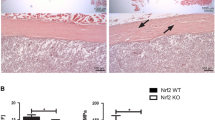
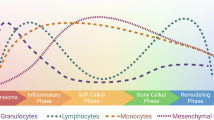
Abstract
Fracture healing is a natural process that recapitulates embryonic skeletal development. In the early phase after fracture, reactive oxygen species (ROS) are produced under inflammatory and ischemic conditions due to vessel injury and soft tissue damage, leading to cell death. Usually, such damage during the course of fracture healing can be largely prevented by protective mechanisms and functions of antioxidant enzymes. However, intrinsic oxidative stress can cause excessive toxic radicals, resulting in irreversible damage to cells associated with bone repair during the fracture healing process. Clinically, patients with type-2 diabetes mellitus, osteoporosis, habitual drinkers, or heavy smokers are at risk of impaired fracture healing due to elevated oxidative stress. Although increased levels of oxidative stress markers upon fracture and effects of antioxidants on fracture healing have been reported, a detailed understanding of what causes impaired fracture healing under intrinsic conditions of oxidative stress is lacking. Nuclear factor erythroid 2-related factor 2 (Nrf2) has been identified as a key transcriptional regulator of the expression of antioxidants and detoxifying enzymes. It further not only plays a crucial role in preventing degenerative diseases in multiple organs, but also during fracture healing. This narrative review evaluates the influence of intrinsic oxidative stress on fracture healing and sheds new light on the intriguing role of Nrf2 during bone regeneration in pathological fractures.



Similar content being viewed by others
References
Turgut A, Gokturk E, Kose N, Kacmaz M, Ozturk HS, Seber S, Acar S (1999) Oxidant status increased during fracture healing in rats. Acta Orthop Scand 70:487–490
Yeler H, Tahtabas F, Candan F (2005) Investigation of oxidative stress during fracture healing in the rats. Cell Biochem Funct 23:137–139
Ilyas A, Odatsu T, Shah A, Monte F, Kim HK, Kramer P, Aswath PB, Varanasi VG (2016) Amorphous silica: a new antioxidant role for rapid critical-sized bone defect healing. Adv Healthc Mater 5(17):2199–2213. https://doi.org/10.1002/adhm.201600203
Kelpke SS, Reiff D, Prince CW, Thompson JA (2001) Acidic fibroblast growth factor signaling inhibits peroxynitrite-induced death of osteoblasts and osteoblast precursors. J Bone Miner Res 16:1917–1925
Hamada Y, Fujii H, Fukagawa M (2009) Role of oxidative stress in diabetic bone disorder. Bone 45(Suppl 1):S35–S38. https://doi.org/10.1016/j.bone.2009.02.004.review
Dai K, Hao Y (2007) Quality of healing compared between osteoporotic fracture and normal traumatic fracture. In: Qin L, Genant HK, Griffith JF, Leung KS (eds) Advanced bioimaging technologies in assessment of the quality of bone and scaffold materials techniques and applications. Springer, Berlin Heidelberg, pp 531–541
Chakkalakal DA, Novak JR, Fritz ED, Mollner TJ, McVicker DL, Garvin KL, McGuire MH, Donohue TM (2005) Inhibition of bone repair in a rat model for chronic and excessive alcohol consumption. Alcohol 36(3):201–214
Patel RA, Wilson RF, Patel PA, Palmer RM (2013) The effect of smoking on bone healing: a systematic review. Bone Joint Res 2(6):102–111. https://doi.org/10.1302/2046-3758.26.2000142
Sies H (1997) Oxidative stress: oxidants and antioxidants. Exp Physiol 82(2):291–295
Mody N, Parhami F, Sarafian TA, Demer LL (2001) Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med 31(4):509–519
