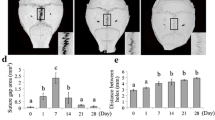
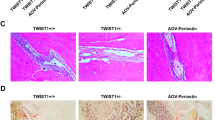
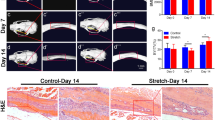
Abstract
Trans-sutural distraction osteogenesis has been proposed as an alternative technique of craniofacial remodelling surgery for craniosynostosis correction. Many studies have defined the contribution of a series of biological events to distraction osteogenesis, such as changes in gene expression, changes in suture cell behaviour and changes in suture collagen fibre characteristics. However, few studies have elucidated the systematic molecular and cellular mechanisms of trans-sutural distraction osteogenesis, and no study has highlighted the contribution of cell–cell or cell–matrix interactions with respect to the whole expansion process to date. Therefore, it is difficult to translate largely primary mechanistic insights into clinical applications and optimize the clinical outcome of trans-sutural distraction osteogenesis. In this review, we carefully summarize in detail the literature related to the effects of mechanical stretching on osteoblasts, endothelial cells, fibroblasts, immune cells (macrophages and T cells), mesenchymal stem cells and collagen fibres in sutures during the distraction osteogenesis process. We also briefly review the contribution of cell–cell or cell–matrix interactions to bone regeneration at the osteogenic suture front from a comprehensive viewpoint.

Similar content being viewed by others
References
Tong H et al (2015) Trans-sutural distraction osteogenesis for midfacial hypoplasia in growing patients with cleft lip and palate: clinical outcomes and analysis of skeletal changes. Plast Reconstr Surg 136:144–155. https://doi.org/10.1097/PRS.0000000000001375
Robin NH (1999) Molecular genetic advances in understanding craniosynostosis. Plast Reconstr Surg 103:1060–1070
Ten Cate AR, Freeman E, Dickinson JB (1977) Sutural development: structure and its response to rapid expansion. Am J Orthod 71:622–636. https://doi.org/10.1016/0002-9416(77)90279-2
Liang W et al (2021) Hydroxyapatite nanoparticles facilitate osteoblast differentiation and bone formation within sagittal suture during expansion in rats. Drug Des Devel Ther 15:905–917. https://doi.org/10.2147/DDDT.S299641
Ekström C, Henrikson CO, Jensen R (1977) Mineralization in the midpalatal suture after orthodontic expansion. Am J Orthod 71:449–455. https://doi.org/10.1016/0002-9416(77)90248-2
Mofid MM et al (2001) Craniofacial distraction osteogenesis: a review of 3278 cases. Plast Reconstr Surg 108:1103–1114. https://doi.org/10.1097/00006534-200110000-00001 (discussion 1115-7)
Wang XX et al (2005) Internal midface distraction in correction of severe maxillary hypoplasia secondary to cleft lip and palate. Plast Reconstr Surg 116:51–60. https://doi.org/10.1097/01.prs.0000169691.22783.29
Tong H et al (2015) Transsutural distraction osteogenesis applied to maxillary complex with new internalized distraction device: analysis of the feasibility and long-term osteogenesis outcome. J Craniofac Surg 26:402–407. https://doi.org/10.1097/SCS.0000000000001263
Opperman LA (2000) Cranial sutures as intramembranous bone growth sites. Dev Dyn 219:472–485. https://doi.org/10.1002/1097-0177(2000)9999:9999%3c::Aid-dvdy1073%3e3.0.Co;2-f
Mao JJ, Wang X, Kopher RA (2003) Biomechanics of craniofacial sutures: orthopedic implications. Angle Orthod 73:128–135. https://doi.org/10.1043/0003-3219(2003)73%3c128:Bocsoi%3e2.0.Co;2
Doro DH, Grigoriadis AE, Liu KJ (2017) Calvarial suture-derived stem cells and their contribution to cranial bone repair. Front Physiol 8:956. https://doi.org/10.3389/fphys.2017.00956
