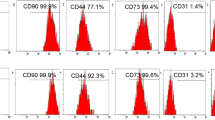
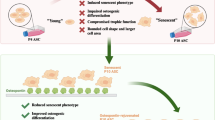
Abstract
The use of BMP-2 in orthopedic surgery is limited by uncertainty surrounding its effects on the differentiation of mesenchymal stem cells (MSCs) and how this is affected by cellular aging. This study compared the effects of recombinant human BMP-2 (rhBMP-2) on osteogenic and adipogenic differentiation between senescent and non-senescent MSCs. Senescent and non-senescent MSCs were cultured in osteogenic and adipogenic differentiation medium containing various concentrations of rhBMP-2. The phenotypes of these cells were compared by performing a calcium assay, adipogenesis assay, staining, real-time PCR, western blotting, and microarray analysis. rhBMP-2 induced osteogenic differentiation to a lesser extent (P < 0.001 and P = 0.005 for alkaline phosphatase activity and Ca2+ release) in senescent MSCs regardless of dose-dependent increase in both cells. However, the induction of adipogenic differentiation by rhBMP-2 was comparable between them. There was no difference between these two groups of cells in the adipogenesis assay (P = 0.279) and their expression levels of PPARγ were similar. Several genes such as CHRDL1, NOG, SMAD1, SMAD7, and FST encoding transcription factors were proposed to underlie the different responses of senescent and non-senescent MSCs to rhBMP-2 in microarray analyses. Furthermore, inflammatory, adipogenic, or cell death-related signaling pathways such as NF-kB or p38-MAPK pathways were upregulated by BMP-2 in senescent MSCs, whereas bone forming signaling pathways involving BMP, SMAD, and TGF- ß were upregulated in non-senescent MSCs as expected. This phenomenon explains bone forming dominance by non-senescent MSCs and possible frequent complications such as seroma, osteolysis, or neuritis in senescent MSCs during BMP-2 use in orthopedic surgery.








Similar content being viewed by others
Data Availability
All data generated or analyzed during this study are included in this article and could be available from the corresponding author on reasonable request.
References
Chamberlain G, Fox J, Ashton B, Middleton J (2007) Concise review: mesenchymal stem cells: their phenotype, differentiation capacity, immunological features, and potential for homing. Stem Cells (Dayton, OH) 25:2739–2749
Fotia C, Massa A, Boriani F, Baldini N, Granchi D (2015) Prolonged exposure to hypoxic milieu improves the osteogenic potential of adipose derived stem cells. J Cell Biochem 116:1442–1453
Berendsen AD, Olsen BR (2015) Regulation of adipogenesis and osteogenesis in mesenchymal stem cells by vascular endothelial growth factor A. J Intern Med 277:674–680
Byun MR, Kim AR, Hwang JH, Kim KM, Hwang ES, Hong JH (2014) FGF2 stimulates osteogenic differentiation through ERK induced TAZ expression. Bone 58:72–80
Wang EA, Israel DI, Kelly S, Luxenberg DP (1993) Bone morphogenetic protein-2 causes commitment and differentiation in C3H10T1/2 and 3T3 cells. Growth Fact (Chur, Switzerland) 9:57–71
Kato S, Kawabata N, Suzuki N, Ohmura M, Takagi M (2009) Bone morphogenetic protein-2 induces the differentiation of a mesenchymal progenitor cell line, ROB-C26, into mature osteoblasts and adipocytes. Life Sci 84:302–310
Scott MA, Nguyen VT, Levi B, James AW (2011) Current methods of adipogenic differentiation of mesenchymal stem cells. Stem Cells Dev 20:1793–1804
Zhuang H, Zhang X, Zhu C, Tang X, Yu F, Shang GW, Cai X (2016) Molecular mechanisms of PPAR-gamma governing MSC osteogenic and adipogenic differentiation. Curr Stem Cell Res Ther 11:255–264