Baek KH, Oh KW, Lee WY, Lee SS, Kim MK, Kwon HS, Rhee EJ, Han JH, Song KH, Cha BY, Lee KW, Kang MI (2010) Association of oxidative stress with postmenopausal osteoporosis and the effects of hydrogen peroxide on osteoclast formation in human bone marrow cell cultures. Calcif Tissue Int 87(3):226–235. https://doi.org/10.1007/s00223-010-9393-9
Domazetovic V, Marcucci G, Iantomasi T, Brandi ML, Vincenzini MT (2017) Oxidative stress in bone remodeling: role of antioxidants. Clin Cases Miner Bone Metab 14(2):209–216. https://doi.org/10.11138/ccmbm/2017.14.1.209 Epub 2017 Oct 25
Shuid AN, Mohamad S, Muhammad N, Fadzilah FM, Mokhtar SA, Mohamed N, Soelaiman IN (2011) Effects of α-tocopherol on the early phase of osteoporotic fracture healing. J Orthop Res 29(11):1732–1738. https://doi.org/10.1002/jor.21452
Yildirimturk S, Batu S, Alatli C, Olgac V, Firat D, Sirin Y (2016) The effects of supplemental melatonin administration on the healing of bone defects in streptozotocin-induced diabetic rats. J Appl Oral Sci 24(3):239–249. https://doi.org/10.1590/1678-775720150570
Volkmer DL, Sears B, Lauing KL, Nauer RK, Roper PM, Yong S, Stover M, Callaci JJ (2011) Antioxidant therapy attenuates deficient bone fracture repair associated with binge alcohol exposure. J Orthop Trauma 25(8):516–521. https://doi.org/10.1097/BOT.0b013e31821f65cc
Taguchi K, Motohashi H, Yamamoto M (2011) Molecular mechanisms of the Keap1-Nrf2 pathway in stress response and cancer evolution. Genes Cells 16:123–140. https://doi.org/10.1111/j.1365-2443.2010.01473.x
Wruck CJ, Gotz ME, Herdegen T, Varoga D, Brandenburg LO, Pufe T (2008) Kavalactones protect neural cells against amyloid beta peptide-induced neurotoxicity via extracellular signal-regulated kinase 1/2-dependent nuclear factor erythroid 2-related factor 2 activation. Mol Pharmacol 73:1785–1795. https://doi.org/10.1124/mol.107.042499
Wruck CJ, Claussen M, Fuhrmann G, Romer L, Schulz A, Pufe T, Waetzig V, Peipp M, Herdegen T, Gotz ME (2007) Luteolin protects rat PC12 and C6 cells against MPP + induced toxicity via an ERK dependent Keap1-Nrf2-ARE pathway. J Neural Transm Suppl 72:57–67
Al-Sawaf O, Clarner T, Fragoulis A, Kan YW, Pufe T, Streetz K, Wruck CJ (2015) Nrf2 in health and disease: current and future clinical implications. Clin Sci (Lond) 129(12):989–999. https://doi.org/10.1042/CS20150436
Lippross S, Beckmann R, Streubesand N, Ayub F, Tohidnezhad M, Campbell G, Kan YW, Horst F, Sönmez TT, Varoga D, Lichte P, Jahr H, Pufe T, Wruck CJ (2014) Nrf2 deficiency impairs fracture healing in mice. Calcif Tissue Int 95(4):349–361. https://doi.org/10.1007/s00223-014-9900-5
Sun YX, Li L, Corry KA, Zhang P, Yang Y, Himes E, Mihuti CL, Nelson C, Dai G, Li J (2015) Deletion of Nrf2 reduces skeletal mechanical properties and decreases load-driven bone formation. Bone 74C:1–9. https://doi.org/10.1016/j.bone.2014.12.066
Kanis JA, Johnell O, Oden A, Johansson H, De Laet C, Eisman JA, Fujiwara S, Kroger H, McCloskey EV, Mellstrom D, Melton LJ, Pols H, Reeve J, Silman A, Tenenhouse A (2004) Smoking and fracture risk: a meta-analysis. Osteoporos Int 16(2):155–162
Strotmeyer ES, Cauley JA (2007) Diabetes mellitus, bone mineral density, and fracture risk. Curr Opin Endocrinol Diabetes Obes 14(6):429–435. https://doi.org/10.1097/MED.0b013e3282f1cba3
Maggi S, Noale M, Giannini S, Adami S, Defeo D, Isaia G, Sinigaglia L, Filipponi P, Crepaldi G, ESOPO Study Group (2006) Quantitative heel ultrasound in a population-based study in Italy and its relationship with fracture history: the ESOPO study. Osteoporos Int 17:237–244