Li W et al (2020) ROCK-TAZ signaling axis regulates mechanical tension-induced osteogenic differentiation of rat cranial sagittal suture mesenchymal stem cells. J Cell Physiol 235:5972–5984. https://doi.org/10.1002/jcp.29522
Xu Y et al (2007) Isolation and characterization of posterofrontal/sagittal suture mesenchymal cells in vitro. Plast Reconstr Surg 119:819–829. https://doi.org/10.1097/01.prs.0000255540.91987.a0
Takeshita N et al (2017) In vivo expression and regulation of genes associated with vascularization during early response of sutures to tensile force. J Bone Miner Metab 35:40–51. https://doi.org/10.1007/s00774-016-0737-z
Li J et al (2020) T cells participate in bone remodeling during the rapid palatal expansion. FASEB J 34:15327–15337. https://doi.org/10.1096/fj.202001078R
Liang W et al (2021) Polarized M2 macrophages induced by mechanical stretching modulate bone regeneration of the craniofacial suture for midfacial hypoplasia treatment. Cell Tissue Res. https://doi.org/10.1007/s00441-021-03533-5
Holmes G et al (2020) Integrated transcriptome and network analysis reveals spatiotemporal dynamics of calvarial suturogenesis. Cell Rep 32:107871. https://doi.org/10.1016/j.celrep.2020.107871
Zhong WJ et al (2011) Periostin-like-factor-induced bone formation within orthopedic maxillary expansion. Orthod Craniofac Res 14:198–205. https://doi.org/10.1111/j.1601-6343.2011.01524.x
Romanyk DL et al (2013) Role of the midpalatal suture in FEA simulations of maxillary expansion treatment for adolescents: a review. Int Orthod 11:119–138. https://doi.org/10.1016/j.ortho.2013.02.001
Meikle MC et al (1982) Rabbit cranial suture fibroblasts under tension express a different collagen phenotype. Arch Oral Biol 27:609–613. https://doi.org/10.1016/0003-9969(82)90078-4
Warren SM et al (2008) Confocal laser scanning microscopic analysis of collagen scaffolding patterns in cranial sutures. J Craniofac Surg 19:198–203. https://doi.org/10.1097/scs.0b013e31815c8a9a
Schipani E et al (2009) Regulation of osteogenesis-angiogenesis coupling by HIFs and VEGF. J Bone Miner Res 24:1347–1353. https://doi.org/10.1359/jbmr.090602
Chaqour B, Goppelt-Struebe M (2006) Mechanical regulation of the Cyr61/CCN1 and CTGF/CCN2 proteins. Febs j 273:3639–3649. https://doi.org/10.1111/j.1742-4658.2006.05360.x
Liu Y, Olsen BR (2014) Distinct VEGF functions during bone development and homeostasis. Arch Immunol Ther Exp (Warsz) 62:363–368. https://doi.org/10.1007/s00005-014-0285-y
Hu K, Olsen BR (2017) Vascular endothelial growth factor control mechanisms in skeletal growth and repair. Dev Dyn 246:227–234. https://doi.org/10.1002/dvdy.24463
Liu Y et al (2012) Intracellular VEGF regulates the balance between osteoblast and adipocyte differentiation. J Clin Invest 122:3101–3113. https://doi.org/10.1172/JCI61209
Hu K, Olsen BR (2016) Osteoblast-derived VEGF regulates osteoblast differentiation and bone formation during bone repair. J Clin Invest 126:509–526. https://doi.org/10.1172/JCI82585
Berendsen AD, Olsen BR (2014) How vascular endothelial growth factor-A (VEGF) regulates differentiation of mesenchymal stem cells. J Histochem Cytochem 62:103–108. https://doi.org/10.1369/0022155413516347
Zhu F et al (2011) Rho kinase inhibitor fasudil suppresses migration and invasion though down-regulating the expression of VEGF in lung cancer cell line A549. Med Oncol 28:565–571. https://doi.org/10.1007/s12032-010-9468-5
