Abstract
Hypophosphatasia (HPP) is a rare genetic disorder characterized by low serum alkaline phosphatase (ALP), its manifestations may include atypical femoral fractures (AFF). However, the prevalence of low serum ALP and HPP in patients with AFF remains unknown. We retrospectively analyzed ALP levels and clinical manifestations compatible with HPP in 72 adult patients with confirmed AFF by chart review. ALP values were compared with those of a control group of patients with prior proximal femoral fracture during antiresorptive treatment (n = 20). Among the AFF patients, 18 (25%) had at least one serum ALP value ≤ 40 IU/L, although in all but one case, at least one ALP value > 40 IU/L was also detected at another time point. Most low ALP values were associated with antiresorptive treatment (P = 0.049) and lowest levels of ALP did not differ between the AFF and the control groups (P = 0.129). However, low ALP values among AFF patients were associated with a higher rate of bilateral AFF (50% vs 22%, P = 0.025), metatarsal fracture (33% vs 7%, P = 0.006), and with trends for more frequent use of glucocorticoid (22% vs 8%, P = 0.089) and proton pump inhibitor (61% vs 44%, P = 0.220). In one AFF patient with low ALP and clinical suspicion of HPP, a rare pathogenic heterozygous variant of the ALPL gene was identified. In conclusion, low ALP values are common among subjects with AFF and mainly related to concomitant antiresorptive medication. Hence, low serum ALP has low specificity for HPP among AFF patients.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Atypical femoral fractures (AFFs) are uncommon and have been associated mainly with prolonged use of bisphosphonates and denosumab, although about 10% occur in the absence of exposure to antiresorptive treatment [1,2,3,4]. The pathophysiological mechanisms of AFF remain poorly understood, but some clinical, biomechanical, and genetic factors have been implicated in the risk of AFF [5, 6]. Among those, recent studies report an increased risk for subtrochanteric and diaphyseal femoral fractures in patients with hypophosphatasia (HPP) [1, 6].
HPP is an inborn error of metabolism characterized by a quantitative deficit of alkaline phosphatase (ALP), due to a loss‐of‐function mutation in the ALPL gene (also known as TNSALP gene) that encodes the tissue non-specific ALP [7]. The adult form of HPP presents with a wide range of clinical manifestations, including skeletal alterations due to defective bone mineralization such as osteopenia, premature tooth loss, rickets, osteomalacia, delayed fracture healing, stress fractures of metatarsal bones, AFF, or cortical stress fractures (pseudo-fractures) which can resemble bisphosphonate‐associated incomplete AFF when localized to the lateral side of the femoral shaft [8]. In addition, HPP manifests with clinical signs and non-specific and mild extra-skeletal symptoms such as seizures, nephrocalcinosis, kidney stones, muscle weakness, chronic pain, and fatigue often overlapping with other metabolic bone diseases. For this reason, many cases of adult HPP remain un- or misdiagnosed, and, subsequently, are treated wrongly or even remain untreated.
Hence, the occurrence of an AFF in adulthood should raise clinical suspicion of an undiagnosed mild form of HPP, particularly when associated with other biochemical and clinical signs consistent with the disease, which should then be confirmed by genetic sequencing of ALPL gene. However, the sensitivity of genetic screening for HPP may be impaired by the lack of patient selection based on the probability of bearing HPP mutations, i.e., on the presence or absence of persistently low serum ALP values [9, 10].
The aim of our study was to investigate the prevalence of low serum ALP values in a cohort of subjects with AFF and compared to a control group of patients with common proximal femur fractures receiving bisphosphonates (BPs). As a secondary outcome, we also explored the influence of antiresorptive medications on ALP values, since these drugs are a major risk factor of AFF. Eventually, TNSFALP gene mutations were screened in AFF patients who presented with persistently low ALP values and clinical manifestations typical of the disease.
