Abstract
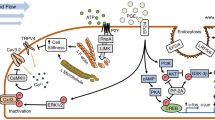
Mechanotransduction is pivotal in the maintenance of homeostasis in different tissues and involves multiple cell signaling pathways. In bone, mechanical stimuli regulate the balance between bone formation and resorption; osteocytes play a central role in this regulation. Dysfunctions in mechanotransduction signaling or in osteocytes response lead to an imbalance in bone homeostasis. This alteration is very relevant in some conditions such as osteoporosis and aging. Both are characterized by increased bone weakness due to different causes, for example, the increase of osteocyte apoptosis that cause an alteration of fluid space, or the alteration of molecular pathways. There are intertwined yet very different mechanisms involved among the cell-intrinsic effects of aging on bone, the cell-intrinsic and tissue-level effects of estrogen/androgen withdrawal on bone, and the effects of reduced mechanical loading on bone, which are all involved to some degree in how aged bone fails to respond properly to stress/strain compared to younger bone. This review aims at clarifying how the cellular and molecular pathways regulated and induced in bone by mechanical stimulation are altered with aging and in osteoporosis, to highlight new possible targets for antiresorptive or anabolic bone therapeutic approaches.


Similar content being viewed by others
References
Rochefort GY, Pallu S, Benhamou CL (2010) Osteocyte: the unrecognized side of bone tissue. Osteoporos Int 21:1457–1469. https://doi.org/10.1007/s00198-010-1194-5
Ingber DE (2006) Cellular mechanotransduction: putting all the pieces together again. FASEB J 20:811–827. https://doi.org/10.1096/fj.05-5424rev
Jaalouk DE, Lammerding J (2009) Mechanotransduction gone awry. Nat Rev Mol Cell Biol 10:63–73. https://doi.org/10.1038/nrm2597
Xiao Z, Quarles LD (2015) Physiological mechanisms and therapeutic potential of bone mechanosensing. Rev Endocr Metab Disord 16:115–129. https://doi.org/10.1007/s11154-015-9313-4
Wu M, Fannin J, Rice KM et al (2011) Effect of aging on cellular mechanotransduction. Ageing Res Rev 10:1–15. https://doi.org/10.1016/j.arr.2009.11.002
Geiger B, Spatz JP, Bershadsky AD (2009) Environmental sensing through focal adhesions. Nat Rev Mol Cell Biol 10:21–33. https://doi.org/10.1038/nrm2593
Yuan Y, Zhang L, Tong X et al (2017) Mechanical stress regulates bone metabolism through microRNAs. J Cell Physiol 232:1239–1245. https://doi.org/10.1002/jcp.25688
Hu M, Yeh R, Lien M et al (2013) Dynamic fluid flow mechanical stimulation modulates bone marrow mesenchymal stem cells. Bone Res 1:98–104. https://doi.org/10.4248/BR201301007
Deepak V, Kayastha P, McNamara LM (2017) Estrogen deficiency attenuates fluid flow–induced [Ca 2+] i oscillations and mechanoresponsiveness of MLO-Y4 osteocytes. FASEB J. https://doi.org/10.1096/fj.201601280R
Goulet GC, Cooper DML, Coombe D, Zernicke RF (2008) Influence of cortical canal architecture on lacunocanalicular pore pressure and fluid flow. Comput Methods Biomech Biomed Eng 11:379–387. https://doi.org/10.1080/10255840701814105
Aguirre JI, Plotkin LI, Stewart SA et al (2006) Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J Bone Miner Res Off J Am Soc Bone Miner Res 21:605–615. https://doi.org/10.1359/jbmr.060107
Dufour C, Holy X, Marie PJ (2007) Skeletal unloading induces osteoblast apoptosis and targets alpha5beta1-PI3K-Bcl-2 signaling in rat bone. Exp Cell Res 313:394–403. https://doi.org/10.1016/j.yexcr.2006.10.021
Matsui H, Fukuno N, Kanda Y et al (2014) The Expression of Fn14 via mechanical stress-activated JNK contributes to apoptosis induction in osteoblasts. J Biol Chem 289:6438–6450. https://doi.org/10.1074/jbc.M113.536300
