification of bone cells in culture are labor-intensive and highly repetitive tasks. Several computer-assisted methods have been proposed to ease these tasks and to take advantage of the increased computational power available today. The present review aimed to provide an overview of contemporary methods utilizing specialized computer software to perform bone histomorphometry or identification of bone cells in culture. In addition, a brief historical perspective on bone histomorphometry is included. We identified ten publications using five different computer-assisted approaches (1) ImageJ and BoneJ; (2) Histomorph: OsteoidHisto, CalceinHisto, and TrapHisto; (3) Fiji/ImageJ2 and Trainable Weka Segmentation (TWS); (4) Visiopharm and artificial intelligence (AI); and (5) Osteoclast identification using deep learning with Single Shot Detection (SSD) architecture, Darknet and You Only Look Once (YOLO), or watershed algorithm (OC_Finder). The review also highlighted a substantial need for more validation studies that evaluate the accuracy of the new computational methods to the manual and conventional analyses of histological bone specimens and cells in culture using microscopy. However, a substantial evolution has occurred during the last decade to identify and separate bone cells and structures of interest. Most early studies have used simple image segmentation to separate structures of interest, whereas the most recent studies have utilized AI and deep learning. AI has been proposed to substantially decrease the amount of time needed for analyses and enable unbiased assessments. Despite the clear advantages of highly sophisticated computational methods, the limited nature of existing validation studies, particularly those that assess the accuracy of the third-generation methods compared to the second-generation methods, appears to be an important reason that these techniques have failed to gain wide acceptance.



Adapted from Emmanuel et al. under the terms of the Creative Commons Attribution License (CC BY) [25]

Adapted from Cohen-Karlik et al. under the terms of the Creative Commons Attribution License (CC BY) [26]

Adapted from Wang et al. under the terms of the Creative Commons Attribution License (CC BY) [27]
Similar content being viewed by others
References
Dempster DW, Compston JE, Drezner MK et al (2013) Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR histomorphometry nomenclature committee. J Bone Miner Res 28:2–17. https://doi.org/10.1002/jbmr.1805
Jee WSS (2005) The past, present, and future of bone morphometry: its contribution to an improved understanding of bone biology. J Bone Miner Metab 23:1–10
Gundersen HJG (1980) Stereology - or how figures for spatial shape and content are obtained by observation of structures in sections. Microsc Acta 83:409–426
Vesterby A, Gundersen HJG, Melsen F (1989) Star volume of marrow space and trabeculae of the first lumbar vertebra: sampling efficiency and biological variation. Bone 10:7–13. https://doi.org/10.1016/8756-3282(89)90140-3
Vesterby A, Kragstrup J, Gundersen HJG, Melsen F (1987) Unbiased stereologic estimation of surface density in bone using vertical sections. Bone 8:13–17. https://doi.org/10.1016/8756-3282(87)90126-8
Mellish RWE, Ferguson-Pell MW, Cochran GVB et al (1991) A new manual method for assessing two-dimensional cancellous bone structure: comparison between iliac crest and lumbar vertebra. J Bone Miner Res 6:689–696. https://doi.org/10.1002/jbmr.5650060706
Garrahan NJ, Mellish RWE, Compston JE (1986) A new method for the two-dimensional analysis of bone structure in human iliac crest biopsies. J Microsc 142:341–349. https://doi.org/10.1111/j.1365-2818.1986.tb04289.x
Huffer WE, Ruegg P, Zhu J-M, Lepoff RB (1994) Semiautomated methods for cancellous bone histomorphometry using a general-purpose video image analysis system. J Microsc 173:53–66. https://doi.org/10.1111/j.1365-2818.1994.tb03427.x