Methods
Study Design and Patients
This was a retrospective observational chart review study. The primary endpoint was the prevalence of low ALP values in patients with AFF. The secondary endpoint of the study was to compare serum levels of ALP of patients with AFF with a control group of patients who presented with prior hip fragility fracture during antiresorptive treatment and to evaluate the influence of antiresorptive treatment on ALP levels.
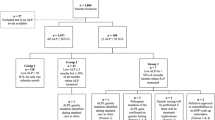
In the AFF cohort 72 patients aged 18 years or older, who were diagnosed with AFF and hospitalized at the Service of Orthopedics at the Geneva University Hospitals between 2000 and 2020 were included. The presence of an AFF was defined according to the criteria of the American Society for Bone and Mineral Research [1]. Exclusion criteria included rare bone disease, current bone, primary liver or metastatic cancer, liver transplantation, autoimmune liver disease, and lack of ALP data. In the control group, adult patients hospitalized with a hip fracture at the Service of Orthopedics at the Geneva University Hospitals between 2007 and 2017 and who had previously been exposed to BPs were included. Three hundred patients with proximal femur fractures were screened for concomitant BP use and only 23 patients were identified as having received antiresorptive treatment at the time of fracture (control group). Of those, 3 patients with current bone or gastrointestinal cancer, previous liver transplantation, and rare bone disease were further excluded.
Clinical and biochemical data were collected before, during, and after hospitalization from medical records of the hospitals and from treating physicians. Previous antiresorptive treatment included oral (alendronate, ibandronate, risedronate, pamidronate) or intravenous (zoledronic acid) bisphosphonates as well as subcutaneous denosumab. Oral vitamin D supplements could be administered alone or together with calcium in doses of 800 UI–1200 UI/day. Additionally, the use of corticosteroids, which can reduce ALP activity and increase the risk of hip fracture and AFF [11], were evaluated. Corticosteroid use included prednisone administration at a range dose 2–30 mg/day. PPIs, including esomeprazole, omeprazole, pantoprazole in standard doses used at least for 1 year, were also evaluated since preclinical studies suggested that IPPs may decrease ALP expression [12] and that IPPs use has been associated with AFF. The study was approved by the Ethics committee Geneva, Switzerland.
Assessments
Clinical data assessed of all included patient demographics, medical history, previous drug treatments, and radiographs. Anamnestic data such as chronic fatigue and chronic pain syndrome, nephrolithiasis, and diagnosis of dementia or other neuropsychiatric diseases were collected during hospitalization or ambulatory visits. Evaluation of osteoporosis was performed using bone densitometry (dual-energy X-ray absorptiometry, DXA) using the available data before and after fracture. Medical records did not include dental history of the patients. At least one serum ALP measurement was prospectively evaluated from every patient. Fractures were diagnosed by conventional radiographs and evaluated by at least one senior consultant of the Service of Orthopedics and of the Division of Bone Diseases at the Geneva University Hospitals.
ALP Measurement
Quantitative determination of ALP in human serum and plasma was performed until 2015 by Beckman Coulter System and after 2015 by a standardized colorimetric assay using a Roche/Hitachi automated clinical chemistry analyzer (Roche Diagnostics, Mannheim, Germany). In short in Roche/Hitachi, p-nitrophenyl phosphate is hydrolyzed by phosphatases in the presence of magnesium and zinc ions to form phosphate and p-nitrophenol. The p-nitrophenol released is proportional to ALP activity and can be measured photometrically. Precision was determined using human samples and controls in an internal protocol. The lower detection limit of ALP was 5 IU/L, the normal range in adults was 30–125 IU/L. However, the cut-off for low ALP was defined as 40 IU/L in our study according to the age-adjusted range of the Canadian Laboratory Initiative in Pediatric Reference Intervals (CALIPER) [13,14,15].
Vitamin D and Vitamin B6 Measurements
Quantitative determination of vitamin D (25-OH vitamin D) in human serum was performed by COBAS 8000ECLIA (Roche Diagnostics, Rotkreuz, Switzerland). In an adult population, values less than 25 nmol/L indicate vitamin D deficiency, values between 25 and 50 nmol/L vitamin D insufficiency and levels over 75 nmol/L reflect optimal supply.