Hirukawa K et al (2005) Effect of tensile force on the expression of IGF-I and IGF-I receptor in the organ-cultured rat cranial suture. Arch Oral Biol 50:367–372. https://doi.org/10.1016/j.archoralbio.2004.07.003
Claeys L et al (2020) Human fibroblasts as a model for the study of bone disorders. Front Endocrinol (Lausanne) 11:394. https://doi.org/10.3389/fendo.2020.00394
Al-Mubarak R, Da Silveira A, Mao JJ (2005) Expression and mechanical modulation of matrix metalloproteinase-1 and -2 genes in facial and cranial sutures. Cell Tissue Res 321:465–471. https://doi.org/10.1007/s00441-005-1136-2
López B et al (2021) Diffuse myocardial fibrosis: mechanisms, diagnosis and therapeutic approaches. Nat Rev Cardiol. https://doi.org/10.1038/s41569-020-00504-1
Green DD et al (1990) Immunolocalization of collagenase and tissue inhibitor of metalloproteinases (TIMP) in mechanically deformed fibrous joints. Am J Orthod Dentofacial Orthop 97:281–288. https://doi.org/10.1016/0889-5406(90)70100-q
Yen EH, Yue CS, Suga DM (1989) Effect of force level on synthesis of type III and type I collagen in mouse interparietal suture. J Dent Res 68:1746–1751. https://doi.org/10.1177/00220345890680120501
Rundhaug JE (2005) Matrix metalloproteinases and angiogenesis. J Cell Mol Med 9:267–285. https://doi.org/10.1111/j.1582-4934.2005.tb00355.x
Nakama T et al (2016) Different roles played by periostin splice variants in retinal neovascularization. Exp Eye Res 153:133–140. https://doi.org/10.1016/j.exer.2016.10.012
Li G et al (2021) Sutural fibroblasts exhibit the function of vascular endothelial cells upon mechanical strain. Arch Biochem Biophys 712:109046. https://doi.org/10.1016/j.abb.2021.109046
Lau LF (2011) CCN1/CYR61: the very model of a modern matricellular protein. Cell Mol Life Sci 68:3149–3163. https://doi.org/10.1007/s00018-011-0778-3
Ikegame M et al (2016) Tensile stress stimulates the expression of osteogenic cytokines/growth factors and matricellular proteins in the mouse cranial suture at the site of osteoblast differentiation. Biomed Res 37:117–126. https://doi.org/10.2220/biomedres.37.117
Ikegame M et al (2001) Tensile stress induces bone morphogenetic protein 4 in preosteoblastic and fibroblastic cells, which later differentiate into osteoblasts leading to osteogenesis in the mouse calvariae in organ culture. J Bone Miner Res 16:24–32. https://doi.org/10.1359/jbmr.2001.16.1.24
Takenouchi H et al (2014) Longitudinal quantitative evaluation of the mid-palatal suture after rapid expansion using in vivo micro-CT. Arch Oral Biol 59:414–423. https://doi.org/10.1016/j.archoralbio.2014.01.010
Park D et al (2012) Endogenous bone marrow MSCs are dynamic, fate-restricted participants in bone maintenance and regeneration. Cell Stem Cell 10:259–272. https://doi.org/10.1016/j.stem.2012.02.003
James AW et al (2008) Proliferation, osteogenic differentiation, and fgf-2 modulation of posterofrontal/sagittal suture-derived mesenchymal cells in vitro. Plast Reconstr Surg 122:53–63. https://doi.org/10.1097/PRS.0b013e31817747b5
Maruyama T et al (2016) Stem cells of the suture mesenchyme in craniofacial bone development, repair and regeneration. Nat Commun 7:10526. https://doi.org/10.1038/ncomms10526
Ekizer A et al (2015) Bone marrow mesenchymal stem cells enhance bone formation in orthodontically expanded maxillae in rats. Angle Orthod 85:394–399. https://doi.org/10.2319/031114-177.1
Yu M et al (2021) Cranial suture regeneration mitigates skull and neurocognitive defects in craniosynostosis. Cell 184(243–256):e18. https://doi.org/10.1016/j.cell.2020.11.037
