Abstract
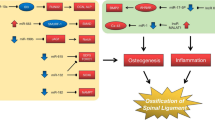
Long non-coding RNAs (lncRNAs) play an important role in the development of bone-related diseases. This study was conducted to investigate the role and mechanism of lncRNA X inactive specific transcript (XIST) in the occurrence of cervical ossification of the posterior longitudinal ligament (OPLL). Here, primary human ligament fibroblasts cells (LFCs) were isolated from 30 cases of OPLL and 30 normal cervical posterior longitudinal ligament (non-OPLL) tissues to perform the qPCR and Western blot assay. We found that the mRNA level of lncRNA XIST was significantly increased in OPLL LFCs compared to non-OPLL LFCs. By bioinformatics analysis, we found that lncRNA XIST has four binding sites for miR-17-5p and found that the mRNA level of miR-17-5p was also significantly decreased in OPLL LFCs compared to non-OPLL LFCs. Since AHNAK is the target gene of miR-17-5p, we further found that the expression of AHNAK was significantly reduced in non-OPLL LFCs after being transfected with miR-17-5p mimic. The qPCR results showed that the mRNA expressions of BMP2 and Runx2 were significantly decreased. After being transfected with lncRNA XIST siRNA in the non-OPLL LFCs, the mRNA levels of lncRNA XIST, AHNAK, BMP2, and Runx2 were significantly decreased and the phosphorylated protein of Smad1/5/8 was reduced. After being cultured by mechanical vibration, the mRNA levels of lncRNA XIST, AHNAK, BMP2, Runx2, COL1, OC, OPN, and Phospho1 were significantly increased, but the mRNA expression of miR-17-5p was significantly decreased. The expression of phosphorylated Smad1/5/8 protein was also significantly increased. Together, this study was the first to determine that XIST gene inhibition plays an important role in the occurrence of cervical OPLL, through the mechanism of regulation of miR-17-5P/AHNAK/BMP2 signaling pathway. Thus, XIST may be a potential target that could be modulated for the treatment of cervical OPLL.






Similar content being viewed by others
Abbreviations
- OPLL:
-
Ossification of posterior longitudinal ligament
- BMI:
-
Body mass index
- lncRNAs:
-
Long non-coding RNAs
- XIST:
-
X inactive specific transcript
- miR-17-5p:
-
microRNA-17-5p
- LFCs:
-
Ligament fibroblast cells
- ceRNA:
-
Competing endogenous RNA
- BMP2:
-
Bone morphogenic protein 2
- IGF:
-
Insulin-like growth factor
- bFGF:
-
Basic fibroblast growth factor
- TGF-β:
-
Transforming growth factor-β
- MAPK:
-
Mitogen-activated protein kinase
- DANCR:
-
Differentiation antagonizing non-protein coding RNA
- miR-141:
-
microRNA-141
- miR-22:
-
microRNA-22
- miR-200a:
-
microRNA-200a
- EZH2:
-
Enhancer of Zeste homolog 2
- RUNX2:
-
Runt-related transcription factor 2
- miR-211:
-
microRNA-211
- CXCR4:
-
C-X-C chemokine receptor type 4
- COL I:
-
Collagen type I
- OC:
-
Osteocalcin
- OPN:
-
Osteopontin
- Phospho1:
-
Phosphatase, orphan 1
References
Xu N, Yu M, Liu X, Sun C, Chen Z, Liu Z (2017) A systematic review of complications in thoracic spine surgery for ossification of the posterior longitudinal ligament. Eur Spine J 26(7):1803–1809
Fujimori T, Le H, Hu SS, Chin C, Pekmezci M, Schairer W, Tay BK, Hamasaki T, Yoshikawa H, Iwasaki M (2015) Ossification of the posterior longitudinal ligament of the cervical spine in 3161 patients: a CT-based study. Spine 40(7):E394–E403
Li ZS, Zhang GB, Sheng HF et al (1999) Incidence of cervical ossification of the posterior longitudinal ligament in neck and shoulder pain patients in northern China. Chin J Spine Spinal Cord. 9(5):285–286 (in Chinese)
Abiola R, Rubery P, Mesfin A (2016) Ossification of the posterior longitudinal ligament: etiology, diagnosis, and outcomes of nonoperative and operative management. Glob Spine J 6(2):195–204
Nakajima M, Takahashi A, Tsuji T et al (2014) A genome-wide association study identifies susceptibility loci for ossification of the posterior longitudinal ligament of the spine. Nat Genet 46(9):1012–1016