For the determination of vitamin B6 (pyridoxal 5′-phosphate, PLP) levels, high-performance liquid chromatography (HPLC)-fluorescence methods were used, the reference range being 12–75 nmol/L.
Molecular Genetic Analysis of the ALPL Gene
All exons and flanking parts of introns (20 bp) of ALPL gene by direct sequencing and compared with refseq: NM_000478.5; promoter and deep intron regions were not analyzed. Possible deletions and duplications (copy number variants—CNV) of ALPL gene were analyzed by MLPA (kit P484-ALPL, lot: A1-0918, MRC Holland, Amsterdam, the Netherlands). These analyses were carried out by Robert Petrovic at the University Hospital of Bratislava.
Statistics
Data are presented as medians with interquartile ranges or percentage. Comparisons between groups were performed using non-parametric Mann–Whitney tests for continuous data and χ2 tests for categorical variables. P values < 0.05 were considered to indicate significant statistical differences between groups. All analyses were performed using STATA software, version 14.0 (StataCorp LP).
Results
Patient Characteristics
The study population included 72 patients (65 females, 7 males) with AFF and at least one available ALP value (Table 1). Among them, 10 patients had not been treated with antiresorptive treatment (AR) while 62 patients were treated with AR, with a duration of AR [median (interquartile range)] of 8 (6) years. These two subgroups were not different concerning age, sex, proportion of bilateral and simultaneous AFF, metatarsal fractures, prevalence of osteoporosis, and corticosteroids or PPIs use. Subjects without AR treatment had a higher body mass index (BMI) (P = 0.017). Compared with the AFF group treated with AR, patients in the control group, i.e., with hip fractures on BPs therapy, were slightly older (P = 0.026), had lower BMI (P = 0.011), a trend for shorter AR duration (P = 0.071), and higher CTX levels (P = 0.002).
ALP Values According to Fracture Type
In order to more precisely address the prevalence of low ALP values in AFF patients, we specifically analyzed the lowest of multiple ALP values assessed. In one patient with alcoholic cirrhosis, four ALP measurements were above the upper limit of normal due to acute liver dysfunction and therefore excluded from analysis.
The median of lowest ALP value was 53 IU/L (range 24–86 IU/L) in the control group, hence not different from all AFF patients (58 IU/L, range 13–322 IU/L, P = 0.129) and AFF patients with AR (56 IU/L, range 13–322 IU/L, P = 0.313). The proportion of patients with low ALP values (≤ 40 IU/L) was also not different between groups (AFF vs control, 25% vs 20%, P = 0.643; AFF with AR vs control, 29% vs 20%, P = 0.428) (Table 1).
Characteristics of AFF Patients According to ALP Values
Fifty-four patients did not have any ALP values ≤ 40 IU/L (median lowest ALP 72 IU/L, range 42–322 IU/L), while 18 AFF patients had at least one ALP value ≤ 40 IU/L (median lowest ALP 38 IU/L, range 13–40 IU/L) (Table 2 and Fig. 1). AFF patients with ALP values ≤ 40 IU/L did not differ from those with values > 40 IU/L for most clinical characteristics, including age, sex, BMI, prevalence of osteoporosis, and duration of AR therapy. However, those with ALP concentrations ≤ 40 IU/L had all been exposed to AR (100% vs 81%, P = 0.049), among whom 17 had received BPs and one denosumab for a median of 8 years. They also experienced more bilateral AFF (50% vs 22%, P = 0.025), which were simultaneous in 67% and more metatarsal fractures (33% vs 7%, P = 0.006), and they were more likely to use glucocorticoids (22% vs 8%, P = 0.089). To note that among those, atypical femur fractures healed without complications in 13 patients and resulted in the development of pseudoarthrosis in four patients.
