Abstract
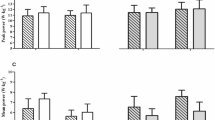
This study examined potential fluctuations in bone metabolic markers across the menstrual cycle both at rest and after a 30-min bout of continuous running at 80% of V̇O2max. Resting and post-exercise (0, 30, 90 min) sclerostin, parathyroid hormone (PTH), carboxy-terminal cross-linking telopeptide of type I collagen (β-CTXI), and procollagen type 1 N propeptide (PINP) were assessed in 10 eumenorrheic women (age: 21 ± 3 y, BMI: 23.2 ± 3.0 kg.m2) during the mid- to late-follicular (FP: day 8.0 ± 1.4) and mid-luteal (LP: day 22.0 ± 2.5) phases of the menstrual cycle. Ovulation was determined using ovulation kits and daily measurement of oral body temperature upon awakening. Menstrual cycle phase was subsequently confirmed by measurement of plasma estradiol and progesterone. On average, resting estradiol concentrations increased from 46.3 ± 8.9 pg·mL−1 in the FP to 67.3 ± 23.4 pg·mL−1 in the LP (p = 0.015), and resting progesterone increased from 4.12 ± 2.36 ng·mL−1 in the FP to 11.86 ± 4.49 ng·mL−1 in the LP (p < 0.001). At rest, there were no differences between menstrual cycle phases in sclerostin (FP: 260.1 ± 135.0 pg·mL−1; LP: 303.5 ± 99.9 pg·mL−1; p = 0.765), PTH (FP: 0.96 ± 0.64 pmol·L−1; LP: 0.79 ± 0.44 pmol·L−1; p = 0.568), β-CTXI (FP: 243.1 ± 158.0 ng·L−1; LP: 202.4 ± 92.3 ng·L−1; p = 0.198), and PINP (FP: 53.6 ± 8.9 μg·L−1; LP: 66.2 ± 20.2 μg·L−1; p = 0.093). Main effects for time (p < 0.05) were shown in sclerostin, PTH, β-CTXI and PINP, without phase or interaction effects. Sclerostin increased from pre- to immediately post-exercise (45%; p = 0.007), and so did PTH (43%; p = 0.011), both returning to resting concentrations 30 min post-exercise. β-CTXI decreased from pre- to post-exercise (20%; p = 0.027) and was still below its pre-exercise concentrations at 90 min post-exercise (17%; p = 0.013). PINP increased immediately post-exercise (29%; p < 0.001), returning to resting concentrations at 30 min post-exercise. These results demonstrate no effect of menstrual cycle phase on resting bone marker concentrations or on the bone metabolic marker response to intense exercise.





Similar content being viewed by others
Data Availability
Data are available only upon request from the corresponding author for researchers who are eligible for accessing confidential data, as all data of this study are restricted due to the Brock University Research Ethics Board privacy policy.
