Abstract
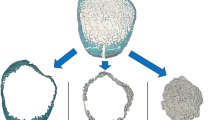
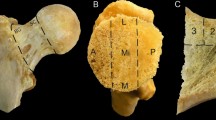
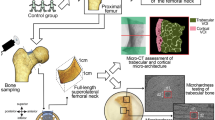
The femoral neck (FN) has been previously characterized by thinner cortices in osteoporotic fracture (HF) when compared to hip osteoarthritis (HOA). The purposes of this study were to complete the previous investigations on FNs from HF and HOA by analyzing the complexity of the cortical structure and to approach the intrinsic properties of cortical bone by assessing the collagen crosslink contents. FN samples were obtained during arthroplasty in 35 postmenopausal women (HF; n = 17; mean age 79 ± 2 years; HOA; n = 18; mean age 66 ± 2 years). The cortical fractal dimension (Ct.FD) and lacunarity (Ct.Lac) derived from high-resolution peripheral quantitative tomography (isotropic voxel size: 82 μm) images of FN by using Ctan software and Fraclac running in ImageJ were analyzed. The collagen crosslinks content [pyridinoline, deoxypyridinoline, pentosidine (PEN)] were assessed in cortical bone. Ct.FD was significantly lower (p < 0.0001) in HF than HOA reflecting a decreased complexity and was correlated to the age and BMD. In two sub-groups, BMD- and age-matched, respectively, Ct.FD remained significantly lower in HF than HOA (p < 0.001). Ct.Lac was not different between HF and HOA. PEN content was two times higher in HF than HOA (p < 0.0001) independently of age. In conclusion, FN with HF was characterized by a less complex cortical texture and higher PEN content than HOA. In addition to the decreased bone mass and BMD previously reported, these modifications contribute to the lower bone quality in HF than HOA in postmenopausal women.

Similar content being viewed by others
References
Bouxsein ML (2001) Biomechanics of age-related fractures. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis, vol 1, 2nd edn. Academic Press, San Diego, pp 509–531
Lotz JC, Cheal EJ, Hayes WC (1995) Stress distributions within the proximal femur during gait and falls: Implications for osteoporotic fracture. Osteoporos Int 5:252–261. https://doi.org/10.1007/BF01774015
Seeman E, Delmas PD (2006) Bone quality—the material and structural basis of bone strength and fragility. N Engl J Med 354:2250–2261. https://doi.org/10.1056/NEJMra053077
Chavassieux P, Seeman E, Delmas PD (2007) Insights into material and structural basis of bone fragility from diseases associated with fractures: how determinants of the biomechanical properties of bone are compromised by disease. Endocr Rev 28:151–164. https://doi.org/10.1210/er.2006-0029
World Health Organisation (1994) Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Report of a WHO Study Group. Tech Rep Ser 843:1–129
Blain H, Chavassieux P, Portero-Muzy N et al (2008) Cortical and trabecular bone distribution in the femoral neck in osteoporosis and osteoarthritis. Bone 43:862–868. https://doi.org/10.1016/j.bone.2008.07.236
Boutroy S, Vilayphiou N, Roux JP et al (2011) Comparison of 2D and 3D bone microarchitecture evaluation at the femoral neck, among postmenopausal women with hip fracture or hip osteoarthritis. Bone 49:1055–1061. https://doi.org/10.1016/j.bone.2011.07.037
Bala Y, Lefèvre E, Roux JP et al (2016) Pore network microarchitecture influences human cortical bone elasticity during growth and aging. J Mech Behav Biomed Mater 63:164–173. https://doi.org/10.1016/j.jmbbm.2016.05.018
Tjong W, Nirody J, Burghardt AJ et al (2014) Structural analysis of cortical porosity applied to HR-pQCT data. Med Phys 41:13701. https://doi.org/10.1118/1.4851575
