Abstract
Our previous gene profiling analysis showed that the transcription cofactor vestigial-like 3 (VGLL3) gene expression was upregulated by mechanical tension in the mouse cranial suture, coinciding with accelerated osteoblast differentiation. Therefore, we hypothesized that VGLL3 plays a significant role in osteogenic differentiation. To clarify the function of VGLL3 in osteoblasts, we examined its expression characteristics in mouse bone tissue and the osteoblastic cell line MC3T3-E1. We further examined the effects of Vgll3 knockdown on osteoblast differentiation and bone morphogenetic protein (BMP) signaling. In the mouse cranial suture, where membranous ossification occurs, VGLL3 was immunohistochemically detected mostly in the nucleus of osteoblasts, preosteoblasts, and fibroblastic cells. VGLL3 expression in MC3T3-E1 cells was transient and peaked at a relatively early stage of differentiation. RNA sequencing revealed that downregulated genes in Vgll3-knockdown cells were enriched in gene ontology terms associated with osteoblast differentiation. Interestingly, most of the upregulated genes were related to cell division. Targeted Vgll3 knockdown markedly suppressed the expression of major osteogenic transcription factors (Runx2, Sp7/osterix, and Dlx5) and osteoblast differentiation. It also attenuated BMP signaling; moreover, exogenous BMP2 partially restore osteogenic transcription factors’ expression in Vgll3-knockdown cells. Furthermore, overexpression of Vgll3 increased the expression of osteogenic transcription factors. These results suggest that VGLL3 plays a critical role in promoting osteoblast differentiation and that part of the process is mediated by BMP signaling. Further elucidation of VGLL3 function will increase our understanding of osteogenesis and skeletal disease etiology.

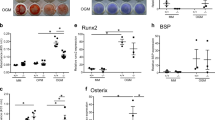
adapted from Ikegame et al., 2019 [26]). The arms were closed to 5-mm distance of each other and then the end of each arm pierced the center of right or left parietal bone to apply 0.2-g tension to the sagittal suture of 4-day-old mouse calvaria. The control arms were fixed by a tape and attached to parietal bones. Lower: A schema of the frontal view of the sagittal suture. Bold arrows indicate the direction of tensile stress exerted by the tension device. Osteoblasts (OB) and preosteoblasts (POB) are alkaline phosphatase (ALP) positive (red line). The area surrounded by a green-dotted line is shown in the following pictures in C. B The gene expression levels of Vgll3 in mouse sagittal sutures cultured with (0.2 g) or without (0 g) tension for 3 h and 6 h, examined by real-time PCR. Values are expressed as means ± SEMs (n = 5). Student’s t test, ***P < 0.001 (6 h–0 g vs 6 h–0.2 g). C In the control groups cultured for 1 h (1 h–0 g) and 6 h (6 h–0 g), the end of parietal bone was surrounded by ALP-positive (red) osteoblasts (OB) and preosteoblasts (POB) (a, d), which were positive for VGLL3 immunostaining (green; b, c, e, f). In the tension group cultured for 6 h (6 h–0.2 g), the number of ALP-positive POB at the end of parietal bone was increased (g, arrow). These newly differentiated POB (arrow) and some fibroblastic cells (FB) in the middle of suture (arrowheads) were positive for VGLL3 staining, mainly in the nucleus (h, i). White-dotted lines indicate the boundary of parietal bones. Nuclear staining: methyl green (light green; a, d, g), DAPI (magenta, pseudo-color; c, f, i). Scale bar: 20 μm. All pictures are shown at the same magnification





Similar content being viewed by others
Data availability
The datasets of the current study are available from the corresponding authors on request.