Morinobu M et al (2003) Osteopontin expression in osteoblasts and osteocytes during bone formation under mechanical stress in the calvarial suture in vivo. J Bone Miner Res 18:1706–1715. https://doi.org/10.1359/jbmr.2003.18.9.1706
Denu RA et al (2016) Fibroblasts and mesenchymal stromal/stem cells are phenotypically indistinguishable. Acta Haematol 136:85–97. https://doi.org/10.1159/000445096
Lynch MD, Watt FM (2018) Fibroblast heterogeneity: implications for human disease. J Clin Invest 128:26–35. https://doi.org/10.1172/jci93555
Dupont S et al (2011) Role of YAP/TAZ in mechanotransduction. Nature 474:179–183. https://doi.org/10.1038/nature10137
Qin X et al (2019) Runx2 regulates cranial suture closure by inducing hedgehog, Fgf, Wnt and Pthlh signaling pathway gene expressions in suture mesenchymal cells. Hum Mol Genet 28:896–911. https://doi.org/10.1093/hmg/ddy386
Kawane T et al (2018) Runx2 is required for the proliferation of osteoblast progenitors and induces proliferation by regulating Fgfr2 and Fgfr3. Sci Rep 8:13551. https://doi.org/10.1038/s41598-018-31853-0
Iseki S, Wilkie AO, Morriss-Kay GM (1999) Fgfr1 and Fgfr2 have distinct differentiation- and proliferation-related roles in the developing mouse skull vault. Development 126:5611–5620
Mikasa M et al (2011) Regulation of Tcf7 by Runx2 in chondrocyte maturation and proliferation. J Bone Miner Metab 29:291–299. https://doi.org/10.1007/s00774-010-0222-z
Merciris D et al (2007) Overexpression of the transcriptional factor Runx2 in osteoblasts abolishes the anabolic effect of parathyroid hormone in vivo. Am J Pathol 170:1676–1685. https://doi.org/10.2353/ajpath.2007.061069
Lee MH et al (2005) Dlx5 specifically regulates Runx2 type II expression by binding to homeodomain-response elements in the Runx2 distal promoter. J Biol Chem 280:35579–35587. https://doi.org/10.1074/jbc.M502267200
Kawane T et al (2014) Dlx5 and mef2 regulate a novel runx2 enhancer for osteoblast-specific expression. J Bone Miner Res 29:1960–1969. https://doi.org/10.1002/jbmr.2240
Mefford HC et al (2010) Copy number variation analysis in single-suture craniosynostosis: multiple rare variants including RUNX2 duplication in two cousins with metopic craniosynostosis. Am J Med Genet A 152:2203–2210. https://doi.org/10.1002/ajmg.a.33557
Clarke B (2008) Normal bone anatomy and physiology. Clin J Am Soc Nephrol 3(Suppl 3):S131–S139. https://doi.org/10.2215/CJN.04151206
El-Rashidy AA et al (2017) Regenerating bone with bioactive glass scaffolds: a review of in vivo studies in bone defect models. Acta Biomater 62:1–28. https://doi.org/10.1016/j.actbio.2017.08.030
Ikegame M, Ejiri S, Okamura H (2019) Expression of non-collagenous bone matrix proteins in osteoblasts stimulated by mechanical stretching in the cranial suture of neonatal mice. J Histochem Cytochem 67:107–116. https://doi.org/10.1369/0022155418793588
Kim HJ et al (1998) FGF-, BMP- and Shh-mediated signalling pathways in the regulation of cranial suture morphogenesis and calvarial bone development. Development 125:1241–1251
Lian JB et al (2006) Networks and hubs for the transcriptional control of osteoblastogenesis. Rev Endocr Metab Disord 7:1–16. https://doi.org/10.1007/s11154-006-9001-5
Chen D, Zhao M, Mundy GR (2004) Bone morphogenetic proteins. Growth Factors 22:233–241. https://doi.org/10.1080/08977190412331279890
Heller JB et al (2007) Cranial suture response to stress: expression patterns of Noggin and Runx2. Plast Reconstr Surg 119:2037–2045. https://doi.org/10.1097/01.prs.0000260589.75706.19