Zhou Y, Guan X, Liu T et al (2015) Whole body vibration improves osseointegration by up-regulating osteoblastic activity but down-regulating osteoblast-mediated osteoclastogenesis via ERK1/2 pathway. Bone 71:17–24. https://doi.org/10.1016/j.bone.2014.09.026
Horstman AM, Dillon EL, Urban RJ, Sheffield-Moore M (2012) The role of androgens and estrogens on healthy aging and longevity. J Gerontol A Biol Sci Med Sci 67:1140–1152. https://doi.org/10.1093/gerona/gls068
Klein-Nulend J, van Oers RFM, Bakker AD, Bacabac RG (2015) Bone cell mechanosensitivity, estrogen deficiency, and osteoporosis. J Biomech 48:855–865. https://doi.org/10.1016/j.jbiomech.2014.12.007
Galea GL, Meakin LB, Harris MA et al (2017) Old age and the associated impairment of bones’ adaptation to loading are associated with transcriptomic changes in cellular metabolism, cell-matrix interactions and the cell cycle. Gene 599:36–52. https://doi.org/10.1016/j.gene.2016.11.006
Jardí F, Laurent M, Kim N et al (2018) Testosterone boosts physical activity in male mice via dopaminergic pathways. Sci Rep 8:957. https://doi.org/10.1038/s41598-017-19104-0
Golds G, Houdek D, Arnason T (2017) Male hypogonadism and osteoporosis: the effects, clinical consequences, and treatment of testosterone deficiency in bone health. Int J Endocrinol 2017:4602129. https://doi.org/10.1155/2017/4602129
Laurent MR, Dubois V, Claessens F et al (2016) Muscle-bone interactions: from experimental models to the clinic? a critical update. Mol Cell Endocrinol 432:14–36. https://doi.org/10.1016/j.mce.2015.10.017
Srinivasan S, Ausk BJ, Prasad J et al (2010) Rescuing loading induced bone formation at senescence. PLoS Comput Biol 6:e1000924. https://doi.org/10.1371/journal.pcbi.1000924
Vernikos J, Schneider VS (2010) Space, gravity and the physiology of aging: parallel or convergent disciplines? A mini-review. Gerontology 56:157–166. https://doi.org/10.1159/000252852
Tan J, Xu X, Tong Z et al (2015) Decreased osteogenesis of adult mesenchymal stem cells by reactive oxygen species under cyclic stretch: a possible mechanism of age related osteoporosis. Bone Res 3:15003. https://doi.org/10.1038/boneres.2015.3
Goggin P, Zygalakis K, Oreffo R, Schneider P (2016) High-resolution 3D imaging of osteocytes and computational modelling in mechanobiology: insights on bone development, ageing, health and disease. Eur Cell Mater 31:264–295. https://doi.org/10.22203/eCM.v031a18
Razi H, Birkhold AI, Weinkamer R et al (2015) Aging leads to a dysregulation in mechanically driven bone formation and resorption. J Bone Miner Res Off J Am Soc Bone Miner Res 30:1864–1873. https://doi.org/10.1002/jbmr.2528
Birkhold AI, Razi H, Duda GN et al (2014) The influence of age on adaptive bone formation and bone resorption. Biomaterials 35:9290–9301. https://doi.org/10.1016/j.biomaterials.2014.07.051
Compston JE, McClung MR, Leslie WD (2019) Osteoporosis. Lancet Lond Engl 393:364–376. https://doi.org/10.1016/S0140-6736(18)32112-3
Nuti R, Brandi ML, Checchia G et al (2019) Guidelines for the management of osteoporosis and fragility fractures. Intern Emerg Med 14:85–102. https://doi.org/10.1007/s11739-018-1874-2
NIH Consensus Development Panel on Osteoporosis Prevention, Diagnosis, and Therapy (2001) Osteoporosis prevention, diagnosis, and therapy. JAMA 285:785–795. https://doi.org/10.1001/jama.285.6.785
Srinivasan S, Gross TS, Bain SD (2012) Bone mechanotransduction may require augmentation in order to strengthen the senescent skeleton. Ageing Res Rev 11:353–360. https://doi.org/10.1016/j.arr.2011.12.007
Spyropoulou A, Karamesinis K, Basdra EK (2015) Mechanotransduction pathways in bone pathobiology. Biochim Biophys Acta BBA Mol Basis Dis 1852:1700–1708. https://doi.org/10.1016/j.bbadis.2015.05.010