References
Ikegame M, Ishibashi O, Yoshizawa T, Shimomura J, Komori T, Ozawa H, Kawashima H (2001) Tensile stress induces bone morphogenetic protein 4 in preosteoblastic and fibroblastic cells, which later differentiate into osteoblasts leading to osteogenesis in the mouse calvariae in organ culture. J Bone Miner Res 16:24–32. https://doi.org/10.1359/jbmr.2001.16.1.24
Ikegame M, Tabuchi Y, Furusawa Y, Kawai M, Hattori A, Kondo T, Yamamoto T (2016) Tensile stress stimulates the expression of osteogenic cytokines/growth factors and matricellular proteins in the mouse cranial suture at the site of osteoblast differentiation. Biomed Res 37:117–126. https://doi.org/10.2220/biomedres.37.117
Simon E, Faucheux C, Zider A, Theze N, Thiebaud P (2016) From vestigial to vestigial-like: the Drosophila gene that has taken wing. Dev Genes Evol 226:297–315. https://doi.org/10.1007/s00427-016-0546-3
Vaudin P, Delanoue R, Davidson I, Silber J, Zider A (1999) TONDU (TDU), a novel human protein related to the product of vestigial (vg) gene of Drosophila melanogaster interacts with vertebrate TEF factors and substitutes for Vg function in wing formation. Development 126:4807–4816
Maeda T, Chapman DL, Stewart AF (2002) Mammalian vestigial-like 2, a cofactor of TEF-1 and MEF2 transcription factors that promotes skeletal muscle differentiation. J Biol Chem 277:48889–48898. https://doi.org/10.1074/jbc.M206858200
Mielcarek M, Piotrowska I, Schneider A, Gunther S, Braun T (2009) VITO-2, a new SID domain protein, is expressed in the myogenic lineage during early mouse embryonic development. Gene Expr Patterns : GEP 9:129–137. https://doi.org/10.1016/j.gep.2008.12.002
Faucheux C, Naye F, Tréguer K, Fédou S, Thiébaud P, Théze N (2010) Vestigial like gene family expression in Xenopus: common and divergent features with other vertebrates. Int J Dev Biol 54:1375–1382. https://doi.org/10.1387/ijdb.103080cf
Simon E, Thézé N, Fédou S, Thiébaud P, Faucheux C (2017) Vestigial-like 3 is a novel Ets1 interacting partner and regulates trigeminal nerve formation and cranial neural crest migration. Biol Open 6:1528–1540. https://doi.org/10.1242/bio.026153
Yamaguchi N (2020) Multiple roles of vestigial-like family members in tumor development. Front Oncol 10:1266. https://doi.org/10.3389/fonc.2020.01266
Arbajian E, Hofvander J, Magnusson L, Mertens F (2020) Deep sequencing of myxoinflammatory fibroblastic sarcoma. Genes Chromosomes Cancer 59:309–317. https://doi.org/10.1002/gcc.22832
Ali NM, Niada S, Brini AT, Morris MR, Kurusamy S, Alholle A, Huen D, Antonescu CR, Tirode F, Sumathi V, Latif F (2019) Genomic and transcriptomic characterisation of undifferentiated pleomorphic sarcoma of bone. J Pathol 247:166–176. https://doi.org/10.1002/path.5176
Liang Y, Tsoi LC, Xing X, Beamer MA, Swindell WR, Sarkar MK, Berthier CC, Stuart PE, Harms PW, Nair RP, Elder JT, Voorhees JJ, Kahlenberg JM, Gudjonsson JE (2017) A gene network regulated by the transcription factor VGLL3 as a promoter of sex-biased autoimmune diseases. Nat Immunol 18:152–160. https://doi.org/10.1038/ni.3643
Billi AC, Gharaee-Kermani M, Fullmer J, Tsoi LC, Hill BD, Gruszka D, Ludwig J, Xing X, Estadt S, Wolf SJ, Rizvi SM, Berthier CC, Hodgin JB, Beamer MA, Sarkar MK, Liang Y, Uppala R, Shao S, Zeng C, Harms PW, Verhaegen ME, Voorhees JJ, Wen F, Ward NL, Dlugosz AA, Kahlenberg JM, Gudjonsson JE (2019) The female-biased factor VGLL3 drives cutaneous and systemic autoimmunity. JCI Insight. https://doi.org/10.1172/jci.insight.127291
Halperin DS, Pan C, Lusis AJ, Tontonoz P (2013) Vestigial-like 3 is an inhibitor of adipocyte differentiation. J Lipid Res 54:473–481. https://doi.org/10.1194/jlr.M032755
Zhang D, Ni N, Wang Y, Tang Z, Gao H, Ju Y, Sun N, He X, Gu P, Fan X (2021) CircRNA-vgll3 promotes osteogenic differentiation of adipose-derived mesenchymal stem cells via modulating miRNA-dependent integrin α5 expression. Cell Death Differ 28:283–302. https://doi.org/10.1038/s41418-020-0600-6
