Abstract
Individuals with diabetes mellitus type 2 (T2DM) have approximately 30% increased risk of hip fracture; however, the main cause of the elevated fracture risk in those subjects remains unclear. Moreover, micromechanical and microarchitectural properties of the superolateral femoral neck—the common fracture-initiating site—are still unknown. We collected proximal femora of 16 men (eight with T2DM and eight controls; age: 61 ± 10 years) at autopsy. After performing post-mortem bone densitometry (DXA), the superolateral neck was excised and scanned with microcomputed tomography (microCT). We also conducted Vickers microindentation testing. T2DM and control subjects did not differ in age (p = 0.605), body mass index (p = 0.114), and femoral neck bone mineral density (BMD) (p = 0.841). Cortical porosity (Ct.Po) was higher and cortical thickness (Ct.Th) was lower in T2DM (p = 0.044, p = 0.007, respectively). Of trabecular microarchitectural parameters, only structure model index (p = 0.022) was significantly different between T2DM subjects and controls. Control group showed higher cortical (p = 0.002) and trabecular bone microhardness (p = 0.005). Increased Ct.Po and decreased Ct.Th in T2DM subjects increase the propensity to femoral neck fracture. Apart from the deteriorated cortical microarchitecture, decreased cortical and trabecular microhardness suggests altered bone composition of the superolateral femoral neck cortex and trabeculae in T2DM. Significantly deteriorated cortical microarchitecture of the superolateral femoral neck is not recognized by standard DXA measurement of the femoral neck.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
In 2017, it was estimated that 6.28% of the world population had diabetes mellitus (DM), and over one million deaths were associated with DM [1]. If the current trends remain, it is expected that more than 10% of the global population will have DM by 2030 [2]. Individuals with type 2 diabetes mellitus (T2DM) have a 1.34-fold higher risk of hip fracture [3]; however, it remains unknown how T2DM increases the fracture risk.
Bone microarchitecture of individuals with T2DM was broadly studied in previous two decades. When scientific community has become aware of the increased fracture risk in T2DM individuals, one more challenge appeared. Namely, it has been shown that dual-energy X-ray absorptiometry (DXA) is not a sufficiently reliable tool to predict the fracture risk in T2DM since bone mineral density (BMD) has often been reported to be equally good [4] as in controls or even higher [5]. Since BMD does not reliably predict the fracture risk, examination of bone microarchitecture was a natural approach to further understand the origins of the increased fracture risk in T2DM. High-resolution peripheral quantitative computed tomography (HR-pQCT) has frequently been used for the assessment of bone microarchitecture at distal tibia and radius [5,6,7,8,9]. While increased cortical porosity in T2DM is often found in these clinical studies of bone microarchitecture, this is not a consistent finding, particularly in T2DM subjects without microvascular disease [5, 7]. Another issue is that tibia, which is highly accessible for examination by HR-pQCT, is not a typical fracture site in T2DM subjects [10]. As the femoral neck microarchitecture can only be crudely examined by conventional computed tomography [11], the majority of studies used bone samples for microcomputed tomography (microCT) assessment [12,13,14,15]. However, the available microCT studies that examined the femoral neck have evaluated trabecular [12, 14, 15] or cortical bone of the inferomedial femoral neck [12]. The femoral head trabecular core [12, 13], which has also been often examined, represents a distal continuation of the inferomedial neck trabeculae. However, trabeculae of the inferomedial femoral neck have different aging-related changes [16] and fracture patterns [17] compared with trabeculae of the superolateral neck. Therefore, it is crucial to investigate cortex and trabeculae of the superolateral femoral neck, particularly considering that the superolateral femoral neck is a site where a hip fracture usually starts.