Cruel M, Granke M, Bosser C, Audran M, Hoc T (2017) Chronic alcohol abuse in men alters bone mechanical properties by affecting both tissue mechanical properties and microarchitectural parameters. Morphologie 101(333):88–96. https://doi.org/10.1016/j.morpho.2017.03.001
Marsell R, Einhorn TA (2011) The biology of fracture healing. Injury 42(6):551–555. https://doi.org/10.1016/j.injury.2011.03.031
Einhorn TA, Gerstenfeld LC (2015) Fracture healing: mechanisms and interventions. Nat Rev Rheumatol 11(1):45–54. https://doi.org/10.1038/nrrheum.2014.164
Simmons DJ (1985) Fracture healing perspectives. Clin Orthop 200:100–113
Andrew JG, Andrew SM, Freemont AJ, Marsh DR (1994) Inflammatory cells in normal human fracture healing. Acta Orthop Scand 65(4):462–466
Bolander ME (1992) Regulation of fracture repair by growth factors. Proc Soc Exp Biol Med 200(2):165–170
Schmid GJ, Kobayashi C, Sandell LJ, Ornitz DM (2009) Fibroblast growth factor expression during skeletal fracture healing in mice. Dev Dyn 238:766–774. https://doi.org/10.1002/dvdy.21882
Graham S, Leonidou A, Lester M, Heliotis M, Mantalaris A, Tsiridis E (2009) Investigating the role of PDGF as a potential drug therapy in bone formation and fracture healing. Expert Opin Investig Drugs 18(11):1633–1654. https://doi.org/10.1517/13543780903241607
Pufe T, Wildemann B, Petersen W, Mentlein R, Raschke M, Schmidmaier G (2002) Quantitative measurement of the splice variants 120 and 164 of the angiogenic peptide vascular endothelial growth factor in the time flow of fracture healing: a study in the rat. Cell Tissue Res 309:387–392
Keramaris NC, Calori GM, Nikolaou VS, Schemitsch EH, Giannoudis PV (2008) Fracture vascularity and bone healing: a systematic review of the role of VEGF. Injury 39(Suppl 2):S45–S57. https://doi.org/10.1016/S0020-1383(08)70015-9
Einhorn TA (1998) The cell and molecular biology of fracture healing. Clin Orthop Relat Res 355:S7–S21
Ortega N, Behonick DJ, Werb Z (2004) Matrix remodeling during endochondral ossifi cation. Trends Cell Biol 14:86–93
Lee FY, Choi YW, Behrens FF, DeFouw DO, Einhorn TA (1998) Programmed removal of chondrocytes during endochondral fracture healing. J Orthop Res 16(1):144–150
Vaananen HK, Zhao H, Mulari M, Halleen JM (2000) The cell biology of osteoclast function. J Cell Sci 113(Pt 3):377–381
Gerstenfeld LC, Cullinane DM, Barnes GL, Graves DT, Einhorn TA (2003) Fracture healing as a post-natal developmental process: molecular, spatial, and temporal aspects of its regulation. J Cell Biochem 88(5):873–884
Kular J, Tickner J, Chim SM, Xu J (2012) An overview of the regulation of bone remodelling at the cellular level. Clin Biochem 45(12):863–873. https://doi.org/10.1016/j.clinbiochem.2012.03.021
Carano RA, Filvaroff EH (2003) Angiogenesis and bone repair. Drug Discov Today 8(21):980–989
Zhang DD (2006) Mechanistic studies of the Nrf2-Keap1 signaling pathway. Drug Metab Rev 38(4):769–789
Nguyen T, Nioi P, Pickett CB (2009) The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284(20):13291–13295. https://doi.org/10.1074/jbc.R900010200
Canning P, Sorrell FJ, Bullock AN (2015) Structural basis of Keap1 interactions with Nrf2. Free Radic Biol Med 88(Pt B):101–107. https://doi.org/10.1016/j.freeradbiomed.2015.05.034
Fragoulis A, Laufs J, Müller S, Soppa U, Siegl S, Reiss LK, Tohidnezhad M, Rosen C, Tenbrock K, Varoga D, Lippross S, Pufe T, Wruck CJ (2012) Sulforaphane has opposing effects on TNF-alpha stimulated and unstimulated synoviocytes. Arthr Res Ther 14(5):R220. https://doi.org/10.1186/ar4059