ALP Values According to Antiresorptive Drugs Use in Patients with AFF
Among patients with AFF, ALP values were lower in AR users compared with AR non-users (56 IU/L vs 86 IU/L, P = 0.040). The proportion of patients with low ALP values (≤ 40 IU/L) was also significantly higher in AR users compared with non-users (29% vs 0%, respectively, P = 0.049) (Table 1). In addition, in most of low ALP patients with AFF, ALP concentrations exceeding 40 IU/L were also measured at some point of time (Fig. 1), particularly off antiresorptive therapy (Fig. 2), indicating that AR, rather than HPP, was the main reason for their low serum ALP values.
HPP in AFF Patients with Low ALP
Among the 18 AFF patients with at least one ALP ≤ 40 IU/L, seven patients were considered to have low ALP values in more than one measurement during or after discontinuation of antiresorptive treatment (range 13–40 IU/L). These patients are reported in more detail in Table 3. Vitamin B6 (pyridoxal 5-Phosphate, PLP) level was available only in 2 of these subjects and was not elevated. A number of non-specific clinical signs and symptoms compatible with HPP were found in these patients, including chronic fatigue and primary pain in three patients and gait disorders in one female patient. Two of the seven patients were diagnosed with nephrolithiasis, whereas only one had a metatarsal fracture. Four patients presented with neuropsychiatric disorders, and one patient was suffering from severe heart disease. Three out of seven patients with repeatedly low ALP values were alive at the time of evaluation and consented to provide blood samples for ALPL DNA sequencing. In one patient, a rare pathogenic heterozygous variant (c.787T > C, p.Tyr263His) was identified which is associated with a likely benign form of HPP [16]. In the other two patients no HPP mutation was detected.
Discussion
Our results show that low ALP values are a common finding in patients with AFF and that these values are mainly associated with concurrent antiresorptive medication, similar to BPs-treated subjects with typical hip fractures.
In almost a quarter of AFF patients, at least one low ALP value ≤ 40 IU/L was detected during the observation period. However, ALP values varied greatly and in all patients except one, higher ALP values (> 40 IU/L) were also measured at some time point. Literature data on the prevalence of low ALP levels in AFF patients are scarce and existing data are mostly related to bisphosphonate treatment. In a previous small study, low ALP levels (< 55 IU/L) were observed in five of ten (50%) long-term bisphosphonate-treated patients with AFF and in five out of 13 (38%) bisphosphonate-treated control patients who did not experience any complication [17]. In another study, ALP concentrations were within the reference range in all eight bisphosphonate-treated AFF patients studied [18]. There are no data on serum ALP concentrations in AFF patients in a hospital setting.
In our study 100% of patients with at least one ALP value ≤ 40 IU/L had a history of long-term antiresorptive treatment, mainly with bisphosphonates. These results confirm the well-known association of low ALP values with long-term bisphosphonate treatment [19]. Bisphosphonates reduce bone ALP by about 40% [19, 20] in response to suppression of osteoclast activity, even in patients presenting with excessive ALP levels such as in Paget’s disease [21]. Dose-dependent inhibition of ALP activity by alendronate, pamidronate, and zoledronate by 42–93% was also observed in vitro [22]. Thus, the low ALP values as detected in most of our AFF patients most likely resulted from antiresorptive treatment with bisphosphonates.
However, not all of our patients on antiresorptives show low ALP values, which would suggest that the concurrence of low ALP and antiresorptive treatment may increase the patients’ vulnerability for experiencing AFF, in addition to other risk factors of AFFs such as ethnical group, pretreatment BMD, glucocorticoid use, weight, and height [4]. On the other hand it can be speculated that consistently low ALP values during antiresorptive treatment reflect better adherence and higher exposure to pharmacological treatment. This may then result in an increased risk of experiencing AFF. For this reason, special attention in terms of AFF risk should be paid in the care of patients who present with persistently low serum ALP values while receiving long-term antiresorptive treatment, since the latter has been associated with an absolute risk of up to 100 AFF per 100,000 person-years [1, 3, 4, 23].