Mechanical testing techniques such as and OsteoProbe indentation and Vickers microindentation may offer information about bone micromechanical properties, which are largely related to bone matrix composition. Bone matrix is a highly complex environment, which is mainly composed of collagen fibers; however, many other proteins are also present, such as protein S [18], osteocalcin, osteopontin, bone sialoprotein, and others [19]. Even a subtle imbalance in any of the components, which could be induced by DM, may lead to deteriorated matrix composition, resulting in reduced bone capability to resist fracture. If DM is able to induce changes in bone matrix, it is expected that they would be possible to detect by microindentation measurements, which may evaluate some aspects of bone quality. According to Khosla et al., results of clinical studies have revealed that two most consistent impairments of bone quality in patients with T2DM are altered bone material properties and increased cortical porosity [20, 21]. By using OsteoProbe, Holloway-Kew et al. have recently shown that individuals with T2DM have a lower BMSi compared with non-DM subjects (individuals with normoglycemia and those with impaired fasting glucose), but femoral neck BMD did not differ between the groups [4]. Equal or higher femoral BMD in T2DM individuals is a common finding in BMD studies [4, 8]. The reduced BMSi in individuals with T2DM has been confirmed by other studies [6], definitely linking the increased bone fragility in T2DM individuals with the impaired bone material properties. In contrast, Samakkarnthai et al. did not find differences in BMSi between T2DM and controls [5]. However, all of the studies that examined BMSi in T2DM individuals have used a clinical microindentation testing device and have focused exclusively on the mid-shaft of tibia [4, 5]. As a laboratory method, Vickers microindentation can be applied on bone specimens, which allows for the examination of clinically more relevant skeletal sites that would otherwise be inaccessible to OsteoProbe-based indentation in a clinical context. While Vickers microindentation has not yet been applied in bone specimens of individuals with diabetes, the technique has been successfully used to probe iliac bone biopsies, showing reduced microhardness in osteoporosis compared with control subjects [22].
Nevertheless, typical fractures in T2DM individuals are actually fractures of the hip and proximal humerus [10]. To date, one study has reported lower hardness of the femoral neck trabeculae in T2DM [15]; however, it remains uninvestigated whether hardness of the superolateral femoral neck—a common fracture-originating site [23]—is lower in T2DM subjects than in non-DM subjects.
The aim of this study was to determine the origins of the increased fracture risk in older individuals with T2DM by analyzing bone microarchitecture and microhardness of the superolateral femoral neck trabecular and cortical compartments in T2DM subjects and age- and sex-matched controls.
Materials and Methods
Sample Selection and Preparation
Sixteen proximal femora were obtained postmortem from male donors at the Institute of Forensic Medicine in Belgrade. Eight samples belonged to individuals who had received diagnosis of T2DM prior to death, and eight belonged to control subjects without DM. The age range of the included individuals was 47–83 years, and the mean age was 61 ± 10.41 years. The principal causes of death, as obtained from autopsy reports, included diabetic ketoacidosis, acute cardiac failure, stroke or cerebral hemorrhage, motor vehicle accidents, other sudden traumatic injuries, or natural death. None of the individuals had a history of a bone fracture, metabolic bone disease, chronic liver disease, or cancer.
The inclusion criteria for the T2DM group were as follows: (1) T2DM diagnosed prior to death; (2) no positive history of hip fracture. The exclusion criteria were based on the autopsy reports, and they included the presence of the following: (1) rheumatoid arthritis; (2) inborn skeletal anomalies; (3) chronic kidney or liver disease; (4) any type of malignancy in the moment of death; (5) acromegaly, hypo- or hyperparathyroidism; (6) known treatment with bisphosphonates, glucocorticoids, anticonvulsants, or antipsychotics; (7) hip osteoarthritis; (8) alcoholism. The inclusion criteria for the control group were as follows: (1) absence of T1DM or T2DM; ii) no history of a hip fracture. The exclusion criteria were the same as those for the T2DM group.
Using a water-cooled low-speed diamond saw, we separated the superolateral neck from the rest of the specimen with several precise cuts, as described previously [24]. The approval for the collection of the sample was granted by the institutional Ethics Committee.