Al-Sawaf O, Fragoulis A, Rosen C, Kan YW, Sönmez TT, Pufe T, Wruck CJ (2014) Nrf2 protects against TWEAK-mediated skeletal muscle wasting. Sci Rep 4:3625. https://doi.org/10.1038/srep03625
Al-Sawaf O, Fragoulis A, Rosen C, Keimes N, Liehn EA, Hölzle F, Kan YW, Pufe T, Sönmez TT, Wruck CJ (2014) Nrf2 augments skeletal muscle regeneration after ischaemia-reperfusion injury. J Pathol 234(4):538–547. https://doi.org/10.1002/path.4418
Fragoulis A, Schenkel J, Herzog M, Schellenberg T, Jahr H, Pufe T, Trautwein C, Kensler TW, Streetz KL, Wruck CJ (2019) Nrf2 ameliorates DDC-induced sclerosing cholangitis and biliary fibrosis and improves the regenerative capacity of the liver. Toxicol Sci 169:485. https://doi.org/10.1093/toxsci/kfz055
Fragoulis A, Siegl S, Fendt M, Jansen S, Soppa U, Brandenburg LO, Pufe T, Weis J, Wruck CJ (2017) Oral administration of methysticin improves cognitive deficits in a mouse model of Alzheimer’s disease. Redox Biol 12:843–853. https://doi.org/10.1016/j.redox.2017.04.024
Callaway DA, Jiang JX (2015) Reactive oxygen species and oxidative stress in osteoclastogenesis, skeletal aging and bone diseases. J Bone Miner Metab 33(4):359–370. https://doi.org/10.1007/s00774-015-0656-4
Kim KS, Choi HW, Yoon HE, Kim IY (2010) Reactive oxygen species generated by NADPH oxidase 2 and 4 are required for chondrogenic differentiation. J Biol Chem 285:40294–40302. https://doi.org/10.1074/jbc.M110.126821
Morita K, Miyamoto T, Fujita N, Kubota Y, Ito K, Takubo K, Miyamoto K, Ninomiya K, Suzuki T, Iwasaki R, Yagi M, Takaishi H, Toyama Y, Suda T (2007) Reactive oxygen species induce chondrocyte hypertrophy in endochondral ossification. J Exp Med 204:1613–1623
Manolagas SC, Parfitt AM (2010) What old means to bone. Trends Endocrinol Metab 21(6):369–374. https://doi.org/10.1016/j.tem.2010.01.010
Wauquier F, Leotoing L, Coxam V, Guicheux J, Wittrant Y (2009) Oxidative stress in bone remodelling and disease. Trends Mol Med 15(10):468–477. https://doi.org/10.1016/j.molmed.2009.08.004
Phillips AM (2005) Overview of the fracture healing cascade. Injury 36(Suppl. 3):55–57
Takahata Y, Takarada T, Iemata M, Yamamoto T, Nakamura Y, Kodama A, Yoneda Y (2009) Functional expression of beta2 adrenergic receptors responsible for protection against oxidative stress through promotion of glutathione synthesis after Nrf2 upregulation in undifferentiated mesenchymal C3H10T1/2 stem cells. J Cell Physiol 218(2):268–275. https://doi.org/10.1002/jcp.21594
Kim J-H, Singhal V, Biswal S, Thimmulappa RK, DiGirolamo DJ (2014) Nrf2 is required for normal postnatal bone acquisition in mice. Bone Res 2:14033. https://doi.org/10.1038/boneres.2014.33
Rana T, Schultz MA, Freeman ML, Biswas S (2012) Loss of Nrf2 accelerates ionizing radiation-induced bone loss by upregulating RANKL. Free Radic Biol Med 53(12):2298–2307. https://doi.org/10.1016/j.freeradbiomed.2012.10.536
Park CK, Lee Y, Kim KH, Lee ZH, Joo M, Kim HH (2014) Nrf2 is a novel regulator of bone acquisition. Bone 63:36–46. https://doi.org/10.1016/j.bone.2014.01.025
Hyeon S, Lee H, Yang Y, Jeong W (2013) Nrf2 deficiency induces oxidative stress and promotes RANKL-induced osteoclast differentiation. Free Radic Biol Med 65:789–799. https://doi.org/10.1016/j.freeradbiomed.2013.08.005
Sun YX, Xu AH, Yang Y, Li J (2015) Role of Nrf2 in bone metabolism. J Biomed Sci 22:101. https://doi.org/10.1186/s12929-015-0212-5