When analyzing specific subgroups of patients, bilateral AFF more frequently occurred in patients with at least one ALP value ≤ 40 IU/L than in patients whose ALP levels were > 40 IU/L. This finding would suggest that low ALP may increase the risk for bilateral AFF. Furthermore, the proportion of patients treated with corticosteroids or PPIs among AFF patients tended to be higher in the low ALP group compared with the ALP > 40 IU/L group. This finding may suggest a contribution of PPIs and corticosteroids on bone metabolism in our AFF patients. Corticosteroid treatment has been shown to mainly decrease ALP levels [24, 25], whereas the effect of PPI administration on ALP levels is less clear [26,27,28,29]. An increased risk for fractures has been reported in both patients treated with PPIs and corticosteroids [12, 24,25,26, 30].
When specifically looking for signs and symptoms compatible with HPP in AFF patients with at least one ALP ≤ 40 IU/L during or after discontinuation of antiresorptive treatment , we identified seven patients in whom chronic fatigue, primary pain, nephrolithiasis, neuropsychiatric disorders, and severe heart disease occurred. Out of three patients who finally had genetic testing, a pathogenic heterozygous variant associated with HPP [16] could eventually be detected in one patient. Since we could not perform genetic testing in all low ALP patients with AFF, we are not able to calculate the actual prevalence of HPP in our cohort. However, HPP appears to be uncommon in our setting. Studies on a possible association between AFF and HPP show contradicting results. On one hand a genome-wide association and candidate gene study failed to identify common genetic variants of AFF consistent with HPP (n = 51) [9]. On the other hand, another study showed that AFF is common in HPP patients with 10% of patients with confirmed HPP (n = 150) having had at least one AFF [10]. Hence, although AFF is recognized as a common complication of HPP, the prevalence of HPP is rather low in the population of AFF patients receiving antiresorptive treatment. Antiresorptive treatment is not recommended in HPP patients.
There are some limitations of this study that can affect the interpretation of the results. First, the design is retrospective, which may have increased the risk for selection bias. This is, however, unlikely since during the whole observation period virtually all AFF cases were referred by orthopedic surgeons to the bone diseases team for consultation. Also due to the retrospective nature of this study, we were not able to perform genetic testing in all patients of our cohort with clinical suspicion of HPP. Second, HPP clinical signs and symptoms used to screen for HPP are non-specific and data were missing for some of them such as dental anomalies as well as vitamin B6 and vitamin D measurements that were only available in some participants. Therefore, we cannot exclude having missed a patient with clinical and/or biochemical suspicion of HPP in our cohort. Third, the number of controls, i.e., the group of patients having typical hip fractures with antiresorptive treatment is low. Strengths of this study are the large number of AFF patients included with a long follow-up period and the inclusion of an appropriate control group.
In conclusion, among patients with AFF, the prevalence of low ALP values < 40 IU/L is quite common (22.5%). However, low ALP values are most commonly associated with antiresorptive treatment and values measured prior and/or after this treatment generally are within the normal range. Hence, from a clinical perspective, ALP values in patients with AFF should be reappraised at a later time point, i.e., when the fractures are healed and the antiresorptive withdrawn, in order to avoid excessive genetic screening for HPP.