Woodbury LA, Woodbury NA, Wronski T, Jee WSS (1976) Preliminary studies on the use of the Quantimet-720 for the measurement of radiographs of bone sections. Int Nucl Inf Syst. https://inis.iaea.org/search/searchsinglerecord.aspx?recordsFor=SingleRecord&RN=8302068
Hollinger JO, Gee SA (1981) Evaluation of the Parietal Bones in the Rat as a Specific Site for the Testing of Osteogenic Materials. A Simple Animal Model to Study Bone Implant Material. US Army Inst. Dent. Res. https://apps.dtic.mil/sti/citations/ADA107036
Wronski TJ, Cintrón M, Dann LM (1988) Temporal relationship between bone loss and increased bone turnover in ovariectomized rats. Calcif Tissue Int 43:179–183. https://doi.org/10.1007/BF02571317
Chow JWM, Jagger CJ, Chambers TJ (1993) Characterization of osteogenic response to mechanical stimulation in cancellous bone of rat caudal vertebrae. Am J Physiol - Endocrinol Metab. https://doi.org/10.1152/ajpendo.1993.265.2.e340
Schwartz MP, Recker RR (1981) Comparison of surface density and volume of human iliac trabecular bone measured directly and by applied stereology. Calcif Tissue Int 33:561–565. https://doi.org/10.1007/BF02409492
Parfitt AM, Rao DS, Stanciu J et al (1985) Irreversible bone loss in osteomalacia. comparison of radial photon absorptiometry with iliac bone histomorphometry during treatment. J Clin Invest 76:2403–2412. https://doi.org/10.1172/JCI112253
Delmas PD, Fontanges E, Duboeuf F et al (1988) Comparison of bone mass measured by histomorphometry on iliac biopsy and by dual photon absorptiometry of the lumbar spine. Bone 9:209–213. https://doi.org/10.1016/8756-3282(88)90033-6
Wright CDP, Vedi S, Garrahan NJ et al (1992) Combined inter-observer and inter-method variation in bone histomorphometry. Bone 13:205–208. https://doi.org/10.1016/8756-3282(92)90198-6
Compston JE, Vedi S, Stellon AJ (1986) Inter-observer and intra-observer variation in bone histomorphometry. Calcif Tissue Int 38:67–70. https://doi.org/10.1007/BF02556831
Parfitt AM, Drezner MK, Glorieux FH et al (1987) Bone histomorphometry: Standardization of nomenclature, symbols, and units: report of the asbmr histomorphometry nomenclature committee. J Bone Miner Res 2:595–610. https://doi.org/10.1002/jbmr.5650020617
Martin I, Mastrogiacomo M, De Leo G et al (2002) Fluorescence microscopy imaging of bone for automated histomorphometry. Tissue Eng 8:847–852. https://doi.org/10.1089/10763270260424204
Doube M, Klosowski MM, Arganda-Carreras I et al (2010) BoneJ: free and extensible bone image analysis in ImageJ. Bone 47:1076–1079. https://doi.org/10.1016/j.bone.2010.08.023
Hyun Hong S (2012) Computer-automated static, dynamic and cellular bone histomorphometry. J Tissue Sci Eng 05:004. https://doi.org/10.4172/2157-7552.s1-004
Egan KP, Brennan TA, Pignolo RJ (2012) Bone histomorphometry using free and commonly available software. Histopathology 61:1168–1173. https://doi.org/10.1111/j.1365-2559.2012.04333.x
Domander R, Felder AA, Doube M (2021) BoneJ2 - refactoring established research software. Wellcome Open Res 6:1–21. https://doi.org/10.12688/wellcomeopenres.16619.2
Van’t Hof RJ, Rose L, Bassonga E, Daroszewska A (2017) Open source software for semi-automated histomorphometry of bone resorption and formation parameters. Bone 99:69–79. https://doi.org/10.1016/j.bone.2017.03.051
Emmanuel T, Brüel A, Thomsen JS et al (2021) Artificial intelligence-assisted identification and quantification of osteoclasts. MethodsX 8:101272. https://doi.org/10.1016/j.mex.2021.101272
Cohen-Karlik E, Awida Z, Bergman A et al (2021) Quantification of osteoclasts in culture, powered by machine learning. Front Cell Dev Biol 9:1267. https://doi.org/10.3389/fcell.2021.674710
Wang X, Kittaka M, He Y et al (2022) OC_Finder: osteoclast segmentation, counting, and classification using watershed and deep learning. Front Bioinforma 2:6. https://doi.org/10.3389/fbinf.2022.819570