DXA Measurements
We conducted ex vivo DXA scanning on 14 out of 16 partially excised proximal femora (Fig. 1) using Hologic QDR 1000/W DXA apparatus (Hologic, USA). Following previous recommendations [25], these bony samples were water-immersed during scanning to simulate the effects of surrounding soft tissues. The assessment of areal bone mineral density (BMD, g/cm2) of the femoral neck was done using built-in APEX software (version 2.0, Hologic, USA). In addition, we used hip structure analysis (HSA) plug-in to estimate cross-sectional area (CSA; cm2), cross-sectional moment of inertia (CSMI; cm4), buckling ratio (BR, dimensionless), cortical thickness (CTh.n, mm), and section modulus (Zn, cm3) in the standardized region of interest (narrow neck—located at the thinnest part of the femoral neck).
MicroCT
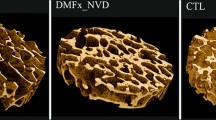
We scanned all of the specimens using a Skyscan 1172 micro-computed tomography system (Bruker microCT, Skyscan, Belgium) under the scanning conditions and parameters previously established: 80 kV, 124 µA, 1200 ms exposure time, aluminum and copper filter, 2 K camera binning, 10 µm voxel size (isotropic), rotation step of 0.40 degrees, and triple frame averaging [24]. The reconstruction of the projection images obtained during the scanning was performed in NRecon software (Bruker microCT, Belgium) on InstaRecon platform (InstaRecon, USA) with suitable thermal drift correction, misalignment compensation, Gaussian smoothing of 3, and appropriate ring artifact and beam hardening corrections. To ensure representativeness of the evaluated bone volume, analyze consistent bone region, and avoid inter-site differences along the superolateral femoral neck [24], we analyzed the middle 60% of the superolateral femoral neck. Namely, if the length of the superolateral femoral neck parallel to longitudinal axis was about 3 cm, in this study we evaluated trabecular and cortical microarchitecture of about 1.8 cm. Volumes of interest (VOIs) were manually marked for cortex and trabeculae separately (Fig. 1). Each VOI included about 1800 slices per sample (middle 60% of the superolateral neck).
The following cortical and trabecular parameters were evaluated using automatic 3D analysis in Ct.An (Skyscan, ver. 1.16.4.1): cortical thickness (Ct.Th, mm), cortical porosity (Ct.Po); trabecular bone volume fraction (BV/TV, %), trabecular number (Tb.N, 1/mm), trabecular thickness (Tb.Th, mm), trabecular separation (Tb.Sp, mm), structure model index (SMI, dimensionless), connectivity density(Conn.D, 1/mm3), fractal dimension (FD), and degree of anisotropy (DA, dimensionless).
Microindentation Testing
To assess tissue-level micromechanical properties of cortical and trabecular bone, we used Vickers microhardness tester (HMV-G version, Schimadzu, Japan). Measurements were conducted under the previously established conditions for microhardness measurement [25] with the load of 50 g and 12 s of indentation time for both the cortical and trabecular regions (Fig. 1). In order to prepare specimens for microindentation testing, all of the samples were embedded in resin and polished. In each specimen, using a 40 × magnifying lens, we made five separate and valid measurements of the cortical compartment and five measurements of the trabecular compartment, and average values were used for intergroup comparisons. For both the cortical and the trabecular compartments, we ensured that the indentations were at least 2.5 diagonals from the margins to avoid boundary effects and that there were at least 2.5 diagonals between adjacent indentations to avoid overlapping of artificial depressions from one indentation to another [26]. The measurements were performed by two researchers independently of each other, and the average value was taken for the analysis.
Statistical Analysis
The Kolmogorov–Smirnov test was used to verify that all of the measured parameters complied with normal distribution. The t test for independent samples was used to check for differences in age, BMI, femoral neck BMD, geometric parameters, microarchitectural bone parameters, and microhardness between the investigated groups (T2DM and controls). To account for possible effects of age and BMI, we conducted analysis of covariance (ANCOVA) for the examined cortical parameters. Linear regression analysis was used to examine the associations between cortical microhardness and cortical porosity. All analyses were performed two-tailed in SPSS software ver. 15 at the significance level of 0.05. The effect size was calculated for each of the microCT parameters in accordance with the guidelines provided by Joseph A. Durlak [27]; we used the Effect Size Calculator for T-Test (https://www.socscistatistics.com/effectsize/default3.aspx).