Tanaka H, Nagai E, Murata H et al (2001) Involvement of bone morphogenetic protein-2 (BMP-2) in the pathological ossification process of the spinal ligament. Rheumayology 40(10):1163–1168
Kawaguchi Y, Furushima K, Sugimori K et al (2003) Association between polymorphism of the transforming growth factor-beta1 gene with the radiologic characteristic and ossification of the posterior longitudinal ligament. Spine 28(13):1424–1426
Sawada T, Kishiya M, Kanemaru K et al (2008) Possible role of extracellular nucleotides in ectopic ossification of human spinal ligaments. J Pharmacol Sci 106(1):152–161
Sun M, Kraus WL (2015) From discovery to function: the expanding roles of long noncoding RNAs in physiology and disease. Endocr Rev 36(1):25–64
Zhang R, Xia LQ, Lu WW, Zhang J, Zhu JS (2016) LncRNAs and cancer. Oncol Lett 12(2):1233–1239
Huynh NP, Anderson BA, Guilak F, McAlinden A (2017) Emerging roles for long noncoding RNAs in skeletal biology and disease. Connect Tissue Res 58(1):116–141
Simionescu-Bankston A, Kumar A (2016) Noncoding RNAs in the regulation of skeletal muscle biology in health and disease. J Mol Med 94(8):853–866
Nie M, Deng ZL, Liu J, Wang DZ (2015) Emerging roles for long noncoding RNAs in skeletal biology and disease. Biomed Res Int 2015:676575
Li L, Liu B, Wapinski OL, Tsai MC, Qu K, Zhang J, Carlson JC, Lin M, Fang F, Gupta RA, Helms JA, Chang HY (2013) Targeted disruption of Hotair leads to homeotic transformation and gene derepression. Cell Rep 5(1):3–12
Saydam O, Shen Y, Würdinger T, Senol O, Boke E, James MF, Tannous BA, Stemmer-Rachamimov AO, Yi M, Stephens RM, Fraefel C, Gusella JF, Krichevsky AM, Breakefield XO (2009) Downregulated microRNA-200a in meningiomas promotes tumor growth by reducing E-cadherin and activating the Wnt/beta-catenin signaling pathway. Mol Cell Biol 29(21):5923–5940
Zhu L, Xu PC (2013) Downregulated LncRNA-ANCR promotes osteoblast differentiation by targeting EZH2 and regulating Runx2 expression. Biochem Biophys Res Commun 432(4):612–617
Li L, Lv G, Wang B, Kuang L (2018) The role of lncRNA XIST/miR-211 axis in modulating the proliferation and apoptosis of osteoarthritis chondrocytes through CXCR1 and MAPK signaling. Biochem Biophys Res Commun 503:2555–2562
Chen CK, Blanco M, Jackson C, Aznauryan E, Ollikainen N, Surka C, Chow A, Crerase A, McDonel P, Guttman M (2016) Xist recruits the X chromosome to the nuclear lamina to enable chromosome-wide silencing. Science 354:468–472
McHugh CA, Chen CK, Chow A et al (2015) The Xist lncRNA interacts directly with SHARP to silence transcription through HDAC3. Nature 521(7551):232–236
Yao Y, Ma J, Xue Y et al (2015) Knockdown of long non-coding RNA XIST exerts tumor-suppressive functions in human glioblastoma stem cells by up-regulating miR-152. Cancer Lett 359(1):75–86
Fang J, Sun CC, Gong C (2016) Long noncoding RNA XIST acts as an oncogene in non-small cell lung cancer by epigenetically repressing KLF2 expression. Biochem Biophys Res Commun 478:811–817
Song P, Ye LF, Zhang C, Peng T, Zhou XH (2016) Long non-coding RNA XIST exerts oncogenic functions in human nasopharyngeal carcinoma by targeting miR-34a-5p. Gene 592:8–14
Zhuang LK, Yang YT, Ma X, Han B, Wang ZS, Zhao QY et al (2016) MicroRNA-92b promotes hepatocellular carcinoma progression by targeting smad7 and is mediated by long non-coding RNA XIST. Cell Death Dis 7:e2203
Ren C, Li X, Wang T et al (2015) Functions and mechanisms of long noncoding RNAs in ovarian cancer. Int J Gynecol Cancer 25:566–569
Wang Z, Liu M, Zhu H et al (2010) Suppression of p21 by c-myc through members of miR-17 family at the post-transcriptional level. Int J Oncol 37(5):1315–1321
Wu Q, Luo G, Yang Z et al (2014) miR-17-5p promotes proliferation by targeting SOCS6 in gastric cancer cells. FEBS Lett 588(12):2055–2062
Fan M, Sethuraman A, Brown M et al (2014) Systematic analysis of metastasisassociated genes identifies miR-17-5p as a metastatic suppressor of basal-like breast cancer. Breast Cancer Res Treat 146(3):487–502