Ibáñez L, Ferrándiz ML, Brines R, Guede D, Cuadrado A, Alcaraz MJ (2014) Effects of Nrf2 deficiency on bone microarchitecture in an experimental model of osteoporosis. Oxid Med Cell Longev 2014:726590. https://doi.org/10.1155/2014/726590 Epub 2014 Jul 7
Hinoi E, Fujimori S, Wang L, Hojo H, Uno K, Yoneda Y (2006) Nrf2 negatively regulates osteoblast differentiation via interfering with Runx2-dependent transcriptional activation. J Biol Chem 281(26):18015–18024
Kanzaki H, Shinohara F, Kajiya M, Kodama T (2013) The Keap1/Nrf2 protein axis plays a role in osteoclast differentiation by regulating intracellular reactive oxygen species signaling. J Biol Chem 288(32):23009–23020. https://doi.org/10.1074/jbc.M113.478545
Yoshida E, Suzuki T, Morita M, Taguchi K, Tsuchida K, Motohashi H, Doita M, Yamamoto M (2018) Hyperactivation of Nrf2 leads to hypoplasia of bone in vivo. Genes Cells 23(5):386–392. https://doi.org/10.1111/gtc.12579
Wruck CJ, Fragoulis A, Gurzynski A, Brandenburg LO, Kan YW, Chan K, Hassenpflug J, Freitag-Wolf S, Varoga D, Lippross S, Pufe T (2011) Role of oxidative stress in rheumatoid arthritis: insights from the Nrf2-knockout mice. Ann Rheum Dis 70(5):844–850. https://doi.org/10.1136/ard.2010.132720
Weiss S, Zimmermann G, Pufe T, Varoga D, Henle P (2009) The systemic angiogenic response during bone healing. Arch Orthop Trauma Surg 129:989–997. https://doi.org/10.1007/s00402-008-0777-5
Mayr-Wohlfart U, Waltenberger J, Hausser H, Kessler S, Gunther KP, Dehio C, Puhl W, Brenner RE (2002) Vascular endothelial growth factor stimulates chemotactic migration of primary human osteoblasts. Bone 30:472–477
Street J, Bao M, deGuzman L, Bunting S, Peale FV, Ferrara N, Steinmetz H, Hoeffel J, Cleland JL, Daugherty A, van Bruggen N, Redmond HP, Carano RAD, Filvaroff EH (2002) Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci USA 99:9656–9661
Kweider N, Fragoulis A, Rosen C, Pecks U, Rath W, Pufe T, Wruck CJ (2011) Interplay between vascular endothelial growth factor (VEGF) and nuclear factor erythroid 2-related factor-2 (Nrf2): implications for preeclampsia. J Biol Chem 286:42863–42872. https://doi.org/10.1074/jbc.M111.286880
Ström O, Borgström F, Kanis JA, Compston J, Cooper C, McCloskey EV, Jönsson B (2011) Osteoporosis: burden, health care provision and opportunities in the EU. Arch Osteoporos 6:59–155. https://doi.org/10.1007/s11657-011-0060-1
Duryee MJ, Dusad A, Hunter CD, Kharbanda KK, Bruenjes JD, Easterling KC, Siebler JC, Thiele GM, Chakkalakal DA (2018) N-Acetyl cysteine treatment restores early phase fracture healing in ethanol-fed rats. Alcohol Clin Exp Res 42(7):1206–1216. https://doi.org/10.1111/acer.13765 Epub 2018 May 27
Roper PM, Abbasnia P, Vuchkovska A, Natoli RM, Callaci JJ (2016) Alcohol-related deficient fracture healing is associated with activation of FoxO transcription factors in mice. J Orthop Res 34(12):2106–2115. https://doi.org/10.1002/jor.23235
Authors/Task Force Members, Rydén L, Grant PJ, Anker SD, Berne C, Cosentino F, Danchin N, Deaton C, Escaned J, Hammes HP, Huikuri H, Marre M, Marx N, Mellbin L, Ostergren J, Patrono C, Seferovic P, Uva MS, Taskinen MR, Tendera M, Tuomilehto J, Valensi P, Zamorano JL, ESC Committee for Practice Guidelines (CPG), Zamorano JL, Achenbach S, Baumgartner H, Bax JJ, Bueno H, Dean V, Deaton C, Erol C, Fagard R, Ferrari R, Hasdai D, Hoes AW, Kirchhof P, Knuuti J, Kolh P, Lancellotti P, Linhart A, Nihoyannopoulos P, Piepoli MF, Ponikowski P, Sirnes PA, Tamargo JL, Tendera