Rajpurkar P, Chen E, Banerjee O, Topol EJ (2022) AI in health and medicine. Nat Med 28:31–38. https://doi.org/10.1038/s41591-021-01614-0
Gulshan V, Peng L, Coram M et al (2016) Development and validation of a deep learning algorithm for detection of diabetic retinopathy in retinal fundus photographs. J Am Med Assoc 316:2402–2410. https://doi.org/10.1001/jama.2016.17216
Esteva A, Kuprel B, Novoa RA et al (2017) Dermatologist-level classification of skin cancer with deep neural networks. Nature 542:115–118. https://doi.org/10.1038/nature21056
Rajpurkar P, Irvin J, Ball RL et al (2018) Deep learning for chest radiograph diagnosis: a retrospective comparison of the CheXNeXt algorithm to practicing radiologists. PLoS Med. https://doi.org/10.1371/journal.pmed.1002686
Hannun AY, Rajpurkar P, Haghpanahi M et al (2019) Cardiologist-level arrhythmia detection and classification in ambulatory electrocardiograms using a deep neural network. Nat Med 25:65–69. https://doi.org/10.1038/s41591-018-0268-3
Gomolin A, Netchiporouk E, Gniadecki R, Litvinov IV (2020) Artificial intelligence applications in dermatology: where do we stand? Front Med 7:100. https://doi.org/10.3389/fmed.2020.00100
Huang P, Lin CT, Li Y et al (2019) Prediction of lung cancer risk at follow-up screening with low-dose CT: a training and validation study of a deep learning method. Lancet Digit Heal 1:e353–e362. https://doi.org/10.1016/S2589-7500(19)30159-1
Wu N, Phang J, Park J et al (2020) Deep neural networks improve radiologists’ performance in breast cancer screening. IEEE Trans Med Imaging 39:1184–1194. https://doi.org/10.1109/TMI.2019.2945514
McKinney SM, Sieniek M, Godbole V et al (2020) International evaluation of an AI system for breast cancer screening. Nature 577:89–94. https://doi.org/10.1038/s41586-019-1799-6
Kather JN, Pearson AT, Halama N et al (2019) Deep learning can predict microsatellite instability directly from histology in gastrointestinal cancer. Nat Med 25:1054–1056. https://doi.org/10.1038/s41591-019-0462-y
Campanella G, Hanna MG, Geneslaw L et al (2019) Clinical-grade computational pathology using weakly supervised deep learning on whole slide images. Nat Med 25:1301–1309. https://doi.org/10.1038/s41591-019-0508-1
Bera K, Schalper KA, Rimm DL et al (2019) Artificial intelligence in digital pathology — new tools for diagnosis and precision oncology. Nat Rev Clin Oncol 16:703–715. https://doi.org/10.1038/s41571-019-0252-y
Brent MB, Brüel A, Thomsen JS (2021) A systematic review of animal models of disuse-induced bone loss. Calcif Tissue Int 108:561–575. https://doi.org/10.1007/s00223-020-00799-9
Brent MB (2021) Abaloparatide: a review of preclinical and clinical studies. Eur J Pharmacol 909:174409. https://doi.org/10.1016/j.ejphar.2021.174409
Brent MB (2022) A review of the skeletal effects of exposure to high altitude and potential mechanisms for hypobaric hypoxia-induced bone loss. Bone 154:116258. https://doi.org/10.1016/j.bone.2021.116258
Brent MB, Brüel A, Thomsen JS (2020) Animal models of disuse-induced bone loss: study protocol for a systematic review. Syst Rev 9:3. https://doi.org/10.1186/s13643-020-01441-3
Emmanuel T, Mistegård J, Bregnhøj A et al (2021) Tissue-resident memory t cells in skin diseases: a systematic review. Int J Mol Sci 22:9004. https://doi.org/10.3390/ijms22169004
Schneider CA, Rasband WS, Eliceiri KW (2012) NIH image to imagej: 25 years of image analysis. Nat Methods 9:671–675. https://doi.org/10.1038/nmeth.2089
Rueden CT, Schindelin J, Hiner MC et al (2017) Image J2: ImageJ for the next generation of scientific image data. BMC Bioinform 18:529. https://doi.org/10.1186/s12859-017-1934-z
Schindelin J, Arganda-Carreras I, Frise E et al (2012) Fiji: an open-source platform for biological-image analysis. Nat Methods 9:676–682. https://doi.org/10.1038/nmeth.2019