Yuan Z, Li Q, Luo S, Liu Z, Luo D, Zhang B, Zhang D, Rao P, Xiao J (2016) PPARgamma and Wnt signaling in adipogenic and osteogenic differentiation of mesenchymal stem cells. Curr Stem Cell Res Ther 11:216–225
Cho JH, Lee JH, Yeom JS, Chang BS, Yang JJ, Koo KH, Hwang CJ, Lee KB, Kim HJ, Lee CK, Kim H, Suk KS, Nam WD, Han J (2017) Efficacy of Escherichia coli-derived recombinant human bone morphogenetic protein-2 in posterolateral lumbar fusion: an open, active-controlled, randomized, multicenter trial. Spine J 17:1866–1874
Donoso O, Pino AM, Seitz G, Osses N, Rodríguez JP (2015) Osteoporosis-associated alteration in the signalling status of BMP-2 in human MSCs under adipogenic conditions. J Cell Biochem 116:1267–1277
Fleet JC, Cashman K, Cox K, Rosen V (1996) The effects of aging on the bone inductive activity of recombinant human bone morphogenetic protein-2. Endocrinology 137:4605–4610
Hara T, Kakudo N, Morimoto N, Horio O, Ogura T, Kusumoto K (2015) Effect of aging on the osteoinductive activity of recombinant human bone morphogenetic protein-2 in rats. J Surg Res 195:377–383
Moerman EJ, Teng K, Lipschitz DA, Lecka-Czernik B (2004) Aging activates adipogenic and suppresses osteogenic programs in mesenchymal marrow stroma/stem cells: the role of PPAR-gamma2 transcription factor and TGF-beta/BMP signaling pathways. Aging Cell 3:379–389
Abuna RP, Stringhetta-Garcia CT, Fiori LP, Dornelles RC, Rosa AL, Beloti MM (2016) Aging impairs osteoblast differentiation of mesenchymal stem cells grown on titanium by favoring adipogenesis. J Appl Oral Sci 24:376–382
Cheng H, Qiu L, Ma J, Zhang H, Cheng M, Li W, Zhao X, Liu K (2010) Replicative senescence of human bone marrow and umbilical cord derived mesenchymal stem cells and their differentiation to adipocytes and osteoblasts. Mol Biol Rep 38:5161–5168
Wagner W, Horn P, Castoldi M, Diehlmann A, Bork S, Saffrich R, Benes V, Blake J, Pfister S, Eckstein V, Ho AD (2008) Replicative senescence of mesenchymal stem cells: a continuous and organized process. PLoS ONE 3:e2213–e2212
Stenderup K, Justesen J, Clausen C, Kassem M (2003) Aging is associated with decreased maximal life span and accelerated senescence of bone marrow stromal cells. Bone 33:919–926
Justesen J, Stenderup K, Eriksen EF, Kassem M (2002) Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int 71:36–44
Chang T-C, Hsu M-F, Wu KK (2015) High glucose induces bone marrow-derived mesenchymal stem cell senescence by upregulating autophagy. PLoS ONE 10:e0126537-e126615
Sabatti C, S Service, Freimer N (2003) False discovery rate in linkage and association genome screens for complex disorders. Genetics 164:829–833
Yue B, Lu B, Dai KR, Zhang XL, Yu CF, Lou JR, Tang TT (2005) BMP2 gene therapy on the repair of bone defects of aged rats. Calcif Tissue Int 77:395–403
James AW (2013) Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013:684736
Bilem I, Chevallier P, Plawinski L, Sone ED, Durrieu MC, Laroche G (2016) RGD and BMP-2 mimetic peptide crosstalk enhances osteogenic commitment of human bone marrow stem cells. Acta Biomater 36:132–142
Menicanin D, Bartold PM, Zannettino AC, Gronthos S (2009) Genomic profiling of mesenchymal stem cells. Stem Cell Rev 5:36–50
de Jong DS, Vaes BL, Dechering KJ, Feijen A, Hendriks JM, Wehrens R, Mummery CL, van Zoelen EJ, Olijve W, Steegenga WT (2004) Identification of novel regulators associated with early-phase osteoblast differentiation. J Bone Miner Res 19:947–958