Knothe Tate M, Adamson J, Tami A, Bauer T (2004) The osteocyte. Int J Biochem Cell Biol 36:1–8. https://doi.org/10.1016/s1357-2725(03)00241-3
Zarrinkalam MR, Mulaibrahimovic A, Atkins GJ, Moore RJ (2012) Changes in osteocyte density correspond with changes in osteoblast and osteoclast activity in an osteoporotic sheep model. Osteoporos Int 23:1329–1336. https://doi.org/10.1007/s00198-011-1672-4
Wang B, Lai X, Price C et al (2014) Perlecan-containing pericellular matrix regulates solute transport and mechanosensing within the osteocyte lacunar-canalicular system. J Bone Miner Res Off J Am Soc Bone Miner Res 29:878–891. https://doi.org/10.1002/jbmr.2105
Hagan ML, Yu K, Zhu J et al (2020) Decreased pericellular matrix production and selection for enhanced cell membrane repair may impair osteocyte responses to mechanical loading in the aging skeleton. Aging Cell 19:e13056. https://doi.org/10.1111/acel.13056
Ciani C, Sharma D, Doty SB, Fritton SP (2014) Ovariectomy enhances mechanical load-induced solute transport around osteocytes in rat cancellous bone. Bone 59:229–234. https://doi.org/10.1016/j.bone.2013.11.026
Qin Y-X, Lin W, Rubin C (2002) The pathway of bone fluid flow as defined by in vivo intramedullary pressure and streaming potential measurements. Ann Biomed Eng 30:693–702. https://doi.org/10.1114/1.1483863
Zaman G, Saxon LK, Sunters A et al (2010) Loading-related regulation of gene expression in bone in the contexts of estrogen deficiency, lack of estrogen receptor alpha and disuse. Bone 46:628–642. https://doi.org/10.1016/j.bone.2009.10.021
Geoghegan IP, Hoey DA, McNamara LM (2019) Estrogen deficiency impairs integrin αvβ3-mediated mechanosensation by osteocytes and alters osteoclastogenic paracrine signalling. Sci Rep 9:4654. https://doi.org/10.1038/s41598-019-41095-3
Zeng D, Yao P, Zhao H (2019) P2X7, a critical regulator and potential target for bone and joint diseases. J Cell Physiol 234:2095–2103. https://doi.org/10.1002/jcp.27544
Jørgensen NR, Husted LB, Skarratt KK et al (2012) Single-nucleotide polymorphisms in the P2X7 receptor gene are associated with post-menopausal bone loss and vertebral fractures. Eur J Hum Genet EJHG 20:675–681. https://doi.org/10.1038/ejhg.2011.253
Li J, Liu D, Ke H et al (2005) The P2X7 nucleotide receptor mediates skeletal mechanotransduction. J Biol Chem 280:42952–42959. https://doi.org/10.1074/jbc.M506415200
Wang N, Agrawal A, Jørgensen NR, Gartland A (2018) P2X7 receptor regulates osteoclast function and bone loss in a mouse model of osteoporosis. Sci Rep 8:3507. https://doi.org/10.1038/s41598-018-21574-9
Laurent M, Antonio L, Sinnesael M et al (2014) Androgens and estrogens in skeletal sexual dimorphism. Asian J Androl 16:213–222. https://doi.org/10.4103/1008-682X.122356
Almeida M, Laurent MR, Dubois V et al (2017) Estrogens and androgens in skeletal physiology and pathophysiology. Physiol Rev 97:135–187. https://doi.org/10.1152/physrev.00033.2015
Castillo AB, Triplett JW, Pavalko FM, Turner CH (2014) Estrogen receptor-beta regulates mechanical signaling in primary osteoblasts. AJP Endocrinol Metab 306:E937–E944. https://doi.org/10.1152/ajpendo.00458.2013
Frost HM (1999) On the estrogen-bone relationship and postmenopausal bone loss: a new model. J Bone Miner Res Off J Am Soc Bone Miner Res 14:1473–1477. https://doi.org/10.1359/jbmr.1999.14.9.1473
Bass SL, Saxon L, Daly RM et al (2002) The effect of mechanical loading on the size and shape of bone in pre-, peri-, and postpubertal girls: a study in tennis players. J Bone Miner Res Off J Am Soc Bone Miner Res 17:2274–2280. https://doi.org/10.1359/jbmr.2002.17.12.2274
Lanyon L, Skerry T (2001) Postmenopausal osteoporosis as a failure of bone’s adaptation to functional loading: a hypothesis. J Bone Miner Res Off J Am Soc Bone Miner Res 16:1937–1947. https://doi.org/10.1359/jbmr.2001.16.11.1937