References
Shane E, Burr D, Abrahamsen B, Adler RA, Brown TD, Cheung AM, Cosman F, Curtis JR, Dell R, Dempster DW, Ebeling PR, Einhorn TA, Genant HK, Geusens P, Klaushofer K, Lane JM, McKiernan F, McKinney R, Ng A, Nieves J, O’Keefe R, Papapoulos S, Howe TS, van der Meulen MC, Weinstein RS, Whyte MP (2014) Atypical subtrochanteric and diaphyseal femoral fractures: second report of a task force of the American Society for Bone and Mineral Research. J Bone Miner Res 29:1–23
Tan SC, Koh SB, Goh SK, Howe TS (2011) Atypical femoral stress fractures in bisphosphonate-free patients. Osteoporos Int 22:2211–2212
Schilcher J, Koeppen V, Aspenberg P, Michaelsson K (2014) Risk of atypical femoral fracture during and after bisphosphonate use. N Engl J Med 371:974–976
Black DM, Geiger EJ, Eastell R, Vittinghoff E, Li BH, Ryan DS, Dell RM, Adams AL (2020) Atypical femur fracture risk versus fragility fracture prevention with bisphosphonates. N Engl J Med 383:743–753
Starr J, Tay YKD, Shane E (2018) Current understanding of epidemiology, pathophysiology, and management of atypical femur fractures. Curr Osteoporos Rep 16:519–529
Nguyen HH, van de Laarschot DM, Verkerk A, Milat F, Zillikens MC, Ebeling PR (2018) Genetic risk factors for Atypical Femoral Fractures (AFFs): a systematic review. JBMR Plus 2:1–11
Whyte MP (2016) Hypophosphatasia—aetiology, nosology, pathogenesis, diagnosis and treatment. Nat Rev Endocrinol 12:233–246
Whyte MP (2009) Atypical femoral fractures, bisphosphonates, and adult hypophosphatasia. J Bone Miner Res 24:1132–1134
Kharazmi M, Michaelsson K, Schilcher J, Eriksson N, Melhus H, Wadelius M, Hallberg P (2019) A genome-wide association study of bisphosphonate-associated atypical femoral fracture. Calcif Tissue Int 105:51–67
Genest F, Seefried L (2018) Subtrochanteric and diaphyseal femoral fractures in hypophosphatasia-not atypical at all. Osteoporos Int 29:1815–1825
Yang YX, Lewis JD, Epstein S, Metz DC (2006) Long-term proton pump inhibitor therapy and risk of hip fracture. JAMA 296:2947–2953
Costa-Rodrigues J, Reis S, Teixeira S, Lopes S, Fernandes MH (2013) Dose-dependent inhibitory effects of proton pump inhibitors on human osteoclastic and osteoblastic cell activity. FEBS J 280:5052–5064
Colantonio DA, Kyriakopoulou L, Chan MK, Daly CH, Brinc D, Venner AA, Pasic MD, Armbruster D, Adeli K (2012) Closing the gaps in pediatric laboratory reference intervals: a CALIPER database of 40 biochemical markers in a healthy and multiethnic population of children. Clin Chem 58:854–868
Estey MP, Cohen AH, Colantonio DA, Chan MK, Marvasti TB, Randell E, Delvin E, Cousineau J, Grey V, Greenway D, Meng QH, Jung B, Bhuiyan J, Seccombe D, Adeli K (2013) CLSI-based transference of the CALIPER database of pediatric reference intervals from Abbott to Beckman, Ortho, Roche and Siemens Clinical Chemistry Assays: direct validation using reference samples from the CALIPER cohort. Clin Biochem 46:1197–1219
Chan MK, Seiden-Long I, Aytekin M, Quinn F, Ravalico T, Ambruster D, Adeli K (2009) Canadian Laboratory Initiative on Pediatric Reference Interval Database (CALIPER): pediatric reference intervals for an integrated clinical chemistry and immunoassay analyzer, Abbott ARCHITECT ci8200. Clin Biochem 42:885–891
Mornet E, Nunes ME (1993) Hypophosphatasia. In: Adam MP, Ardinger HH, Pagon RA, Wallace SE, Bean LJH, Mirzaa G, Amemiya A (eds) GeneReviews((R)). Seattle (WA)
Bhattacharyya T, Jha S, Wang H, Kastner DL, Remmers EF (2016) Hypophosphatasia and the risk of atypical femur fractures: a case-control study. BMC Musculoskelet Disord 17:332
Bhadada SK, Sridhar S, Muthukrishnan J, Mithal A, Sharma DC, Bhansali A, Dhiman V (2014) Predictors of atypical femoral fractures during long term bisphosphonate therapy: a case series & review of literature. Indian J Med Res 140:46–54
Eastell R, Hannon RA (2008) Biomarkers of bone health and osteoporosis risk. Proc Nutr Soc 67:157–162