Results
T2DM and control subjects did not differ in age (p = 0.605) or BMI (p = 0.114). Femoral neck BMD did not differ between the examined groups either (p = 0.841). CSAn, BR, CSMI, CTh.n, and Zn also did not differ between the investigated groups (p = 0.357, p = 0.465, p = 0.140, p = 0.722, and p = 0.274, respectively) (Table 1).
Cortical Microarchitecture and Microindentation
We found that Ct.Po was significantly higher in T2DM subjects compared with controls (p = 0.044). Moreover, Ct.Th was significantly lower in T2DM than in control individuals (p = 0.007) (Table 2). After adjusting for BMI and age, the significant intergroup difference in Ct.Po was lost, but P-values were still close to significance (p = 0.090). Even after adjusting for age and BMI, Ct.Th remained significantly lower in T2DM (p = 0.032). Microindentation testing revealed that control specimens had higher cortical bone microhardness compared with T2DM specimens (p = 0.002) (Fig. 2).
We next used linear regression analysis to examine the associations of cortical microhardness and cortical porosity, and showed that cortical microhardness negatively correlated with Co.Po (r = − 0.6, p = 0.039).
Trabecular Microarchitecture and Microindentation
Majority of trabecular microarchitectural parameters were not significantly different between T2DM and control groups. Specifically, BV/TV, Tb.N, Tb.Th, Tb.Sp, DA, FD, and Conn.D did not vary between the groups (p = 0.695, p = 0.581, p = 0.930, p = 0.234, p = 0.355, p = 0.516, and p = 0.124, respectively) (Table 2). Only SMI was significantly higher in controls than in T2DM group (p = 0.022). Control specimens had higher trabecular bone microhardness than T2DM specimens (p = 0.005) (Fig. 2).
Discussion
To the best of our knowledge, this is the first evaluation of bone micromechanical properties and microarchitecture of cortical and trabecular compartments of the superolateral femoral neck in individuals with T2DM. Investigations of the superolateral femoral neck are particularly relevant considering that simulations of a sideways fall in fresh frozen human cadaveric femora unequivocally confirmed that a fracture most often initiated at the superolateral femoral neck [28]. Therefore, it is essential to evaluate T2DM effects at this region of the skeleton so as to better understand the basis for the increased hip fracture risk in T2DM. We found that individuals with T2DM have impaired bone micromechanical properties of cortical and trabecular compartments of the superolateral neck, coupled with deteriorated cortical microarchitecture, indicating the microstructural origins of an increased fracture risk in subjects with T2DM. However, none of the DXA and HSA parameters differed between the examined groups. Indeed, bone fractures occur when the applied force exceeds bone strength. Therefore, apart from the magnitude and direction of a force, bone intrinsic characteristics are essential to understand the individual’s bone fracture risk. Two fundamental components of cortical bone strength are cortical thickness and cortical porosity [29, 30]. For example, in a CT-based finite element study based on 44 right femora from female individuals, Endo et al. showed that the reduced cortical bone thickness caused powerful stresses and strains, finally resulting in an increased risk of hip fracture [31]. Also, two HR-pQCT studies conducted by Sundh et al. emphasized that increased cortical porosity was associated with a higher prevalence of multiple osteoporotic fractures in men [32] and women [33]. Hence, our findings reflect an increased fracture risk in individuals with T2DM.