Sapir-Koren R, Livshits G (2013) Is interaction between age-dependent decline in mechanical stimulation and osteocyte–estrogen receptor levels the culprit for postmenopausal-impaired bone formation? Osteoporos Int 24:1771–1789. https://doi.org/10.1007/s00198-012-2208-2
Aguirre JI, Plotkin LI, Gortazar AR et al (2007) A novel ligand-independent function of the estrogen receptor is essential for osteocyte and osteoblast mechanotransduction. J Biol Chem 282:25501–25508. https://doi.org/10.1074/jbc.M702231200
Klein-Nulend J, Sterck JGH, Semeins CM et al (2002) Donor age and mechanosensitivity of human bone cells. Osteoporos Int 13:137–146
Klein-Nulend J, van Oers RFM, Bakker AD, Bacabac RG (2014) Nitric oxide signaling in mechanical adaptation of bone. Osteoporos Int 25:1427–1437. https://doi.org/10.1007/s00198-013-2590-4
Galea GL, Meakin LB, Sugiyama T et al (2013) Estrogen receptor α mediates proliferation of osteoblastic cells stimulated by estrogen and mechanical strain, but their acute down-regulation of the Wnt antagonist sost is mediated by estrogen receptor β. J Biol Chem 288:9035–9048. https://doi.org/10.1074/jbc.M112.405456
Lee K, Jessop H, Suswillo R et al (2003) Endocrinology: bone adaptation requires oestrogen receptor-alpha. Nature 424:389. https://doi.org/10.1038/424389a
Melville KM, Kelly NH, Surita G et al (2015) Effects of deletion of ERα in osteoblast-lineage cells on bone mass and adaptation to mechanical loading differ in female and male mice. J Bone Miner Res Off J Am Soc Bone Miner Res 30:1468–1480. https://doi.org/10.1002/jbmr.2488
Laurent MR, Jardí F, Dubois V et al (2016) Androgens have antiresorptive effects on trabecular disuse osteopenia independent from muscle atrophy. Bone 93:33–42. https://doi.org/10.1016/j.bone.2016.09.011
Sinnesael M, Laurent MR, Jardi F et al (2015) Androgens inhibit the osteogenic response to mechanical loading in adult male mice. Endocrinology 156:1343–1353. https://doi.org/10.1210/en.2014-1673
Saita Y, Nakamura T, Mizoguchi F et al (2009) Combinatory effects of androgen receptor deficiency and hind limb unloading on bone. Horm Metab Res Horm Stoffwechselforsch Horm Metab 41:822–828. https://doi.org/10.1055/s-0029-1231056
Lin C, Jiang X, Dai Z et al (2009) Sclerostin mediates bone response to mechanical unloading through antagonizing Wnt/β-catenin signaling. J Bone Miner Res 24:1651–1661. https://doi.org/10.1359/jbmr.090411
Robinson JA, Chatterjee-Kishore M, Yaworsky PJ et al (2006) Wnt/beta-catenin signaling is a normal physiological response to mechanical loading in bone. J Biol Chem 281:31720–31728. https://doi.org/10.1074/jbc.M602308200
Gardinier JD, Rostami N, Juliano L, Zhang C (2018) Bone adaptation in response to treadmill exercise in young and adult mice. Bone Rep 8:29–37. https://doi.org/10.1016/j.bonr.2018.01.003
Holguin N, Brodt MD, Silva MJ (2016) Activation of wnt signaling by mechanical loading is impaired in the bone of old mice. J Bone Miner Res Off J Am Soc Bone Miner Res 31:2215–2226. https://doi.org/10.1002/jbmr.2900
Macias BR, Aspenberg P, Agholme F (2013) Paradoxical Sost gene expression response to mechanical unloading in metaphyseal bone. Bone 53:515–519. https://doi.org/10.1016/j.bone.2013.01.018
Gu Q, Tian H, Zhang K et al (2018) Wnt5a/FZD4 mediates the mechanical stretch-induced osteogenic differentiation of bone mesenchymal stem cells. Cell Physiol Biochem Int J Exp Cell Physiol Biochem Pharmacol 48:215–226. https://doi.org/10.1159/000491721
Charoenpanich A, Wall ME, Tucker CJ et al (2014) Cyclic tensile strain enhances osteogenesis and angiogenesis in mesenchymal stem cells from osteoporotic donors. Tissue Eng Part A 20:67–78. https://doi.org/10.1089/ten.TEA.2013.0006