Arganda-Carreras I, Kaynig V, Rueden C et al (2017) Trainable Weka Segmentation: a machine learning tool for microscopy pixel classification. Bioinformatics 33:2424–2426. https://doi.org/10.1093/bioinformatics/btx180
Malhan D, Muelke M, Rosch S et al (2018) An optimized approach to perform bone histomorphometry. Front Endocrinol (Lausanne). https://doi.org/10.3389/fendo.2018.00666
Brent MB, Emmanuel T, Simonsen U et al (2022) Hypobaric hypoxia deteriorates bone mass and strength in mice. Bone 154:116203. https://doi.org/10.1016/j.bone.2021.116203
Jiang J, Zhang X (2021) Research on moving object tracking technology of sports video based on deep learning algorithm. ACM Int Conf Proceeding Ser. https://doi.org/10.1145/3482632.3487433
Menze M, Geiger A (2015) Object scene flow for autonomous vehicles. Proc IEEE Comput Soc Conf Comput Vis Pattern Recognit 07–12:3061–3070. https://doi.org/10.1109/CVPR.2015.7298925
Jia Z, Saxena A, Chen T (2011) Robotic object detection: learning to improve the classifiers using sparse graphs for path planning. IJCAI Int Jt Conf Artif Intell. https://doi.org/10.5591/978-1-57735-516-8/IJCAI11-347
Ren S, He K, Girshick R, Sun J (2015) Faster R-CNN: towards real-time object detection with region proposal networks. IEEE Trans Pattern Anal Mach Intell 39:1137–1149. https://doi.org/10.1109/TPAMI.2016.2577031
Liu W, Anguelov D, Erhan D, et al (2016) SSD: Single shot multibox detector. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics) 9905 LNCS:21–37. https://doi.org/10.1007/978-3-319-46448-0_2#Sec2
Redmon J, Divvala S, Girshick R, Farhadi A (2016) You only look once: unified, real-time object detection. Proc IEEE Comput Soc Conf Comput Vis Pattern Recognit. https://doi.org/10.1109/CVPR.2016.91
Everingham M, Van Gool L, Williams CKI et al (2010) The pascal visual object classes (VOC) challenge. Int J Comput Vis 88:303–338. https://doi.org/10.1007/s11263-009-0275-4
Xia GS, Bai X, Ding J et al (2018) DOTA: a large-scale dataset for object detection in aerial images. Proc IEEE Comput Soc Conf Comput Vis Pattern Recognit. https://doi.org/10.1109/CVPR.2018.00418
Møgelmose A, Trivedi MM, Moeslund TB (2012) Vision-based traffic sign detection and analysis for intelligent driver assistance systems: perspectives and survey. IEEE Trans Intell Transp Syst 13:1484–1497. https://doi.org/10.1109/TITS.2012.2209421
Lin TY, Maire M, Belongie S, et al (2014) Microsoft COCO: Common objects in context. Lect Notes Comput Sci (including Subser Lect Notes Artif Intell Lect Notes Bioinformatics) 8693 LNCS:740–755. https://doi.org/10.1007/978-3-319-10602-1_48
Kohtala S, Nedal TMV, Kriesi C et al (2022) Automated quantification of human osteoclasts using object detection. Front Cell Dev Biol. https://doi.org/10.3389/FCELL.2022.941542
Zhang L, Chang M, Beck CA et al (2016) Analysis of new bone, cartilage, and fibrosis tissue in healing murine allografts using whole slide imaging and a new automated histomorphometric algorithm. Bone Res 4:1–9. https://doi.org/10.1038/boneres.2015.37
Funding
This research received no external funding.
Author information
Authors and Affiliations
Contributions
Conceptualization, MBB and TE; methodology, MBB and TE; investigation, MBB and TE; resources, MBB and TE; data curation, MBB and TE; writing—original draft preparation, MBB and TE; writing—review and editing, MBB and TE; visualization, MBB and TE. All authors have read and agreed to the published version of the manuscript.
Corresponding author
Ethics declarations
Conflicts of interest
M. B. Brent and T. Emmanuel declare no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Brent, M.B., Emmanuel, T. Contemporary Advances in Computer-Assisted Bone Histomorphometry and Identification of Bone Cells in Culture. Calcif Tissue Int 112, 1–12 (2023). https://doi.org/10.1007/s00223-022-01035-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01035-2