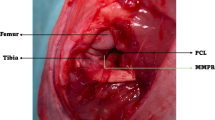
Abstract
Medial meniscus posterior root tears (MMPRT) are often associated with osteoarthritis (OA) progression and subchondral bone insufficiency fractures. This study aimed to develop the first MMPRT mouse model. The MMPRT model was created by sectioning the medial meniscus posterior root of 12-week-old CL57BL/6J male mice under stereomicroscopic observation. The sham operation and the destabilization of the medial meniscus (DMM) model groups were also created. OA progression and subchondral bone changes were evaluated histologically using the Osteoarthritis Research Society International (OARSI) subchondral bone scoring system at 2, 4, 8, and 12 weeks after surgery. Microcomputed tomography (µCT) was performed to evaluate the presence of insufficient fractures. OA progression and medial meniscus extrusion were observed in the MMPRT and DMM models 12 weeks after surgery. OA progressed in both models during the time course, without a significant difference in the OARSI score between the two groups. The subchondral bone score was significantly higher at 12 weeks than at 2 and 4 weeks in the MMPRT group, while no significant difference was found between the two groups. In the µCT analysis, destruction of the medial tibial plateau was observed in 4/40 knees, while none were observed in the DMM group. Of the four knees, destruction of the medial femoral condyle was also observed in three knees. Characteristic pathological changes were observed in the mouse MMPRT model. The mouse MMPRT model may be useful for investigating pathological changes after MMPRT.







Similar content being viewed by others
References
Furumatsu T, Okazaki Y, Okazaki Y et al (2019) Injury patterns of medial meniscus posterior root tears. Orthop Traumatol Surg Res 105:107–111. https://doi.org/10.1016/j.otsr.2018.10.001
Allaire R, Muriuki M, Gilbertson L, Harner CD (2008) Biomechanical consequences of a tear of the posterior root of the medial meniscus: similar to total meniscectomy. J Bone Jt Surg 90:1922–1931. https://doi.org/10.2106/JBJS.G.00748
Laprade CM, Foad A, Smith SD et al (2015) Biomechanical consequences of a nonanatomic posterior medial meniscal root repair. Am J Sports Med 43:912–920. https://doi.org/10.1177/0363546514566191
Krych AJ, Reardon PJ, Johnson NR et al (2017) Non-operative management of medial meniscus posterior horn root tears is associated with worsening arthritis and poor clinical outcome at 5-year follow-up. Knee Surg Sport Traumatol Arthrosc 25:383–389. https://doi.org/10.1007/s00167-016-4359-8
Sayyid S, Younan Y, Sharma G et al (2019) Subchondral insufficiency fracture of the knee: grading, risk factors, and outcome. Skeletal Radiol 48:1961–1974. https://doi.org/10.1007/s00256-019-03245-6
Okazaki Y, Furumatsu T, Hiranaka T et al (2020) Medial meniscus posterior root repair prevents the progression of subchondral insufficiency fracture of the knee. J Orthop Sci 26:1051–1055. https://doi.org/10.1016/j.jos.2020.10.008
Clements KM, Price JS, Chambers MG et al (2003) Gene deletion of either interleukin-1β, interleukin-1β -converting enzyme, inducible nitric oxide synthase, or stromelysin 1 accelerates the development of knee osteoarthritis in mice after surgical transection of the medial collateral ligament and partia. Arthritis Rheum 48:3452–3463. https://doi.org/10.1002/art.11355
Kamekura S, Hoshi K, Shimoaka T et al (2005) Osteoarthritis development in novel experimental mouse models induced by knee joint instability. Osteoarthr Cartil 13:632–641. https://doi.org/10.1016/j.joca.2005.03.004
Glasson SS, Askew R, Sheppard B et al (2005) Deletion of active ADAMTS5 prevents cartilage degradation in a murine model of osteoarthritis. Nature 434:644–648. https://doi.org/10.1038/nature03369
Glasson SS, Blanchet TJ, Morris EA (2007) The surgical destabilization of the medial meniscus (DMM) model of osteoarthritis in the 129/SvEv mouse. Osteoarthr Cartil 15:1061–1069. https://doi.org/10.1016/j.joca.2007.03.006
Glasson SS, Chambers MG, Van Den BWB, Little CB (2010) The OARSI histopathology initiative e recommendations for histological assessments of osteoarthritis in the mouse. Osteoarthr Cartil 18:S17–S23. https://doi.org/10.1016/j.joca.2010.05.025
Nagira K, Ikuta Y, Shinohara M et al (2020) Histological scoring system for subchondral bone changes in murine models of joint aging and osteoarthritis. Sci Rep 10:1–14. https://doi.org/10.1038/s41598-020-66979-7
Fang H, Huang L, Welch I et al (2018) Early changes of articular cartilage and subchondral bone in the DMM mouse model of osteoarthritis. Sci Rep 8:1–9. https://doi.org/10.1038/s41598-018-21184-5
Lampropoulou-Adamidou K, Lelovas P, Karadimas EV et al (2014) Useful animal models for the research of osteoarthritis. Eur J Orthop Surg Traumatol 24:263–271. https://doi.org/10.1007/s00590-013-1205-2
McCoy AM (2015) Animal models of osteoarthritis: comparisons and key considerations. Vet Pathol 52:803–818. https://doi.org/10.1177/0300985815588611