Robling AG, Niziolek PJ, Baldridge LA et al (2008) Mechanical stimulation of bone in vivo reduces osteocyte expression of Sost/sclerostin. J Biol Chem 283:5866–5875. https://doi.org/10.1074/jbc.M705092200
Albiol L, Büttner A, Pflanz D et al (2020) Effects of long-term sclerostin deficiency on trabecular bone mass and adaption to limb loading differ in male and female mice. Calcif Tissue Int 106:415–430. https://doi.org/10.1007/s00223-019-00648-4
Niziolek PJ, Bullock W, Warman ML, Robling AG (2015) Missense mutations in LRP5 associated with high bone mass protect the mouse skeleton from disuse- and ovariectomy-induced osteopenia. PLoS ONE 10:e0140775. https://doi.org/10.1371/journal.pone.0140775
Gaudio A, Pennisi P, Bratengeier C et al (2010) Increased sclerostin serum levels associated with bone formation and resorption markers in patients with immobilization-induced bone loss. J Clin Endocrinol Metab 95:2248–2253. https://doi.org/10.1210/jc.2010-0067
Norwitz NG, Mota AS, Misra M, Ackerman KE (2019) LRP5, bone density, and mechanical stress: a case report and literature review. Front Endocrinol 10:184. https://doi.org/10.3389/fendo.2019.00184
Gerbaix M, Vico L, Ferrari SL, Bonnet N (2015) Periostin expression contributes to cortical bone loss during unloading. Bone 71:94–100. https://doi.org/10.1016/j.bone.2014.10.011
Bonnet N, Garnero P, Ferrari S (2016) Periostin action in bone. Mol Cell Endocrinol 432:75–82. https://doi.org/10.1016/j.mce.2015.12.014
You J (2001) Osteopontin gene regulation by oscillatory fluid flow via intracellular calcium mobilization and activation of mitogen-activated protein kinase in MC3T3-E1 osteoblasts. J Biol Chem 276:13365–13371. https://doi.org/10.1074/jbc.M009846200
Tan SD, Bakker AD, Semeins CM et al (2008) Inhibition of osteocyte apoptosis by fluid flow is mediated by nitric oxide. Biochem Biophys Res Commun 369:1150–1154. https://doi.org/10.1016/j.bbrc.2008.03.007
Rangaswami H, Schwappacher R, Tran T et al (2012) Protein kinase G and focal adhesion kinase converge on Src/Akt/β-catenin signaling module in osteoblast mechanotransduction. J Biol Chem 287:21509–21519. https://doi.org/10.1074/jbc.M112.347245
Gao Q, Xu M, Alander CB et al (2009) Effects of prostaglandin E2 on bone in mice in vivo. Prostagland Other Lipid Mediat 89:20–25. https://doi.org/10.1016/j.prostaglandins.2009.03.003
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455–498. https://doi.org/10.1146/annurev.bioeng.8.061505.095721
Damoulis PD, Hauschka PV (1997) Nitric oxide acts in conjunction with proinflammatory cytokines to promote cell death in osteoblasts. J Bone Miner Res 12:412–422
Rangaswami H, Schwappacher R, Marathe N et al (2010) Cyclic GMP and protein kinase G control a Src-containing mechanosome in osteoblasts. Sci Signal 3:ra91. https://doi.org/10.1126/scisignal.2001423
Kalyanaraman H, Schall N, Pilz RB (2018) Nitric oxide and cyclic GMP functions in bone. Nitric Oxide Biol Chem 76:62–70. https://doi.org/10.1016/j.niox.2018.03.007
Joiner DM, Tayim RJ, McElderry J-D et al (2014) Aged male rats regenerate cortical bone with reduced osteocyte density and reduced secretion of nitric oxide after mechanical stimulation. Calcif Tissue Int 94:484–494. https://doi.org/10.1007/s00223-013-9832-5
Chalil S, Jaspers RT, Manders RJ et al (2015) Increased endoplasmic reticulum stress in mouse osteocytes with aging alters Cox-2 response to mechanical stimuli. Calcif Tissue Int 96:123–128. https://doi.org/10.1007/s00223-014-9944-6
Joldersma M, Burger EH, Semeins CM, Klein-Nulend J (2000) Mechanical stress induces COX-2 mRNA expression in bone cells from elderly women. J Biomech 33:53–61