Majumdar S, Lin J, Link T et al (1999) Fractal analysis of radiographs: assessment of trabecular bone structure and prediction of elastic modulus and strength. Med Phys 26:1330–1340. https://doi.org/10.1118/1.598628
Gudea A, Stefan A (2013) Histomorphometric, fractal and lacunarity comparative analysis of sheep (Ovis aries), goat (Capra hircus) and roe deer (Capreollus capreollus) compact bone samples. Folia Morphol (Warsz) 72:239–248. https://doi.org/10.5603/FM.2013.0039
De Melo RHC, Conci A (2013) How Succolarity could be used as another fractal measure in image analysis. Telecommun Syst 52:1643–1655. https://doi.org/10.1007/s11235-011-9657-3
Dong P (2000) Lacunarity for spatial heterogeneity measurement in GIS. Geogr Inf Sci 6:20–26. https://doi.org/10.1080/10824000009480530
Sanchez-Molina D, Velazquez-Ameijide J, Quintana V et al (2013) Fractal dimension and mechanical properties of human cortical bone. Med Eng Phys 35:576–582. https://doi.org/10.1016/j.medengphy.2012.06.024
Bauer JS, Kohlmann S, Eckstein F et al (2006) Structural analysis of trabecular bone of the proximal femur using multislice computed tomography: a comparison with dual X-ray absorptiometry for predicting biomechanical strength in vitro. Calcif Tissue Int 78:78–89. https://doi.org/10.1007/s00223-005-0070-3
Viguet-Carrin S, Garnero P, Delmas PD (2006) The role of collagen in bone strength. Osteoporos Int 17(3):319–336. https://doi.org/10.1007/s00198-005-2035-9
Saito M, Marumo K (2015) Effects of collagen crosslinking on bone material properties in health and disease. Calcif Tissue Int 97(3):242–261. https://doi.org/10.1007/s00223-015-9985-5
Karim L, Vashishth D (2012) Heterogeneous glycation of cancellous bone and its association with bone quality and fragility. PLoS ONE 7:e35047. https://doi.org/10.1371/journal.pone.0035047
Viguet-Carrin S, Follet H, Gineyts E et al (2010) Association between collagen cross-links and trabecular microarchitecture properties of human vertebral bone. Bone 46:342–347. https://doi.org/10.1016/j.bone.2009.10.001
De Laet C, Reeve J (2001) Epidemiology of osteoporotic fractures in Europe. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis, vol 1, 2nd edn. Academic Press, San Diego, pp 585–597
Greendale GA, Barrett-Connor E (2001) Outcomes of osteoporotic fractures. In: Marcus R, Feldman D, Kelsey J (eds) Osteoporosis, vol 1, 2nd edn. Academic Press, San Diego, CA, pp 819–829
Smith TG, Lange GD, Marks WB (1996) Fractal methods and results in cellular morphology—dimensions, lacunarity and multifractals. J Neurosci Methods 69:123–136. https://doi.org/10.1016/S0165-0270(96)00080-5
Melo RHC, Vieira ED, Conci A (2006) Characterizing the lacunarity of objects and image sets and its use as a technique for the analysis of textural patterns. Adv Concepts Intell Vis Syst Proc 4179:208–219. https://doi.org/10.1007/11864349
Karperien AL, Jelinek HF (2015) Fractal, multifractal, and lacunarity analysis of microglia in tissue engineering. Front Bioeng Biotechnol 3:51. https://doi.org/10.3389/fbioe.2015.00051
Viguet-Carrin S, Gineyts E, Bertholon C, Delmas PD (2009) Simple and sensitive method for quantification of fluorescent enzymatic mature and senescent crosslinks of collagen in bone hydrolysate using single-column high performance liquid chromatography. J Chromatogr B Analyt Technol Biomed Life Sci 877:1–7. https://doi.org/10.1016/j.jchromb.2008.10.043
Link TM, Majumdar S, Lin JC et al (1998) Assessment of trabecular structure using high resolution CT images and texture analysis. J Comput Assist Tomogr 22:15–24
Topoliński T, Mazurkiewicz A, Jung S et al (2012) Microarchitecture parameters describe bone structure and its strength better than BMD. ScientificWorldJournal 2012:502781. https://doi.org/10.1100/2012/502781