Fang Y, Xu C, Fu Y (2015) MicroRNA-17-5p induces drug resistance and invasion of ovarian carcinoma cells by targeting PTEN signaling. J Biol Res 22(1):1–10
Li H, Li T, Wang S et al (2013) miR-17-5p and miR-106a are involved in the balance between osteogenic and adipogenic differentiation of adipose-derived mesenchymal stem cells. Stem Cell Rev 10:313–324
Fang T, Wu Q, Zhou L et al (2016) miR-106b-5p and miR-17-5p suppress osteogenic differentiation by targeting Smad5 and inhibit bone formation. Exp Cell Res 347:74–82
Shin S, Seong JK, Bae YS (2016) Ahnak stimulates BMP2-mediated adipocyte differentiation through Smad1 activation. Obesity 24:398–407
Kon T, Yamazaki M, Tagawa M et al (1997) Bone morphogenetic protein-2 stimulates differentiation of cultured spinal ligament cells from patients with ossification of the posterior longitudinal ligament. Calcif Tissue Int 60:291–296
Tanno M, Furukawa KI, Ueyama K et al (2003) Uniaxial cyclic stretch induces osteogenic differentiation and synthesis of bone morphogenetic proteins of spinal ligament cells derived from patients with ossification of the posterior longitudinal ligaments. Bone 33:475–484
Iwasawa T, Iwasaki K, Sawada T et al (2006) Pathophysiological role of endothelin in ectopic ossification of human spinal ligaments induced by mechanical stress. Calcif Tissue Int 79:422–430
Iwasaki K, Furukawa KI, Tanno M et al (2004) Uni-axial cyclic stretch induces Cbfa1 expression in spinal ligament cells derived from patients with ossification of the posterior longitudinal ligament. Calcif Tissue Int 74:448–457
Ohishi H, Furukawa K, Iwasaki K et al (2003) Role of prostaglandin I2 in the gene expression induced by mechanical stress in spinal ligament cells derived from patients with ossification of the posterior longitudinal ligament. J Pharmacol Exp Ther 305:818–824
Tsukamoto N, Maeda T, Miura H et al (2006) Repetitive tensile stress to rat caudal vertebrae inducing cartilage formation in the spinal ligaments: a possible role of mechanical stress in the development of ossification of the spinal ligaments. Spine 5:234–242
Ikegawa S (2014) Genomic study of ossification of the posterior longitudinal ligament of the spine. Proc Jpn Acad Ser B Phys Biol Sci 90:405–412
Yang HS, Lu XH, Chen DY et al (2011) Upregulated expression of connexin43 in spinal ligament fibroblasts derived from patients presenting ossification of the posterior longitudinal ligament. Spine 36:2267–2274
Yang HS, Lu XH, Chen DY et al (2011) Mechanical strain induces Cx43 expression in spinal ligament fibroblasts derived from patients presenting ossification of the posterior longitudinal ligament. Eur Spine J 20:1459–1465
Acknowledgements
We would like to thank the patients and their caregivers for taking part in the study.
Author information
Authors and Affiliations
Contributions
Designed the study: LXY, TDZ, YLL, CXS. Performed the study: LXY, YHS, CY, CDY, JLS. Analyzed the data: LXY, TDZ, YLL, CXS. Wrote the paper: LXY, TDZ, YLL, CXS.
Corresponding authors
Ethics declarations
Conflict of interest
Liao Xinyuan, Tang Dezhi, Yang Haisong, Chen Yu, Chen Deyu, Jia Lianshun, Yang Lili and Chen Xiongsheng declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This study was approved by the Ethics Committee of Shanghai Changzheng Hospital and conducted strictly in accordance with the Helsinki Declaration. All patients signed an informed consent for sample collection prior to study.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Liao, X., Tang, D., Yang, H. et al. Long Non-coding RNA XIST May Influence Cervical Ossification of the Posterior Longitudinal Ligament Through Regulation of miR-17-5P/AHNAK/BMP2 Signaling Pathway. Calcif Tissue Int 105, 670–680 (2019). https://doi.org/10.1007/s00223-019-00608-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00608-y