Corrigan MA, Coyle S, Eichholz KF et al (2019) Aged osteoporotic bone marrow stromal cells demonstrate defective recruitment, mechanosensitivity, and matrix deposition. Cells Tissues Organs 207:83–96. https://doi.org/10.1159/000503444
Joiner DM, Tayim RJ, Kadado A, Goldstein SA (2012) Bone marrow stromal cells from aged male rats have delayed mineralization and reduced response to mechanical stimulation through nitric oxide and ERK1/2 signaling during osteogenic differentiation. Biogerontology 13:467–478. https://doi.org/10.1007/s10522-012-9391-6
Cardoso L, Herman BC, Verborgt O et al (2009) Osteocyte apoptosis controls activation of intracortical resorption in response to bone fatigue. J Bone Miner Res Off J Am Soc Bone Miner Res 24:597–605. https://doi.org/10.1359/jbmr.081210
Liu W, Xu C, Zhao H et al (2015) Osteoprotegerin induces apoptosis of osteoclasts and osteoclast precursor cells via the fas/fas ligand pathway. PLoS ONE 10:e0142519. https://doi.org/10.1371/journal.pone.0142519
Grimston SK, Watkins MP, Stains JP, Civitelli R (2013) Connexin43 modulates post-natal cortical bone modeling and mechano-responsiveness. BoneKEy Rep 2:446. https://doi.org/10.1038/bonekey.2013.180
Loiselle AE, Jiang JX, Donahue HJ (2013) Gap junction and hemichannel functions in osteocytes. Osteocyte 54:205–212. https://doi.org/10.1016/j.bone.2012.08.132
Plotkin LI, Manolagas SC, Bellido T (2002) Transduction of cell survival signals by connexin-43 hemichannels. J Biol Chem 277:8648–8657. https://doi.org/10.1074/jbc.M108625200
Zhang Y, Paul EM, Sathyendra V et al (2011) Enhanced osteoclastic resorption and responsiveness to mechanical load in gap junction deficient bone. PLoS ONE 6:e23516. https://doi.org/10.1371/journal.pone.0023516
Bivi N, Condon KW, Allen MR et al (2012) Cell autonomous requirement of connexin 43 for osteocyte survival: consequences for endocortical resorption and periosteal bone formation. J Bone Miner Res Off J Am Soc Bone Miner Res 27:374–389. https://doi.org/10.1002/jbmr.548
Davis HM, Pacheco-Costa R, Atkinson EG et al (2017) Disruption of the Cx43/miR21 pathway leads to osteocyte apoptosis and increased osteoclastogenesis with aging. Aging Cell 16:551–563. https://doi.org/10.1111/acel.12586
Kar R, Riquelme M, Werner S, Jiang J (2013) Connexin 43 channels protect osteocytes against oxidative stress-induced cell death. J Bone Min Res 28:1611–1621. https://doi.org/10.1002/jbmr.1917
Plotkin LI, Speacht TL, Donahue HJ (2015) Cx43 and mechanotransduction in bone. Curr Osteoporos Rep 13:67–72. https://doi.org/10.1007/s11914-015-0255-2
Moriishi T, Fukuyama R, Ito M et al (2012) Osteocyte network; a negative regulatory system for bone mass augmented by the induction of rankl in osteoblasts and sost in osteocytes at unloading. PLoS ONE 7:e40143. https://doi.org/10.1371/journal.pone.0040143
Wang Y, Liu W, Masuyama R et al (2012) Pyruvate dehydrogenase kinase 4 induces bone loss at unloading by promoting osteoclastogenesis. Bone 50:409–419. https://doi.org/10.1016/j.bone.2011.07.012
Lim WH, Wong G, Lim EM et al (2015) Circulating lipocalin 2 levels predict fracture-related hospitalizations in elderly women: a prospective cohort study. J Bone Miner Res Off J Am Soc Bone Miner Res 30:2078–2085. https://doi.org/10.1002/jbmr.2546
Rucci N, Capulli M, Piperni SG et al (2015) Lipocalin 2: a new mechanoresponding gene regulating bone homeostasis. J Bone Miner Res Off J Am Soc Bone Miner Res 30:357–368. https://doi.org/10.1002/jbmr.2341
Wang C, Shan S, Wang C et al (2017) Mechanical stimulation promote the osteogenic differentiation of bone marrow stromal cells through epigenetic regulation of Sonic Hedgehog. Exp Cell Res 352:346–356. https://doi.org/10.1016/j.yexcr.2017.02.021
Wang J, Wang CD, Zhang N et al (2016) Mechanical stimulation orchestrates the osteogenic differentiation of human bone marrow stromal cells by regulating HDAC1. Cell Death Dis 7:e2221. https://doi.org/10.1038/cddis.2016.112