Rosen CJ, Hochberg MC, Bonnick SL, McClung M, Miller P, Broy S, Kagan R, Chen E, Petruschke RA, Thompson DE, de Papp AE, Trial FAC, I, (2005) Treatment with once-weekly alendronate 70 mg compared with once-weekly risedronate 35 mg in women with postmenopausal osteoporosis: a randomized double-blind study. J Bone Miner Res 20:141–151
Ralston SH, Corral-Gudino L, Cooper C, Francis RM, Fraser WD, Gennari L, Guañabens N, Javaid MK, Layfield R, O’Neill TW, Russell RGG, Stone MD, Simpson K, Wilkinson D, Wills R, Zillikens MC, Tuck SP (2019) Diagnosis and management of Paget’s disease of bone in adults: a clinical guideline. J Bone Miner Res 34:e3657
Vaisman DN, McCarthy AD, Cortizo AM (2005) Bone-specific alkaline phosphatase activity is inhibited by bisphosphonates. Biol Trace Elem Res 104:131–140
Adler RA (2018) Management of endocrine disease: atypical femoral fractures: risks and benefits of long-term treatment of osteoporosis with anti-resorptive therapy. Eur J Endocrinol 178:R81–R87
Lo Cascio V, Kanis JA, Beneton MN, Bertoldo F, Adami S, Poggi G, Zanolin ME (1995) Acute effects of deflazacort and prednisone on rates of mineralization and bone formation. Calcif Tissue Int 56:109–112
Morrison D, Ali NJ, Routledge PA, Capewell S (1992) Bone turnover during short course prednisolone treatment in patients with chronic obstructive airways disease. Thorax 47:418–420
Thong BKS, Ima-Nirwana S, Chin KY (2019) Proton pump inhibitors and fracture risk: a review of current evidence and mechanisms involved. Int J Environ Res Public Health 16:1571
Poly TN, Islam MM, Yang HC, Wu CC, Li YJ (2019) Proton pump inhibitors and risk of hip fracture: a meta-analysis of observational studies. Osteoporos Int 30:103–114
Briganti SI, Naciu AM, Tabacco G, Cesareo R, Napoli N, Trimboli P, Castellana M, Manfrini S, Palermo A (2021) Proton pump inhibitors and fractures in adults: a critical appraisal and review of the literature. Int J Endocrinol 2021:8902367
Compston J (2018) Glucocorticoid-induced osteoporosis: an update. Endocrine 61:7–16
Sharara AI, El-Halabi MM, Ghaith OA, Habib RH, Mansour NM, Malli A, El Hajj-Fuleihan G (2013) Proton pump inhibitors have no measurable effect on calcium and bone metabolism in healthy young males: a prospective matched controlled study. Metabolism 62:518–526
Acknowledgements
The authors thank all patients involved in the study, as well as their caregivers, care team, investigators, and staff in participating institutions. The authors thank Dr. Joachim Sauer of DP-Medsystems AG for providing medical writing support, which was sponsored by Alexion, Zürich, Switzerland, in accordance with Good Publication Practice. This study was sponsored by an unrestricted grant from Alexion.
Funding
Open access funding provided by University of Geneva.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
ET reported grant from Alexion for this study and personal fees from AMGEN, outside the submitted work; SF reported grants and other from AMGEN, other from UCB, other from Radius, grants and other from Agnovos, outside the submitted work; EB reported personal fees from AMGEN, Nestlé, outside the submitted work. All other authors did not report any disclosures. Eleni Tsiantouli, Emmanuel Biver, Thierry Chevalley, Robert Petrovic, Didier Hannouche, and Serge Ferrari declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
Ethical approval was waived by Ethics Comittee of Geneva and all procedures performed were part of the routine care.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Tsiantouli, E., Biver, E., Chevalley, T. et al. Prevalence of Low Serum Alkaline Phosphatase and Hypophosphatasia in Adult Patients with Atypical Femur Fractures. Calcif Tissue Int 110, 703–711 (2022). https://doi.org/10.1007/s00223-022-00949-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-00949-1