Sinha KM, Zhou X (2013) Genetic and molecular control of osterix in skeletal formation. J Cell Biochem 114:975–984. https://doi.org/10.1002/jcb.24439
Komori T (2006) Regulation of osteoblast differentiation by transcription factors. J Cell Biochem 99:1233–1239. https://doi.org/10.1002/jcb.20958
Hojo H, Chung UI, Ohba S (2017) Identification of the gene-regulatory landscape in skeletal development and potential links to skeletal regeneration. Regen Ther 6:100–107. https://doi.org/10.1016/j.reth.2017.04.001
Macsai CE, Foster BK, Xian CJ (2008) Roles of Wnt signalling in bone growth, remodelling, skeletal disorders and fracture repair. J Cell Physiol 215:578–587. https://doi.org/10.1002/jcp.21342
Nishimura R, Hata K, Matsubara T, Wakabayashi M, Yoneda T (2012) Regulation of bone and cartilage development by network between BMP signalling and transcription factors. J Biochem 151:247–254. https://doi.org/10.1093/jb/mvs004
Wu M, Chen G, Li YP (2016) TGF-β and BMP signaling in osteoblast, skeletal development, and bone formation, homeostasis and disease. Bone Res 4:16009. https://doi.org/10.1038/boneres.2016.9
Kawane T, Komori H, Liu W, Moriishi T, Miyazaki T, Mori M, Matsuo Y, Takada Y, Izumi S, Jiang Q, Nishimura R, Kawai Y, Komori T (2014) Dlx5 and mef2 regulate a novel runx2 enhancer for osteoblast-specific expression. J Bone Miner Res 29:1960–1969. https://doi.org/10.1002/jbmr.2240
Acampora D, Merlo GR, Paleari L, Zerega B, Postiglione MP, Mantero S, Bober E, Barbieri O, Simeone A, Levi G (1999) Craniofacial, vestibular and bone defects in mice lacking the Distal-less-related gene Dlx5. Development 126:3795–3809
Greenblatt MB, Shim JH, Glimcher LH (2013) Mitogen-activated protein kinase pathways in osteoblasts. Annu Rev Cell Dev Biol 29:63–79. https://doi.org/10.1146/annurev-cellbio-101512-122347
Rodríguez-Carballo E, Gámez B, Ventura F (2016) p38 MAPK signaling in osteoblast differentiation. Front Cell Dev Biol 4:40. https://doi.org/10.3389/fcell.2016.00040
Ikegame M, Ejiri S, Okamura H (2019) Expression of non-collagenous bone matrix proteins in osteoblasts stimulated by mechanical stretching in the cranial suture of neonatal mice. J Histochem Cytochem 67:107–116. https://doi.org/10.1369/0022155418793588
Burstone MS (1961) Histochemical demonstration of phosphatases in frozen sections with naphthol AS-phosphates. J Histochem Cytochem 9:146–153
Love MI, Huber W, Anders S (2014) Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol 15:550. https://doi.org/10.1186/s13059-014-0550-8
Tosa I, Yamada D, Yasumatsu M, Hinoi E, Ono M, Oohashi T, Kuboki T, Takarada T (2019) Postnatal Runx2 deletion leads to low bone mass and adipocyte accumulation in mice bone tissues. Biochem Biophys Res Commun 516:1229–1233. https://doi.org/10.1016/j.bbrc.2019.07.014
Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, Shimizu Y, Bronson RT, Gao YH, Inada M, Sato M, Okamoto R, Kitamura Y, Yoshiki S, Kishimoto T (1997) Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell 89:755–764. https://doi.org/10.1016/s0092-8674(00)80258-5
Nakashima K, Zhou X, Kunkel G, Zhang Z, Deng JM, Behringer RR, de Crombrugghe B (2002) The novel zinc finger-containing transcription factor osterix is required for osteoblast differentiation and bone formation. Cell 108:17–29. https://doi.org/10.1016/s0092-8674(01)00622-5
Robledo RF, Rajan L, Li X, Lufkin T (2002) The Dlx5 and Dlx6 homeobox genes are essential for craniofacial, axial, and appendicular skeletal development. Genes Dev 16:1089–1101. https://doi.org/10.1101/gad.988402
Baek K, Baek JH (2013) The transcription factors myeloid elf-1-like factor (MEF) and distal-less homeobox 5 (Dlx5) inversely regulate the differentiation of osteoblasts and adipocytes in bone marrow. Adipocyte 2:50–54. https://doi.org/10.4161/adip.22019
Suo J, Feng X, Li J, Wang J, Wang Z, Zhang L, Zou W (2020) VGLL4 promotes osteoblast differentiation by antagonizing TEADs-inhibited Runx2 transcription. Sci Adv 6:eaba4147. https://doi.org/10.1126/sciadv.aba4147