Yamazaki M, Fukushima H, Shin M et al (2009) Tumor necrosis factor alpha represses bone morphogenetic protein (BMP) signaling by interfering with the DNA binding of Smads through the activation of NF-kappaB. J Biol Chem 284:35987–35995. https://doi.org/10.1074/jbc.M109.070540
Nakamura H, Aoki K, Masuda W et al (2013) Disruption of NF-κB1 prevents bone loss caused by mechanical unloading. J Bone Miner Res Off J Am Soc Bone Miner Res 28:1457–1467. https://doi.org/10.1002/jbmr.1866
Santos A, Bakker AD, Willems HME et al (2011) Mechanical loading stimulates BMP7, but not BMP2, production by osteocytes. Calcif Tissue Int 89:318–326. https://doi.org/10.1007/s00223-011-9521-1
Lambers FM, Kuhn G, Weigt C et al (2015) Bone adaptation to cyclic loading in murine caudal vertebrae is maintained with age and directly correlated to the local micromechanical environment. J Biomech 48:1179–1187. https://doi.org/10.1016/j.jbiomech.2014.11.020
Leppänen OV, Sievänen H, Jokihaara J et al (2008) Pathogenesis of age-related osteoporosis: impaired mechano-responsiveness of bone is not the culprit. PLoS ONE 3:e2540. https://doi.org/10.1371/journal.pone.0002540
Silva MJ, Brodt MD, Lynch MA et al (2012) Tibial loading increases osteogenic gene expression and cortical bone volume in mature and middle-aged mice. PLoS ONE 7:e34980. https://doi.org/10.1371/journal.pone.0034980
Järvinen TLN, Pajamäki I, Sievänen H et al (2003) Femoral neck response to exercise and subsequent deconditioning in young and adult rats. J Bone Miner Res Off J Am Soc Bone Miner Res 18:1292–1299. https://doi.org/10.1359/jbmr.2003.18.7.1292
Brodt MD, Silva MJ (2010) Aged mice have enhanced endocortical response and normal periosteal response compared with young-adult mice following 1 week of axial tibial compression. J Bone Miner Res Off J Am Soc Bone Miner Res 25:2006–2015. https://doi.org/10.1002/jbmr.96
Wu Y, Zhang P, Dai Q et al (2013) Effect of mechanical stretch on the proliferation and differentiation of BMSCs from ovariectomized rats. Mol Cell Biochem 382:273–282. https://doi.org/10.1007/s11010-013-1744-1
Ouyang N, Zhang P, Fu R et al (2018) Mechanical strain promotes osteogenic differentiation of bone mesenchymal stem cells from ovariectomized rats via the phosphoinositide 3-kinase/Akt signaling pathway. Mol Med Rep 17:1855–1862. https://doi.org/10.3892/mmr.2017.8030
Acknowledgements
Valeria Carina, Viviana Costa and Daniele Bellavia contributed to the manuscript by working at the Technology Platform of Tissue Engineering, Theranostic and Oncology (Lab. Manager Dr. Gianluca Giavaresi), a laboratory started up by the Rizzoli Orthopedic Institute in Palermo (Italy) with the grants also of National Operative Program projects (PON). AO Foundation.
Funding
No benefits in any form were received or will be received from a commercial party.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. VC, EDB, MF, and GG had the idea for the article, VC, EDB, VC, DB, and SC performed the literature search and data analysis, VC, EDB, VC, and DB drafted the work. Finally, FV, MF and GG critically revised the work. All authors red and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
Valeria Carina, Elena Della Bella, Viviana Costa, Daniele Bellavia, Francesca Veronesi, Simona Cepollaro, Milena Fini, Gianluca Giavaresi, declare that they have no conflict of interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Carina, V., Della Bella, E., Costa, V. et al. Bone's Response to Mechanical Loading in Aging and Osteoporosis: Molecular Mechanisms. Calcif Tissue Int 107, 301–318 (2020). https://doi.org/10.1007/s00223-020-00724-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-020-00724-0