References
Holesh J, Lord M (2020) Physiology, ovulation—statpearls—NCBI bookshelf. StatPearls Publishing LLC., Treasure Island
Reed BG, Carr BR (2018) The normal menstrual cycle and the control of ovulation. In: Feingold KR, Anawalt B, Boyce A, Chrousos G, de Herder WW, Dhatariya K, Dungan K, Hershman JM, Hofland J, Kalra S, Kaltsas G, Koch C, Kopp P, Korbonits M, Kovacs CS, Kuohung W, Laferrère B, Levy M, McGee EA, McLachlan R, Morley JE, New M, Purnell J, Sahay R, Singer F, Sperling MA, Stratakis CA, Trence DL, Wilson DP (eds) Endotext [Internet]. MDText.com, Inc., South Dartmouth, MA
Mihm M, Gangooly S, Muttukrishna S (2011) The normal menstrual cycle in women. Anim Reprod Sci 124(3–4):229–236. https://doi.org/10.1016/j.anireprosci.2010.08.030
Oosthuyse T, Bosch AN (2010) The effect of the menstrual cycle on exercise metabolism. Sport Med 40(3):207–227. https://doi.org/10.2165/11317090-000000000-00000
Leonard AN, Shill AL, Thackray AE, Stensel DJ, Bishop NC (2021) Fasted plasma asprosin concentrations are associated with menstrual cycle phase, oral contraceptive use and training status in healthy women. Eur J Appl Physiol 121(3):793–801. https://doi.org/10.1007/s00421-020-04570-8
Ugur K, Aydin S (2019) Saliva and blood asprosin hormone concentration associated with obesity. Int J Endocrinol Mar 27: 2521096. https://doi.org/10.1155/2019/2521096
Vaananen K, Harkonene P (1996) Estrogen and bone metabolism. J Climacteric Postmenopause 23:65–69
Zallone A (2006) Direct and indirect estrogen actions on osteoblasts and osteoclasts. Ann N Y Acad Sci 1068(1):173–179. https://doi.org/10.1196/annals.1346.019
Robinson JA, Harris SA, Riggs BL, Spelsberg TC (1997) Estrogen regulation of human osteoblastic cell proliferation and differentiation. Endocrinology 138(7):2919–2927. https://doi.org/10.1210/endo.138.7.5277
Barbieri RL (1992) Hormone treatment of endometriosis: the estrogen threshold hypothesis. Am J Obstet Gynecol 166(2):740–745. https://doi.org/10.1016/0002-9378(92)91706-G
Weitzmann MN, Pacifici R (2006) Estrogen deficiency and bone loss: an inflammatory tale. J Clin Invest 116(5):1186–1194. https://doi.org/10.1172/JCI28550
Southmayd EA, Mallinson RJ, Williams NI, Mallinson DJ, De Souza MJ (2017) Unique effects of energy versus estrogen deficiency on multiple components of bone strength in exercising women. Osteoporos Int 28(4):1365–1376. https://doi.org/10.1007/s00198-016-3887-x
Cauley JA (2015) Estrogen and bone health in men and women. Steroids 99:11–15. https://doi.org/10.1016/j.steroids.2014.12.010
Khosla S, Oursler MJ, Monroe DG (2012) Estrogen and the skeleton. Trends Endocrinol Metab 23(11):576–581. https://doi.org/10.1016/j.tem.2012.03.008
Fazzalari NL (2008) Bone remodeling: a review of the bone microenvironment perspective for fragility fracture (osteoporosis) of the hip. Semin Cell Dev Biol 19(5):467–472. https://doi.org/10.1016/j.semcdb.2008.08.003
Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y (2009) Oxidative stress in bone remodelling and disease. Trends Mol Med 15(10):468–477. https://doi.org/10.1016/j.molmed.2009.08.004
Oursler MJ, Pederson L, Fitzpatrick L, Riggs BL, Spelsberg T (1994) Human giant cell tumors of the bone (osteoclastomas) are estrogen target cells. Proc Natl Acad Sci USA 91(12):5227–5231. https://doi.org/10.1073/pnas.91.12.5227
Gass ML, Kagan R, Kohles JD, Martens MG (2008) Bone turnover marker profile in relation to the menstrual cycle of premenopausal healthy women. Menopause 15(4):667–675. https://doi.org/10.1097/gme.0b013e31815f8917