Vaes BL, Dechering KJ, Feijen A, Hendriks JM, Lefevre C, Mummery CL, Olijve W, van Zoelen EJ, Steegenga WT (2002) Comprehensive microarray analysis of bone morphogenetic protein 2-induced osteoblast differentiation resulting in the identification of novel markers for bone development. J Bone Miner Res 17:2106–2118
Menni C, Kiddle SJ, Mangino M, Vinuela A, Psatha M, Steves C, Sattlecker M, Buil A, Newhouse S, Nelson S, Williams S, Voyle N, Soininen H, Kloszewska I, Mecocci P, Tsolaki M, Vellas B, Lovestone S, Spector TD, Dobson R, Valdes AM (2015) Circulating proteomic signatures of chronological age. J Gerontol A Biol Sci Med Sci 70:809–816
Talavera-Adame D, Gupta A, Kurtovic S, Chaiboonma KL, Arumugaswami V, Dafoe DC (2013) Bone morphogenetic protein-2/-4 upregulation promoted by endothelial cells in coculture enhances mouse embryoid body differentiation. Stem Cells Dev 22:3252–3260
Albers CE, Hofstetter W, Sebald HJ, Sebald W, Siebenrock KA, Klenke FM (2012) L51P - A BMP2 variant with osteoinductive activity via inhibition of Noggin. Bone 51:401–406
Jin W, Takagi T, Kanesashi SN, Kurahashi T, Nomura T, Harada J, Ishii S (2006) Schnurri-2 controls BMP-dependent adipogenesis via interaction with Smad proteins. Dev Cell 10:461–471
Zhang S, Fei T, Zhang L, Zhang R, Chen F, Ning Y, Han Y, Feng XH, Meng A, Chen YG (2007) Smad7 antagonizes transforming growth factor beta signaling in the nucleus by interfering with functional Smad-DNA complex formation. Mol Cell Biol 27:4488–4499
Layliev J, Sagebin F, Weinstein A, Marchac A, Szpalski C, Saadeh PB, Warren SM (2013) Percutaneous gene therapy heals cranial defects. Gene Ther 20:922–929
Choy L, Derynck R (2003) Transforming growth factor-beta inhibits adipocyte differentiation by Smad3 interacting with CCAAT/enhancer-binding protein (C/EBP) and repressing C/EBP transactivation function. J Biol Chem 278:9609–9619
Choy L, Skillington J, Derynck R (2000) Roles of autocrine TGF-beta receptor and Smad signaling in adipocyte differentiation. J Cell Biol 149:667–682
Flanagan JN, Linder K, Mejhert N, Dungner E, Wahlen K, Decaunes P, Ryden M, Bjorklund P, Arver S, Bhasin S, Bouloumie A, Arner P, Dahlman I (2009) Role of follistatin in promoting adipogenesis in women. J Clin Endocrinol Metab 94:3003–3009
Kawabata N, Kamiya N, Suzuki N, Matsumoto M, Takagi M (2007) Changes in extracellular activin A:follistatin ratio during differentiation of a mesenchymal progenitor cell line, ROB-C26 into osteoblasts and adipocytes. Life Sci 81:8–18
Mai Y, Zhang Z, Yang H, Dong P, Chu G, Yang G, Sun S (2014) BMP and activin membrane-bound inhibitor (BAMBI) inhibits the adipogenesis of porcine preadipocytes through Wnt/beta-catenin signaling pathway. Biochem Cell Biol 92:172–182
Zheng GB, Yoon BH, Lee JH (2017) Comparison of the osteogenesis and fusion rates between activin A/BMP-2 chimera (AB204) and rhBMP-2 in a beagle’s posterolateral lumbar spine model. Spine J 17:1529–1536
Jain RG, Phelps KD, Pekala PH (1999) Tumor necrosis factor-alpha initiated signal transduction in 3T3-L1 adipocytes. J Cell Physiol 179:58–66
Cortez M, Carmo LS, Rogero MM, Borelli P, Fock RA (2013) A high-fat diet increases IL-1, IL-6, and TNF-alpha production by increasing NF-kappaB and attenuating PPAR-gamma expression in bone marrow mesenchymal stem cells. Inflammation 36:379–386
Segales J, Perdiguero E, Munoz-Canoves P (2016) Regulation of muscle stem cell functions: a focus on the p38 MAPK signaling pathway. Front Cell Dev Biol 4:91