Recently, Wölfel et al. have analyzed hardness of the femoral mid-shaft cortex in T2DM, high-porosity T2DM, and control groups [34]. Those subjects were older than our subjects and included both sexes; however, nanoindentation analyses did not reveal any variations in hardness between the groups. Even the group consisting of T2DM subjects with high Ct.Po did not differ in nanohardness from the other two groups [34], which may indicate that the deterioration of bone microarchitecture is independent of changes in nanostructure. Recently, Holloway-Kew et al. have speculated that the assessment of bone material properties is better than DXA for bone quality evaluation in T2DM individuals; their hypothesis was based on the fact that in their study on 340 men, no differences in femoral neck BMD were found, while distal tibia BMSi was lower in T2DM individuals than in non-DM group [4]. Similar to Holloway-Kew et al., we also analyzed only male subjects, and found that femoral neck BMD was similar between the investigated groups; in our study, microhardness was reduced in T2DM, but we examined the femoral neck of individuals with T2DM, whereas Holloway-Kew et al. investigated the distal tibia [4]. Samakkarnthai et al. conducted a series of analyses, including measuring BMSi (using microindentation of the distal tibia), measurement of skin advanced glycation end-products (AGEs) (using autofluorescence), and HR-pQCT of the distal radius and tibia, in 171 T2DM patients and 108 age-matched non-DM subjects [5]. However, they showed that BMSi of the distal tibia did not vary between the investigated groups. Samakkarnthai et al. also showed that T2DM subjects with vascular complications had a significantly more porous cortex of the distal tibia compared with controls, whereas the presence of vascular complications did not affect BMSi [5]. Farr et al. conducted a study on BMSi and microarchitecture of the distal tibia and radius in 60 postmenopausal women; they found that T2DM patients had a significantly lower BMSi than controls, which was coupled with a higher cortical thickness of the distal radius and tibia. Farr et al. also found improved trabecular microarchitecture of the distal radius in T2DM individuals, before adjusting for BMI. However, after adjusting the data for BMI, Tb.N was no longer higher and Tb.Sp was not lower in T2DM subjects [21]. Nilsson et al. performed a cross-sectional study that assessed microarchitecture of the distal tibia and radius in 99 women with T2DM and 954 control subjects [6]. They reported a significantly higher trabecular BV/TV in T2DM group at both skeletal sites. While Ct.Po in the tibia did not differ between the groups, Ct.Po of the radius was either better (at a more proximal site) or worse (at a standard site) in T2DM compared with controls [6]. Finite element analysis revealed significantly higher failure loads in T2DM than in control group [6], and BMSi by reference point indentation was lower in T2DM [6]. By using microCT, Karim et al. analyzed trabecular microarchitecture of the femoral core of the head in 20 T2DM subjects undergoing elective total hip replacement surgery and 33 non-DM controls [12]. Trabecular microarchitecture did not differ between the groups for any of the examined parameters [12]. The authors also found that T2DM group demonstrated impaired cortical bone biomechanical properties, as evidenced by some cyclic reference point indentation properties [12]. Our findings regarding the femoral neck trabecular microarchitecture in T2DM subjects are mostly in agreement with previous studies [13]. Namely, previous studies that examined trabecular microarchitecture of the femoral head (trabecular cores that are actually derived from inferomedial trabeculae of the femoral neck) did not find significant intergroup differences for any of the investigated parameters [12,13,14]. However, Sihota et al. reported deteriorated trabecular microarchitecture of the femoral head (lower BV/TV, Tb.N, and Tb.Th; higher SMI and Tb.Sp) as well as lower modulus and hardness obtained by nanoindentation testing [15]. This could be partly explained by the choice of the examination site given that Sihota et al. examined trabeculae from subchondral compartment of the femoral head—the compartment located on the opposite part of the femoral head than the trabecular cores examined by the previously mentioned studies [12,13,14]. To summarize, regardless of the used methodology (microCT or pQCT), the results of the previous studies that examined bone microarchitecture (T2DM vs controls) are not uniform, but the majority of the studies agree that bone microstructure and mechanical properties are altered in T2DM individuals.
To the best of our knowledge, our study was the first to examine micromechanical and microarchitectural properties of the superolateral femoral neck trabeculae, and examination of the cortical compartment of the superolateral femoral neck may be considered the main strength of our study. Particularly, our assessment of cortical bone is important because its deterioration was not detectable by clinical tools, considering that we found similar BMD and hip geometry parameters between the groups with the current sample size.