Mozzanega B et al (2013) Cyclic variations of bone resorption mediators and markers in the different phases of the menstrual cycle. J Bone Miner Metab 31(4):461–467. https://doi.org/10.1007/s00774-013-0430-4
Zittermann A et al (2000) Physiologic fluctuations of serum estradiol levels influence biochemical markers of bone resorption in young women. J Clin Endocrinol Metab 85(1):95–101. https://doi.org/10.1210/jcem.85.1.6250
Nielsen HK, Brixen K, Bouillon R, Mosekilde L (1990) Changes in biochemical markers of osteoblastic activity during the menstrual cycle. J Clin Endocrinol Metab 70(5):1431–1437. https://doi.org/10.1210/jcem-70-5-1431
Chiu KM et al (2000) Correlation of estradiol, parathyroid hormone, interleukin-6, and soluble interleukin-6 receptor during the normal menstrual cycle. Bone. https://doi.org/10.1016/S8756-3282(99)00243-4
Chiu KM, Ju J, Mayes D, Bacchetti P, Weitz S, Arnaud CD (1999) Changes in bone resorption during the menstrual cycle. J Bone Miner Res 14(4):609–615. https://doi.org/10.1359/jbmr.1999.14.4.609
Suzuki N et al (2007) Effect of acute resistance exercise on bone metabolism during menstrual cycle. Japanese J Phys Fit Sport Med. https://doi.org/10.7600/jspfsm.56.215
Pitkin RM, Reynolds WA, Williams GA, Hargis GK (1978) Calcium-regulating hormones during the menstrual cycle*. J Clin Endocrinol Metab 47(3):626–632. https://doi.org/10.1210/jcem-47-3-626
Martin D, Cooper SB, Tang JCY, Fraser WD, Sale C, Elliott-Sale KJ (2021) Bone metabolic marker concentrations across the menstrual cycle and phases of combined oral contraceptive use. Bone 145:115864. https://doi.org/10.1016/j.bone.2021.115864
Gorai I et al (1998) Serum soluble interleukin-6 receptor and biochemical markers of bone metabolism show significant variations during the menstrual cycle. J Clin Endocrinol Metab 83(2):326–332. https://doi.org/10.1210/jcem.83.2.4584
Baran DT, Whyte MP, Haussler MR, Leonard J, Slatopolsky E, Avioli LV (2015) Hormones in the normal young woman. J Clin Endocrinol Metab 50(2):377–379
Buchanan JR, Santen RJ, Cavaliere A, Cauffman SW, Greer RB, Demers LM (1986) Interaction between parathyroid hormone and endogenous estrogen in normal women. Metabolism 35(6):489–494. https://doi.org/10.1016/0026-0495(86)90003-X
Musef KENN, Manolagas SC, Deftos LJ, Alexander N, Yen SSC (1986) Calcium regulating hormones across the menstrual cycle. J Clin Endocrinol Metab 62(6):1313–1316
Liakou CG et al (2016) Changes of serum sclerostin and Dickkopf-1 levels during the menstrual cycle. A pilot study. Endocrine 54(2):543–551. https://doi.org/10.1007/s12020-016-1056-9
Cidem M, Usta TA, Karacan I, Kucuk SH, Uludag M, Gun K (2013) Effects of sex steroids on serum sclerostin levels during the menstrual cycle. Gynecol Obstet Invest 75(3):179–184. https://doi.org/10.1159/000347013
Niethammer B, Körner C, Schmidmayr M, Luppa PB, Seifert-Klauss VR (2015) Non-reproductive effects of anovulation: bone metabolism in the luteal phase of premenopausal women differs between ovulatory and anovulatory cycles. Geburtshilfe Frauenheilkd 75(12):1250–1257
Canadian Society for Exercise Physiology (2017) Get active questionnaire. Can Soc Exerc Physiol. http://www.csep.ca/CMFiles/GAQ_CSEPPATHReadinessForm_2pages.pdf
Forsyth J, Reilly T (2008) The effect of menstrual cycle on 2000-m rowing ergometry performance. Eur J Sport Sci 8(6):351–357. https://doi.org/10.1080/17461390802308644
Godin G (2011) Godin leisure-time exercise questionnaire. Health Fit J Canada 4(1):18–22. https://doi.org/10.14288/hfjc.v4i1.82