Qin X et al (2021) Runt-related transcription factor-2 (Runx2) is required for bone matrix protein gene expression in committed osteoblasts in mice. J Bone Miner Res. https://doi.org/10.1002/jbmr.4386
Komori T (2010) Regulation of bone development and extracellular matrix protein genes by RUNX2. Cell Tissue Res 339:189–195. https://doi.org/10.1007/s00441-009-0832-8
McCarthy TL, Centrella M, Canalis E (1989) Regulatory effects of insulin-like growth factors I and II on bone collagen synthesis in rat calvarial cultures. Endocrinology 124:301–309. https://doi.org/10.1210/endo-124-1-301
Whyte MP (1994) Hypophosphatasia and the role of alkaline phosphatase in skeletal mineralization. Endocr Rev 15:439–461. https://doi.org/10.1210/edrv-15-4-439
Hunter GK, Goldberg HA (1993) Nucleation of hydroxyapatite by bone sialoprotein. Proc Natl Acad Sci USA 90:8562–8565. https://doi.org/10.1073/pnas.90.18.8562
Chen J, Shapiro HS, Sodek J (1992) Development expression of bone sialoprotein mRNA in rat mineralized connective tissues. J Bone Miner Res 7:987–997. https://doi.org/10.1002/jbmr.5650070816
Hunter GK (2013) Role of osteopontin in modulation of hydroxyapatite formation. Calcif Tissue Int 93:348–354. https://doi.org/10.1007/s00223-013-9698-6
Boskey AL et al (1998) Fourier transform infrared microspectroscopic analysis of bones of osteocalcin-deficient mice provides insight into the function of osteocalcin. Bone 23:187–196. https://doi.org/10.1016/s8756-3282(98)00092-1
Zhao S et al (2015) Effects of strontium ranelate on bone formation in the mid-palatal suture after rapid maxillary expansion. Drug Des Devel Ther 9:2725–2734. https://doi.org/10.2147/DDDT.S82892
Geissmann F et al (2010) Development of monocytes, macrophages, and dendritic cells. Science 327:656–661. https://doi.org/10.1126/science.1178331
Murray PJ, Wynn TA (2011) Protective and pathogenic functions of macrophage subsets. Nat Rev Immunol 11:723–737. https://doi.org/10.1038/nri3073
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11:889–896. https://doi.org/10.1038/ni.1937
Yan Y et al (2015) T cells are required for orthodontic tooth movement. J Dent Res 94:1463–1470. https://doi.org/10.1177/0022034515595003
Liu Y et al (2017) Aspirin blocks orthodontic relapse via inhibition of CD4(+) T lymphocytes. J Dent Res 96:586–594. https://doi.org/10.1177/0022034516685527
Pawelec KM, Best SM, Cameron RE (2016) Collagen: a network for regenerative medicine. J Mater Chem B 4:6484–6496. https://doi.org/10.1039/c6tb00807k
Hosseini HS et al (2020) Biomechanical signaling and collagen fiber reorientation during distraction enterogenesis. J Mech Behav Biomed Mater 101:103425. https://doi.org/10.1016/j.jmbbm.2019.103425
Liu SS et al (2011) Is there an optimal force level for sutural expansion? Am J Orthod Dentofacial Orthop 139:446–455. https://doi.org/10.1016/j.ajodo.2009.03.056
Wu BH et al (2017) Stretch force guides finger-like pattern of bone formation in suture. PLoS ONE 12:e0177159. https://doi.org/10.1371/journal.pone.0177159
Bazrafshan Z, Stylios GK (2019) Spinnability of collagen as a biomimetic material: a review. Int J Biol Macromol 129:693–705. https://doi.org/10.1016/j.ijbiomac.2019.02.024
Najafi M, Farhood B, Mortezaee K (2019) Extracellular matrix (ECM) stiffness and degradation as cancer drivers. J Cell Biochem 120:2782–2790. https://doi.org/10.1002/jcb.27681
Doyle AD, Yamada KM (2016) Mechanosensing via cell-matrix adhesions in 3D microenvironments. Exp Cell Res 343:60–66. https://doi.org/10.1016/j.yexcr.2015.10.033
Fu X et al (2015) Anchorage-dependent binding of integrin I-domain to adhesion ligands. J Mol Recognit 28:385–392. https://doi.org/10.1002/jmr.2453