Here, we showed that 36% of the variability in cortical microhardness was explainable by the variability in cortical porosity. As for the remaining portion of the variability in mechanical properties in T2DM, it could be dependent on other aspects of bone quality that could be affected by the disease. On iliac crest biopsies of osteoporosis and control subjects, Boivin et al. showed that Vickers microhardness positively correlated with the degree of bone mineralization (DMB); nevertheless, the lower microhardness in individuals with osteoporosis cannot be fully explained by DMB alone [22]. In this context, organic matrix was also shown to partly determine microhardness values (about 30%) [22]. The reduced Vickers microhardness in individuals with T2DM in our study may reflect the higher fracture risk in these individuals, particularly having in mind that the reduced microhardness is determined by the alterations to both inorganic and organic components of the bone matrix and that Vickers microhardness correlates with tissue-level Young’s modulus [22]. Of note, there are also other potential contributors to the increased fracture risk in T2DM that may or may not be captured by microindentation. For example, in addition to the altered mineralization pattern, T2DM individuals may present lower osteon density compared with control subjects [34], as well as low bone turnover coupled with a decreased number of osteoblast precursors and increased number of osteoclast precursors [35]. Indeed, additional research is warranted to comprehensively study the effects of T2DM on other aspects of bone quality and further clarify the mechanisms of the increased fracture risk.
However, there are few limitations, such as missing data regarding the duration of disease and HbA1c, and a relatively small number of samples. The sample size was conditioned by the COVID-19 outbreak and avoidance of autopsies in COVID-19–positive individuals; nevertheless, our study was sensitive enough to demonstrate that cortical microarchitecture and cortical and trabecular microhardness are significantly reduced in older individuals with T2DM. While clinical studies have used OsteoProbe for microindentation, here we used Vickers microhardness testing as a traditional and standardized method that has been broadly used for material testing and has been also used for the evaluation of bone specimens [22, 25, 26]. We used standardized forces as suggested in previous studies on bones, which were able to measure the tissue-level hardness of the examined bone specimens. Although different, both of these methods are obviously able to distinguish between diabetes and controls specimens. Moreover, it has recently been shown that the BMSi, as measured by OsteoProbe indentation, is a valid measurement of hardness [36], and correlates strongly and significantly with Vickers and Rockwell microhardness values (r = 0.94, r = 0.93, respectively) [36]. However, it should be noted that OsteoProbe assesses the outer cortical surface only, as this is the only possible testing direction in a clinical setting; in contrast, Vickers microindentation on the excised bone specimens allowed us to examine the cross-section material properties of the superolateral femoral neck, which allowed us to separately evaluate its cortical and trabecular bony compartments. The superolateral femoral neck—the typical fracture-initiating site—is nonaccessible to the clinical indentation methods, so gaining direct knowledge on the superolateral femoral neck micromechanical properties is only possible on excised bone specimens obtained from surgery or autopsy.
Conclusions
T2DM individuals have deteriorated cortical microarchitecture of the superolateral femoral neck, and cortical and trabecular micromechanical properties are impaired in T2DM subjects as well. These findings offer explanation for the increased hip fracture risk in T2DM subjects. Considering that the significantly deteriorated cortical microarchitecture of the superolateral femoral neck is not recognized by DXA measurement, analyses of bone microarchitecture and bone material properties offer better understanding of the reduced bone strength in T2DM subjects.
Data Availability
The data that support the findings of this study are available from the corresponding author upon reasonable request.