Swain DP (2000) Energy cost calculations for exercise prescription: an update. Sport Med 30(1):17–22. https://doi.org/10.2165/00007256-200030010-00002
Field A (2009) Discovering statistics using SPSS, 3rd edn. Sage Publications Ltd., London
Cohen J (1988) Statistical power analysis for the behavioural sciences. Routledge, New York. https://doi.org/10.4324/9780203771587
Cohen J (1992) Statistical power analysis. Curr Dir Psychol Sci 1(3):98–101. https://doi.org/10.1111/1467-8721.ep10768783
Riggs BL, Khosla S, Melton LJ (2002) Sex steroids and the construction and conservation of the adult skeleton. Endocr Rev 23(3):279–302. https://academic.oup.com/edrv/article/23/3/279/2424167
Lasley BL et al (2002) The relationship of circulating dehydroepiandrosterone, testosterone, and estradiol to stages of the menopausal transition and ethnicity. J Clin Endocrinol Metab 7(8):3760–3767. https://academic.oup.com/jcem/article/87/8/3760/2846984
Kouvelioti R, Kurgan N, Falk B, Ward WE, Josse AR, Klentrou P (2019) Cytokine and sclerostin response to high-intensity interval running versus cycling. Med Sci Sports Exerc 51(12):2458–2464. https://doi.org/10.1249/MSS.0000000000002076
Pickering ME et al (2017) Serum sclerostin increases after acute physical activity. Calcif Tissue Int 101(2):170–173. https://doi.org/10.1007/s00223-017-0272-5
Kurgan N et al (2020) Cytokines, adipokines, and bone markers at rest and in response to plyometric exercise in obese vs normal weight adolescent females. Front Endocrinol 11:1. https://doi.org/10.3389/fendo.2020.531926
Smit MA, Van Kinschot CMJ, Van Der Linden J, Van Noord C, Kos S (2019) Clinical guidelines and PTH measurement: does assay generation matter? Endocr Rev 40(6):1468–1480. https://doi.org/10.1210/er.2018-00220
Khosla S, Atkinson EJ, Melton LJ, Riggs BL (1997) Effects of age and estrogen status on serum parathyroid hormone levels and biochemical markers of bone turnover in women: a population-based study 1. J Clin Endocrinol Metab 82(5):1522–1527. https://doi.org/10.1210/jcem.82.5.3946
Scott JPR, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD (2011) The role of exercise intensity in the bone metabolic response to an acute bout of weight-bearing exercise. J Appl Physiol 110(2):423–432. https://doi.org/10.1152/japplphysiol.00764.2010
Barry DW, Kohrt WM (2007) Acute effects of 2 hours of moderate-intensity cycling on serum parathyroid hormone and calcium. Calcif Tissue Int 80(6):359–365. https://doi.org/10.1007/s00223-007-9028-y
Scott JPR, Sale C, Greeves JP, Casey A, Dutton J, Fraser WD (2010) The effect of training status on the metabolic response of bone to an acute bout of exhaustive treadmill running. J Clin Endocrinol Metab 95(8):3918–3925. https://doi.org/10.1210/jc.2009-2516
Kohrt WM et al (2018) Maintenance of serum ionized calcium during exercise attenuates parathyroid hormone and bone resorption responses. J Bone Miner Res 33(7):1326–1334. https://doi.org/10.1002/jbmr.3428
Kohrt WM et al (2019) Dermal calcium loss is not the primary determinant of parathyroid hormone secretion during exercise. Med Sci Sports Exerc 51(10):2117–2124. https://doi.org/10.1249/MSS.0000000000002017
Townsend R et al (2016) Parathyroid hormone secretion is controlled by both ionized calcium and phosphate during exercise and recovery in men. J Clin Endocrinol Metab 101(8):3231–3239. https://doi.org/10.1210/jc.2016-1848