Goring A et al (2019) Regulation of the bone vascular network is sexually dimorphic. J Bone Miner Res 34:2117–2132. https://doi.org/10.1002/jbmr.3825
Wan Y et al (2018) Prickle1 regulates differentiation of frontal bone osteoblasts. Sci Rep 8:18021. https://doi.org/10.1038/s41598-018-36742-0
Feng R et al (2015) Positive effect of IGF-1 injection on gastrocnemius of rat during distraction osteogenesis. J Orthop Res 33:1424–1432. https://doi.org/10.1002/jor.22796
Alzahrani MM et al (2014) The effect of altering the mechanical loading environment on the expression of bone regenerating molecules in cases of distraction osteogenesis. Front Endocrinol (Lausanne) 5:214. https://doi.org/10.3389/fendo.2014.00214
Zhang WB et al (2011) Expression of bone morphogenetic protein, vascular endothelial growth factor, and basic fibroblast growth factor in irradiated mandibles during distraction osteogenesis. J Oral Maxillofac Surg 69:2860–2871. https://doi.org/10.1016/j.joms.2010.12.037
Weiss S et al (2005) Systemic regulation of angiogenesis and matrix degradation in bone regeneration–distraction osteogenesis compared to rigid fracture healing. Bone 37:781–790. https://doi.org/10.1016/j.bone.2005.06.014
Zhang F et al (2020) Inflammatory macrophages facilitate mechanical stress-induced osteogenesis. Aging (Albany NY) 12:3617–3625. https://doi.org/10.18632/aging.102833
Chen X et al (2005) Stretch-induced PTH-related protein gene expression in osteoblasts. J Bone Miner Res 20:1454–1461. https://doi.org/10.1359/jbmr.2005.20.8.1454
Zeng Q et al (2015) Integrin-β1, not integrin-β5, mediates osteoblastic differentiation and ECM formation promoted by mechanical tensile strain. Biol Res 48:25. https://doi.org/10.1186/s40659-015-0014-y
Zhang J et al (2019) Effects of equibiaxial mechanical stretch on extracellular matrix-related gene expression in human calvarial osteoblasts. Eur J Oral Sci 127:10–18. https://doi.org/10.1111/eos.12595
Shah HN et al (2021) Craniofacial and long bone development in the context of distraction osteogenesis. Plast Reconstr Surg 147:54e–65e. https://doi.org/10.1097/PRS.0000000000007451
Funding
The authors have no relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in this article. This project was supported by the Key Clinical Projects of Peking University Third Hospital (BYSY2018061).
Author information
Authors and Affiliations
Contributions
WL proposed the idea of this article; WL and GL explored reviews and wrote the manuscript. EZ drafted the scheme figure. HB and ZZ revised this work critically for important contents according the reviewer’s comments, and ZZ supported this research.
Corresponding authors
Ethics declarations
Conflict of interest
There will be no conflict of commercial interest for authors Wei Liang, Enzhe Zhao, Guan Li, Hongsen Bi and Zhenmin Zhao with the publication of the manuscript.
Human and Animal Rights and Informed Consent
Ethical clearance was obtained from the Peking University Biomedical Ethics Committee (No. LA2019177). All the in vivo and in vitro cellular experiments were performed according to the National Institutes of Health Regulations for the care and use of animals.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liang, W., Zhao, E., Li, G. et al. Suture Cells in a Mechanical Stretching Niche: Critical Contributors to Trans-sutural Distraction Osteogenesis. Calcif Tissue Int 110, 285–293 (2022). https://doi.org/10.1007/s00223-021-00927-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00927-z