M, Torbicki A, Wijns W, Windecker S, Reviewers Document, De Backer G, Sirnes PA, Ezquerra EA, Avogaro A, Badimon L, Baranova E, Baumgartner H, Betteridge J, Ceriello A, Fagard R, Funck-Brentano C, Gulba DC, Hasdai D, Hoes AW, Kjekshus JK, Knuuti J, Kolh P, Lev E, Mueller C, Neyses L, Nilsson PM, Perk J, Ponikowski P, Reiner Z, Sattar N, Schächinger V, Scheen A, Schirmer H, Strömberg A, Sudzhaeva S, Tamargo JL, Viigimaa M, Vlachopoulos C, Xuereb RG (2013) ESC Guidelines on diabetes, pre-diabetes, and cardiovascular diseases developed in collaboration with the EASD: the Task Force on diabetes, pre-diabetes, and cardiovascular diseases of the European Society of Cardiology (ESC) and developed in collaboration with the European Association for the Study of Diabetes (EASD). Eur Heart J 34(39):3035–3087. https://doi.org/10.1093/eurheartj/eht108
American Diabetes Association (2014) Diagnosis and classification of diabetes mellitus. Diabetes Care 37(Suppl 1):S81–S90. https://doi.org/10.2337/dc14-S081
Marin C, Luyten FP, Van der Schueren B, Kerckhofs G, Vandamme K (2018) The impact of type 2 diabetes on bone fracture healing. Front Endocrinol (Lausanne) 9:6. https://doi.org/10.3389/fendo.2018.00006
Brown ML, Yukata K, Farnsworth CW, Chen DG, Awad H, Hilton MJ, O’Keefe RJ, Xing L, Mooney RA (2014) Zuscik MJ (2014) Delayed fracture healing and increased callus adiposity in a C57BL/6 J murine model of obesity-associated type 2 diabetes mellitus. PLoS ONE 9(6):e99656. https://doi.org/10.1371/journal.pone.0099656
Fujii H, Hamada Y, Fukagawa M (2008) Bone formation in spontaneously diabetic Torii-newly established model of non-obese type 2 diabetes rats. Bone 42(2):372–379
Waddington RJ, Alraies A, Colombo JS, Sloan AJ, Okazaki J, Moseley R (2011) Characterization of oxidative stress status during diabetic bone healing. Cells Tissues Organs 194(2–4):307–312. https://doi.org/10.1159/000324251
Machura P, Grymowicz M, Rudnicka E, Pięta W, Calik-Ksepka A, Skórska J, Smolarczyk R (2018) Premature ovarian insufficiency—hormone replacement therapy and management of long-term consequences. Prz Menopauzalny 17(3):135–138. https://doi.org/10.5114/pm.2018.78559
(2000) Osteoporosis prevention, diagnosis, and therapy. NIH Consens Statement 17:1–45
Lean JM, Davies JT, Fuller K, Jagger CJ, Kirstein B, Partington GA, Urry ZL, Chambers TJ (2003) A crucial role for thiol antioxidants in estrogen-deficiency bone loss. J Clin Invest 112(6):915–923
Muthusami S, Ramachandran I, Muthusamy B, Vasudevan G, Prabhu V, Subramaniam V, Jagadeesan A, Narasimhan S (2005) Ovariectomy induces oxidative stress and impairs bone antioxidant system in adult rats. Clin Chim Acta 360(1–2):81–86
Li H, Huang C, Zhu J, Gao K, Fang J, Li H (2018) Lutein suppresses oxidative stress and inflammation by Nrf2 activation in an osteoporosis rat model. Med Sci Monit 24:5071–5075. https://doi.org/10.12659/MSM.908699
Pellegrini GG, Cregor M, McAndrews K, Morales CC, McCabe LD, McCabe GP, Peacock M, Burr D, Weaver C, Bellido T (2017) Nrf2 regulates mass accrual and the antioxidant endogenous response in bone differently depending on the sex and age. PLoS ONE 12(2):e0171161. https://doi.org/10.1371/journal.pone.0171161
Natoli RM, Yu H, Meislin MC, Abbasnia P, Roper P, Vuchkovska A, Xiao X, Stock SR, Callaci JJ (2018) Alcohol exposure decreases osteopontin expression during fracture healing and osteopontin-mediated mesenchymal stem cell migration in vitro. J Orthop Surg Res 13(1):101. https://doi.org/10.1186/s13018-018-0800-7