Shea KL, Barry DW, Sherk VD, Hansen KC, Wolfe P, Kohrt WM (2014) Calcium supplementation and parathyroid hormone response to vigorous walking in postmenopausal women. Med Sci Sports Exerc 46(10):2007–2013. https://doi.org/10.1249/MSS.0000000000000320
Guillemant J, Accarie C, Peres G, Guillemant S (2004) Acute effects of an oral calcium load on markers of bone metabolism during endurance cycling exercise in male athletes. Calcif Tissue Int 74(5):407–414. https://doi.org/10.1007/s00223-003-0070-0
Sherk VD, Wherry SJ, Barry DW, Shea KL, Wolfe P, Kohrt WM (2017) Calcium supplementation attenuates disruptions in calcium homeostasis during exercise. Med Sci Sport Exerc 49(7):1437–1442. https://doi.org/10.1249/MSS.0000000000001239.Calcium
Haakonssen EC et al (2015) The effects of a calcium-rich pre-exercise meal on biomarkers of calcium homeostasis in competitive female cyclists a randomised crossover trial. PLoS ONE 10(5):1–16. https://doi.org/10.1371/journal.pone.0123302
Barry DW, Hansen KC, Van Pelt RE, Witten M, Wolfe P, Kohrt WM (2011) Acute calcium ingestion attenuates exercise-induced disruption of calcium homeostasis. Med Sci Sports Exerc 43(4):617–623. https://doi.org/10.1249/MSS.0b013e3181f79fa8
Bhattarai HK, Shrestha S, Rokka K, Shakya R (2020) Vitamin D, calcium, parathyroid hormone, and sex steroids in bone health and effects of aging. J Osteoporos. https://doi.org/10.1155/2020/9324505
Bjarnason NH, Henriksen EEG, Alexandersen P, Christgau S, Henriksen DB, Christiansen C (2002) Mechanism of circadian variation in bone resorption. Bone 30(1):307–313. https://doi.org/10.1016/S8756-3282(01)00662-7
Clowes JA, Hannon RA, Yap TS, Hoyle NR, Blumsohn A, Eastell R (2002) Effect of feeding on bone turnover markers and its impact on biological variability of measurements. Bone 30(6):886–890. https://doi.org/10.1016/S8756-3282(02)00728-7
Szulc P, Naylor K, Hoyle NR, Eastell R, Leary ET (2017) Use of CTX-I and PINP as bone turnover markers: national bone health alliance recommendations to standardize sample handling and patient preparation to reduce pre-analytical variability. Osteoporos Int 28(9):2541–2556. https://doi.org/10.1007/s00198-017-4082-4
Shetty S, Kapoor N, Bondu J, Thomas N, Paul T (2016) Bone turnover markers: emerging tool in the management of osteoporosis. Indian J Endocrinol Metab 20(6):846–852. https://doi.org/10.4103/2230-8210.192914
Qvist P, Christgau S, Pedersen BJ, Schlemmer A, Christiansen C (2002) Circadian variation in the serum concentration of C-terminal telopeptide of type I collagen (serum CTx): effects of gender, age, menopausal status, posture, daylight, serum cortisol, and fasting. Bone 31(1):57–61. https://doi.org/10.1016/S8756-3282(02)00791-3
Chubb SAP, Mandelt CD, Vasikaran SD (2015) Comparison of results from commercial assays for plasma CTX: the need for harmonization. Clin Biochem 48(7–8):519–524. https://doi.org/10.1016/j.clinbiochem.2015.03.002
Miller WG, Myers GL, Rej R (2006) Why commutability matters. Clin Chem 52(4):553–554. https://doi.org/10.1373/clinchem.2005.063511
Kouvelioti R, LeBlanc P, Falk B, Ward WE, Josse AR, Klentrou P (2019) Effects of high-intensity interval running versus cycling on sclerostin, and markers of bone turnover and oxidative stress in young men. Calcif Tissue Int 104(6):582–590. https://doi.org/10.1007/s00223-019-00524-1
Scott JPR et al (2012) Effect of fasting versus feeding on the bone metabolic response to running. Orig Full Length Artic. https://doi.org/10.1016/j.bone.2012.08.128