Aouadi M, Jager J, Laurent K, Gonzalez T, Cormont M, Binetruy B, Le Marchand-Brustel Y, Tanti JF, Bost F (2007) p38MAP Kinase activity is required for human primary adipocyte differentiation. FEBS Lett 581:5591–5596
Lee JS, Park JH, Kwon IK, Lim JY (2011) Retinoic acid inhibits BMP4-induced C3H10T1/2 stem cell commitment to adipocyte via downregulating Smad/p38MAPK signaling. Biochem Biophys Res Commun 409:550–555
Wilde JM, Gumucio JP, Grekin JA, Sarver DC, Noah AC, Ruehlmann DG, Davis ME, Bedi A, Mendias CL (2016) Inhibition of p38 mitogen-activated protein kinase signaling reduces fibrosis and lipid accumulation after rotator cuff repair. J Shoulder Elbow Surg 25:1501–1508
Matsumoto A, Yamaji K, Kawanami M, Kato H (2001) Effect of aging on bone formation induced by recombinant human bone morphogenetic protein-2 combined with fibrous collagen membranes at subperiosteal sites. J Periodontal Res 36:175–182
Beane OS, Fonseca VC, Cooper LL, Koren G, Darling EM (2014) Impact of aging on the regenerative properties of bone marrow-, muscle-, and adipose-derived mesenchymal stem/stromal cells. PLoS ONE 9:e115963
Tannoury CA, An HS (2014) Complications with the use of bone morphogenetic protein 2 (BMP-2) in spine surgery. Spine J 14:552–559
Mesfin A, Buchowski JM, Zebala LP, Bakhsh WR, Aronson AB, Fogelson JL, Hershman S, Kim HJ, Ahmad A, Bridwell KH (2013) High-dose rhBMP-2 for adults: major and minor complications: a study of 502 spine cases. J Bone Joint Surg Am 95:1546–1553
Khan H, Mafi P, Mafi R, Khan W (2016) The effects of ageing on differentiation and characterisation of human mesenchymal stem cells. Curr Stem Cell Res Ther 13:378–383
Acknowledgements
This study was supported by a Grant-in-Aid (No.06- 2011-218) from the SNUH Research Fund. We specially thank to Pf. In-Gyu Kim for his supervision of this study.
Funding
This study was supported by a Grant-in-Aid (No.06-2011-218) from the SNUH Research Fund.
Author information
Authors and Affiliations
Contributions
The concept was designed by JHC and JHL. Data acquisition was done by KML and DMS. Data were analyzed by JHL and DMS. Data interpretation was performed by JHC, JHL, CKL, and DMS. Initial manuscript was drafted by JHC. All authors contributed to revising the draft for intellectual content. All authors approved the final version of the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
All authors listed above (Jae Hwan Cho, Jae Hyup Lee, Kyung Mee Lee, Choon-Ki Lee, Dong-Myung Shin) have no conflicts of interest to declare.
Ethical Approval
All procedures performed in this study were under confirmation of Institutional Review Board of SMG-SNUBMC (Seoul Metropolitan Government Seoul National University Boramae Medical Center) (IRB No: 06-2011-218) with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
223_2021_923_MOESM1_ESM.docx
Supplementary file1 (DOCX 20 kb). Supplementary material 1. Specific methodology for comparisons between senescent and non-senescent MSCs induced by rhBMP-2
223_2021_923_MOESM2_ESM.pptx
Supplementary file2 (PPTX 259 kb). Supplementary material 2. GSEA leading edge subset analysis for P4 MSC (sample CD) vs senescent MSC (sample AB)
Rights and permissions
About this article
Cite this article
Cho, J.H., Lee, J.H., Lee, K.M. et al. BMP-2 Induced Signaling Pathways and Phenotypes: Comparisons Between Senescent and Non-senescent Bone Marrow Mesenchymal Stem Cells. Calcif Tissue Int 110, 489–503 (2022). https://doi.org/10.1007/s00223-021-00923-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-021-00923-3