Weinstein RS, Majumdar S (1994) Fractal geometry and vertebral compression fractures. J Bone Miner Res 9:1797–1802. https://doi.org/10.1002/jbmr.5650091117
Zebaze R, Seeman E (2015) Cortical bone: a challenging geography. J Bone Miner Res 30:24–29. https://doi.org/10.1002/jbmr.2419
Bernhard A, Milovanovic P, Zimmermann EA et al (2013) Micro-morphological properties of osteons reveal changes in cortical bone stability during aging, osteoporosis, and bisphosphonate treatment in women. Osteoporos Int 24:2671–2680. https://doi.org/10.1007/s00198-013-2374-x
Saito M, Fujii K, Soshi S, Tanaka T (2006) Reductions in degree of mineralization and enzymatic collagen cross-links and increases in glycation-induced pentosidine in the femoral neck cortex in cases of femoral neck fracture. Osteoporos Int 17:986–995. https://doi.org/10.1007/s00198-006-0087-0
Vashishth D, Gibson GJ, Khoury JI et al (2001) Influence of nonenzymatic glycation on biomechanical properties of cortical bone. Bone 28:195–201. https://doi.org/10.1016/S8756-3282(00)00434-8
Hernandez CJ, Tang SY, Baumbach BM et al (2005) Trabecular microfracture and the influence of pyridinium and non-enzymatic glycation-mediated collagen cross-links. Bone 37:825–832. https://doi.org/10.1016/j.bone.2005.07.019
Sroga GE, Wu PC, Vashishth D (2015) Insulin-like growth factor 1, glycation and bone fragility: Implications for fracture resistance of bone. PLoS ONE 10:e0117240. https://doi.org/10.1371/journal.pone.0117046
Issever AS, Link TM, Kentenich M et al (2010) Assessment of trabecular bone structure using MDCT: comparison of 64- and 320-slice CT using HR-pQCT as the reference standard. Eur Radiol 20:458–468. https://doi.org/10.1007/s00330-009-1571-7
Issever AS, Link TM, Kentenich M et al (2009) Trabecular bone structure analysis in the osteoporotic spine using a clinical in vivo setup for 64-slice MDCT imaging: comparison to microCT imaging and microFE modeling. J Bone Miner Res 24:1628–1637. https://doi.org/10.1359/JBMR.090311
Berteau JP, Gineyts E, Pithioux M et al (2015) Ratio between mature and immature enzymatic cross-links correlates with post-yield cortical bone behavior: an insight into greenstick fractures of the child fibula. Bone 79:190–195. https://doi.org/10.1016/j.bone.2015.05.045
Acknowledgements
The author Gustavo Davi Rabelo thanks the “Ciência sem Fronteiras - Conselho Nacional de Desenvolvimento Científico e Tecnológico/Brasil” (Processo número 245336/2012-5) for the Post-doc scholarship.
Author information
Authors and Affiliations
Contributions
GDR and PC designed the study and were evolved in all phases of the study and in writing the manuscript. JPR, NPM, and EG contributed to the experimental work and drafting the results. GDR, JPR, and PC were responsible for statistical analysis of the data. RC was responsible for the discussion of the results and supervision. All authors revised the paper critically for intellectual content and approved the final version. All authors agree to be accountable for the work and to ensure that any questions relating to the accuracy and integrity of the paper are investigated and properly resolved.
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Rights and permissions
About this article
Cite this article
Rabelo, G.D., Roux, JP., Portero-Muzy, N. et al. Cortical Fractal Analysis and Collagen Crosslinks Content in Femoral Neck After Osteoporotic Fracture in Postmenopausal Women: Comparison with Osteoarthritis. Calcif Tissue Int 102, 644–650 (2018). https://doi.org/10.1007/s00223-017-0378-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0378-9