Kuyinu EL, Narayanan G, Nair LS, Laurencin CT (2016) Animal models of osteoarthritis: classification, update, and measurement of outcomes. J Orthop Surg Res 11:1–27. https://doi.org/10.1186/s13018-016-0346-5
Pond MJ, Nuki G (1973) Experimentally-induced osteoarthritis in the dog. Ann Rheum Dis 32:387–388. https://doi.org/10.1136/ard.32.4.387
Miyaji N, Nishida K, Tanaka T et al (2020) Inhibition of knee osteoarthritis progression in mice by administering SRT2014, an activator of silent information regulator 2 ortholog 1. Cartilage 13:1356–1366. https://doi.org/10.1177/1947603519900795
David MA, Smith MK, Pilachowski RN et al (2017) Early, focal changes in cartilage cellularity and structure following surgically induced meniscal destabilization in the mouse. J Orthop Res 35:537–547. https://doi.org/10.1002/jor.23443
Botter SM, Van Osch GJVM, Clockaerts S et al (2011) Osteoarthritis induction leads to early and temporal subchondral plate porosity in the tibial plateau of mice: an in vivo microfocal computed tomography study. Arthritis Rheum 63:2690–2699. https://doi.org/10.1002/art.30307
Goldring SR, Goldring MB (2016) Changes in the osteochondral unit during osteoarthritis: structure, function and cartilage bone crosstalk. Nat Rev Rheumatol 12:632–644. https://doi.org/10.1038/nrrheum.2016.148
Finnilä MAJ, Thevenot J, Aho OM et al (2017) Association between subchondral bone structure and osteoarthritis histopathological grade. J Orthop Res 35:785–792. https://doi.org/10.1002/jor.23312
Padalecki JR, Jansson KS, Smith SD et al (2014) Biomechanical consequences of a complete radial tear adjacent to the medial meniscus posterior root attachment site: in situ pull-out repair restores derangement of joint mechanics. Am J Sports Med 42:699–707. https://doi.org/10.1177/0363546513499314
Marzo JM, Gurske-DePerio J (2009) Effects of medial meniscus posterior horn avulsion and repair on tibiofemoral contact area and peak contact pressure with clinical implications. Am J Sports Med 37:124–129. https://doi.org/10.1177/0363546508323254
Seitz AM, Lubomierski A, Friemert B et al (2012) Effect of partial meniscectomy at the medial posterior horn on tibiofemoral contact mechanics and meniscal hoop strains in human knees. J Orthop Res 30:934–942. https://doi.org/10.1002/jor.22010
Okazaki Y, Furumatsu T, Yamauchi T et al (2020) Medial meniscus posterior root repair restores the intra-articular volume of the medial meniscus by decreasing posteromedial extrusion at knee flexion. Knee Surg Sport Traumatol Arthrosc 28:3435–3442. https://doi.org/10.1007/s00167-020-05953-2
Ahlback S, Bauer GCH, Bohne WH (1968) Arthritis and rheumatism spontaneous osteonecrosis of the knee. Arthritis Rheum 11:705–733. https://doi.org/10.1002/art.1780110602
Hansson CJ (1938) On insufficiency fractures of femur and tibia. Acta radiol 19:554–559. https://doi.org/10.3109/00016923809137785
Pentecost RL, Murray RA, Brindley HH (1964) Fatigue, insufficiency, and pathologic fractures. JAMA J Am Med Assoc 187:1001–1004. https://doi.org/10.1001/jama.1964.03060260029006
Yamamoto T, Bullough PG (2000) Spontaneous osteonecrosis of the knee: the result of subchondral insufficiency fracture. J Bone Jt Surg 82:858–866
Takeda M, Higuchi H, Kimura M et al (2008) Spontaneous osteonecrosis of the knee. J Bone Jt Surg 90:324–329. https://doi.org/10.1302/0301-620X.90B3.18629
Kosaka H, Maeyama A, Nishio J et al (2021) Histopathologic evaluation of bone marrow lesions in early stage subchondral insufficiency fracture of the medial femoral condyle. Int J Clin Exp Pathol 14:819–826
Acknowledgements
We thank Ms. Minako Nagata, Ms. Maya Yasuda, and Ms. KyokoTanaka for their expert technical assistance. We would like to thank Editage (www. editage.com) for English language editing.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
Koji Nukuto, Takehiko Matsushita, Kohei Kamada, Kyohei Nishida, Kanto Nagai, Noriyuki Kanzaki, Yuichi Hoshino, Tomoyuki Matsumoto, Takahiro Niikura, Ryosuke Kuroda have no conflicts of interest in the authorship and publication of this article.
Human and Animal Rights and Informed Consent
All procedures were approved by the Institutional Animal Care and Use Committee of Kobe University Graduate School of Medicine (approval number P200410). No human data were used in this study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
223_2022_1028_MOESM1_ESM.tif
Supplementary file1 (TIF 2700 KB) Supplemental Fig. 1 Sample allocation and collection in the medial meniscus root tear (MMPRT) model. Excld. Excluded from the analysis. PD patellar dislocation, NPD no patellar dislocation, FX Fracture, NFX no fracture
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Nukuto, K., Matsushita, T., Kamada, K. et al. Development and Analysis of Mouse Medial Meniscus Posterior Root Tear Model. Calcif Tissue Int 112, 55–65 (2023). https://doi.org/10.1007/s00223-022-01028-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01028-1