Helias-Rodzewicz Z, Perot G, Chibon F, Ferreira C, Lagarde P, Terrier P, Coindre JM, Aurias A (2010) YAP1 and VGLL3, encoding two cofactors of TEAD transcription factors, are amplified and overexpressed in a subset of soft tissue sarcomas. Genes Chromosomes Cancer 49:1161–1171. https://doi.org/10.1002/gcc.20825
Hori N, Okada K, Takakura Y, Takano H, Yamaguchi N, Yamaguchi N (2020) Vestigial-like family member 3 (VGLL3), a cofactor for TEAD transcription factors, promotes cancer cell proliferation by activating the Hippo pathway. J Biol Chem 295:8798–8807. https://doi.org/10.1074/jbc.RA120.012781
Cody NA, Shen Z, Ripeau JS, Provencher DM, Mes-Masson AM, Chevrette M, Tonin PN (2009) Characterization of the 3p12.3-pcen region associated with tumor suppression in a novel ovarian cancer cell line model genetically modified by chromosome 3 fragment transfer. Mol Carcinog 48:1077–1092. https://doi.org/10.1002/mc.20535
Gambaro K, Quinn MC, Wojnarowicz PM, Arcand SL, de Ladurantaye M, Barres V, Ripeau JS, Killary AM, Davis EC, Lavoie J, Provencher DM, Mes-Masson AM, Chevrette M, Tonin PN (2013) VGLL3 expression is associated with a tumor suppressor phenotype in epithelial ovarian cancer. Mol Oncol 7:513–530. https://doi.org/10.1016/j.molonc.2012.12.006
Gat-Yablonski G, Frumkin-Ben David R, Bar M, Potievsky O, Phillip M, Lazar L (2011) Homozygous microdeletion of the POU1F1, CHMP2B, and VGLL3 genes in chromosome 3–a novel syndrome. Am J Med Genet A 155A:2242–2246. https://doi.org/10.1002/ajmg.a.34136
Acknowledgements
We would like to express our sincere gratitude to both Dr. Ikue Tosa, Okayama University (Professor Takarada Laboratory), for providing us with bone samples from Runx2-knockout mouse and Prof. Sadakazu Ejiri, Asahi University, for helpful discussions. We also thank Ms. Heriati Sitosari and Mr. Yilin Zheng for editing the manuscript.
Funding
This work was supported by JSPS KAKENHI grants JP2259203 and JP19K07269 to M.I.; a research grant from Koyanagi-Zaidan in 2020 to M.I.; and the National Natural Science Foundation of China grants 81870736 and 81801040 to B.Z. and Y.L., respectively.
Author information
Authors and Affiliations
Contributions
HY contributed to validation, formal analysis, investigation, and visualization. MI contributed to conceptualization, methodology, writing of the original draft, visualization, supervision, and funding acquisition. YF contributed to formal analysis and statistics. FT performed investigation. YY contributed to formal analysis and investigation. JT contributed to methodology and investigation. YW contributed to investigation and editing of the manuscript. JG performed investigation. DY contributed to methodology. TT contributed to methodology and resources. YL contributed to project administration and funding acquisition. HO contributed to supervision, project administration, and editing of the manuscript. BZ contributed to project administration and funding acquisition.
Corresponding authors
Ethics declarations
Conflict of interest
Haoze Yuan, Mika Ikegame, Yoko Fukuhara, Fumiko Takemoto, Yaqiong Yu, Jumpei Teramachi, Yao Weng, Jiajie Guo, Daisuke Yamada, Takeshi Takarada, Ying Li, Hirohiko Okamura, and Bin Zhang declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The protocol for the animal experiment was conducted according to the guidelines of the Animal Care and Use Committee, Okayama University (Approval No. OKU-2016114). These guidelines were established based on NIH guide for the care and use of Laboratory animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Yuan, H., Ikegame, M., Fukuhara, Y. et al. Vestigial-Like 3 Plays an Important Role in Osteoblast Differentiation by Regulating the Expression of Osteogenic Transcription Factors and BMP Signaling. Calcif Tissue Int 111, 331–344 (2022). https://doi.org/10.1007/s00223-022-00997-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-00997-7