Theocharidis A, McKinlay BJ, Vlachopoulos D, Josse AR, Falk B, Klentrou P (2020) Effects of post exercise protein supplementation on markers of bone turnover in adolescent swimmers. J Int Soc Sports Nutr 17(1):1–11. https://doi.org/10.1186/s12970-020-00350-z
Townsend R, Elliott-Sale KJ, Currell K, Tang J, Fraser WD, Sale C (2017) The effect of postexercise carbohydrate and protein ingestion on bone metabolism. Med Sci Sports Exerc 49(6):1209–1218. https://doi.org/10.1249/MSS.0000000000001211
Herrmann M, Müller M, Scharhag J, Sand-Hill M, Kindermann W, Herrmann W (2007) The effect of endurance exercise-induced lactacidosis on biochemical markers of bone turnover. Clin Chem Lab Med 45(10):1381–1389. https://doi.org/10.1515/CCLM.2007.282
De Sousa MV, Pereira RMR, Fukui R, Caparbo VF, Da Silva MER (2014) Carbohydrate beverages attenuate bone resorption markers in elite runners. Metabolism 63(12):1536–1541. https://doi.org/10.1016/j.metabol.2014.08.011
Martin D, Cooper SB, Tang JCY, Fraser WD, Sale C, Elliott-Sale KJ (2021) Bone metabolic marker concentrations across the menstrual cycle and phases of combined oral contraceptive use. Bone. https://doi.org/10.1016/j.bone.2021.115864
Rantalainen T, Heinonen A, Linnamo V, Komi PV, Takala TES, Kainulainen H (2009) Short-term bone biochemical response to a single bout of high-impact exercise. J Sport Sci Med 8(4):553–559
Pomerants J, Tillman T, Karelson V, Jurimae K (2008) Impact of acute exercise on bone turnover and growth hormone/insulin-like growth factor axis in boys. J Sports Med Phys Fitness 48(2):266–271
Prawiradilaga RS, Madsen AO, Jørgensen NR, Helge EW (2020) Acute response of biochemical bone turnover markers and the associated ground reaction forces to high-impact exercise in postmenopausal women. Biol Sport 37(1):41–48. https://doi.org/10.5114/biolsport.2020.91497
Funding
This research was funded by the Natural Sciences and Engineering Research Council of Canada (NSERC Grant to PK # 2020-00014 and TH # 2016-06118). NK holds an NSERC doctoral scholarship.
Author information
Authors and Affiliations
Contributions
AG contributed to the analysis of blood samples, data analysis and interpretation, and prepared the first draft. NK contributed to the analysis of the blood samples. SM, SM and TH designed the original study and completed all data collection. CS, HL-S and KE-S contributed to data interpretation and final draft. PK was the supervisor of the research and contributed to all aspects of data analysis and interpretation and prepared the final draft. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflict of interest
Anne Guzman, Nigel Kurgan, Sara C. Moniz, Seth F. McCarthy, Craig Sale, Heather Logan-Sprenger, Kirsty Elliott-Sale, Tom J. Hazell, Panagiota Klentrou have no conflict of interest to declare.
Ethical Approval
All procedures performed in this study involving human participants were conducted in accordance with the ethical standards of the institutional research committees and with the 1964 Declaration of Helsinki and its later amendments. The study received ethics approval from the Research Ethics Boards of Wilfrid Laurier and Brock Universities.
Informed Consent
All participants agreed to participate in this study by signing a consent form.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Guzman, A., Kurgan, N., Moniz, S.C. et al. Menstrual Cycle Related Fluctuations in Circulating Markers of Bone Metabolism at Rest and in Response to Running in Eumenorrheic Females. Calcif Tissue Int 111, 124–136 (2022). https://doi.org/10.1007/s00223-022-00970-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-00970-4