Chakkalakal DA (2005) Alcohol-induced bone loss and deficient bone repair. Alcohol Clin Exp Res 29(12):2077–2090
Chakkalakal DA, Novak JR, Fritz ED, Mollner TJ, McVicker DL, Garvin KL, McGuire MH, Donohue TM (2005) Inhibition of bone repair in a rat model for chronic and excessive alcohol consumption. Alcohol 36(3):201–214
Trevisiol CH, Turner RT, Pfaff JE, Hunter JC, Menagh PJ, Hardin K, Ho E, Iwaniec UT (2007) Impaired osteoinduction in a rat model for chronic alcohol abuse. Bone 41(2):175–180
Callaci JJ, Juknelis D, Patwardhan A, Sartori M, Frost N, Wezeman FH (2004) The effects of binge alcohol exposure on bone resorption and biomechanical and structural properties are offset by concurrent bisphosphonate treatment. Alcoholism 28(1):182–191
Broulik PD, Rosenkrancova J, Ruzicka P, Sedlacek R, Zima T (2009) The effect of chronic alcohol administration on bone mineral content and bone strength in male rats. Physiol Res 59(4):599–604
Elmali N, Ertem K, Ozen S, Inan M, Baysal T, Güner G, Bora A (2002) Fracture healing and bone mass in rats fed on liquid diet containing ethanol. Alcohol Clin Exp Res 26:509–513
Lauing K, Himes R, Rachwalski M, Strotman P, Callaci JJ (2008) Binge alcohol treatment of adolescent rats followed by alcohol abstinence is associated with site-specific differences in bone loss and incomplete recovery of bone mass and strength. Alcohol 42(8):649–656. https://doi.org/10.1016/j.alcohol.2008.08.005
Rothem DE, Rothem L, Soudry M, Dahan A, Eliakim R (2009) Nicotine modulates bone metabolism-associated gene expression in osteoblast cells. J Bone Miner Metab 27(5):555–561. https://doi.org/10.1007/s00774-009-0075-5
Santiago HA, Zamarioli A, Sousa Neto MD, Volpon JB (2017) Exposure to secondhand smoke impairs fracture healing in rats. Clin Orthop Relat Res 475(3):894–902. https://doi.org/10.1007/s11999-016-5184-6
Akhter MP, Lund AD, Gairola CG (2005) Bone biomechanical property deterioration due to tobacco smoke exposure. Calcif Tissue Int 77(5):319–326
Ueng SW, Lin SS, Wang CR, Liu SJ, Tai CL, Shih CH (1999) Bone healing of tibial lengthening is delayed by cigarette smoking: study of bone mineral density and torsional strength on rabbits. J Trauma 46(1):110–115
Aspera-Werz RH, Ehnert S, Heid D, Zhu S, Chen T, Braun B, Sreekumar V, Arnscheidt C, Nussler AK (2018) Nicotine and cotinine inhibit catalase and glutathione reductase activity contributing to the impaired osteogenesis of SCP-1 cells exposed to cigarette smoke. Oxid Med Cell Longev 2018:3172480. https://doi.org/10.1155/2018/3172480
Author information
Authors and Affiliations
Contributions
YK and HJ designed the study and prepared the first draft of the paper. They are guarantors. CW and TP did the literature search and recommended the papers to be evaluated. All authors contributed in reading and writing sections of the review and all authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflict of interest
Yusuke Kubo, Christoph Jan Wruck, Athanassios Fragoulis, Wolf Drescher, Hans Christoph Pape, Philipp Lichte, Horst Fischer, Mersedeh Tohidnezhad, Frank Hildebrand, Thomas Pufe, and Holger Jahr declare that they have no competing interest.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Kubo, Y., Wruck, C.J., Fragoulis, A. et al. Role of Nrf2 in Fracture Healing: Clinical Aspects of Oxidative Stress. Calcif Tissue Int 105, 341–352 (2019). https://doi.org/10.1007/s00223-019-00576-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-019-00576-3