References
Khan MAB, Hashim MJ, King JK, Govender RD, Mustafa H, Al Kaabi J (2020) Epidemiology of type 2 diabetes—global burden of disease and forecasted trends. J epidemiol glob health 10:107–111
Saeedi P, Petersohn I, Salpea P, Malanda B, Karuranga S, Unwin N, Colagiuri S, Guariguata L, Motala AA, Ogurtsova K, Shaw JE, Bright D, Williams R, Committee IDFDA (2019) Global and regional diabetes prevalence estimates for 2019 and projections for 2030 and 2045: results from the international diabetes federation diabetes atlas, 9(th) edition. Diabetes Res Clin Pract 157:107843
Fan Y, Wei F, Lang Y, Liu Y (2016) Diabetes mellitus and risk of hip fractures: a meta-analysis. Osteoporos Int 27:219–228
Holloway-Kew KL, Betson A, Rufus-Membere PG, Gaston J, Diez-Perez A, Kotowicz MA, Pasco JA (2021) Impact microindentation in men with impaired fasting glucose and type 2 diabetes. Bone 142:115685
Samakkarnthai P, Sfeir JG, Atkinson EJ, Achenbach SJ, Wennberg PW, Dyck PJ, Tweed AJ, Volkman TL, Amin S, Farr JN, Vella A, Drake MT, Khosla S (2020) Determinants of bone material strength and cortical porosity in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 105:e3718-3729
Nilsson AG, Sundh D, Johansson L, Nilsson M, Mellström D, Rudäng R, Zoulakis M, Wallander M, Darelid A, Lorentzon M (2017) Type 2 Diabetes mellitus is associated with better bone microarchitecture but lower bone material strength and poorer physical function in elderly women: a population-based study. J Bone Miner Res 32:1062–1071
Shanbhogue VV, Hansen S, Frost M, Jørgensen NR, Hermann AP, Henriksen JE, Brixen K (2016) Compromised cortical bone compartment in type 2 diabetes mellitus patients with microvascular disease. Eur J Endocrinol 174:115–124
Burghardt AJ, Issever AS, Schwartz AV, Davis KA, Masharani U, Majumdar S, Link TM (2010) High-resolution peripheral quantitative computed tomographic imaging of cortical and trabecular bone microarchitecture in patients with type 2 diabetes mellitus. J Clin Endocrinol Metab 95:5045–5055
Patsch JM, Burghardt AJ, Yap SP, Baum T, Schwartz AV, Joseph GB, Link TM (2013) Increased cortical porosity in type 2 diabetic postmenopausal women with fragility fractures. J Bone Miner Res 28:313–324
Schousboe JT, Morin SN, Kline GA, Lix LM, Leslie WD (2022) Differential risk of fracture attributable to type 2 diabetes mellitus according to skeletal site. Bone 154:116220
Osima M, Kral R, Borgen TT, Høgestøl IK, Joakimsen RM, Eriksen EF, Bjørnerem Å (2017) Women with type 2 diabetes mellitus have lower cortical porosity of the proximal femoral shaft using low-resolution CT than nondiabetic women, and increasing glucose is associated with reduced cortical porosity. Bone 97:252–260
Karim L, Mo-ulton J, Van Vliet M, Velie K, Robbins A, Malekipour F, Abdeen A, Ayres D, Bouxsein ML (2018) Bone microarchitecture, biomechanical properties, and advanced glycation end-products in the proximal femur of adults with type 2 diabetes. Bone 114:32–39
Cirovic A, Vujacic M, Petrovic B, Cirovic A, Zivkovic V, Nikolic S, Djonic D, Bascarevic Z, Djuric M, Milovanovic P (2022) Vascular complications in individuals with type 2 diabetes mellitus additionally increase the risk of femoral neck fractures due to deteriorated trabecular microarchitecture. Calcif Tissue Int 110:65–73
Piccoli A, Cannata F, Strollo R, Pedone C, Leanza G, Russo F, Greto V, Isgrò C, Quattrocchi CC, Massaroni C, Silvestri S, Vadalà G, Bisogno T, Denaro V, Pozzilli P, Tang SY, Silva MJ, Conte C, Papalia R, Maccarrone M, Napoli N (2020) Sclerostin regulation, microarchitecture, and advanced glycation end-products in the bone of elderly women with type 2 diabetes. J Bone Miner Res 35:2415–2422
Sihota P, Yadav RN, Dhaliwal R, Bose JC, Dhiman V, Neradi D, Karn S, Sharma S, Aggarwal S, Goni VG, Mehandia V, Vashishth D, Bhadada SK, Kumar N (2021) Investigation of mechanical, material, and compositional determinants of human trabecular bone quality in type 2 diabetes. J Clin Endocrinol Metab 106:e2271–e2289
Djuric M, Djonic D, Milovanovic P, Nikolic S, Marshall R, Marinkovic J, Hahn M (2010) Region-specific sex-dependent pattern of age-related changes of proximal femoral cancellous bone and its implications on differential bone fragility. Calcif Tissue Int 86:192–201
Milovanovic P, Djonic D, Marshall RP, Hahn M, Nikolic S, Zivkovic V, Amling M, Djuric M (2012) Micro-structural basis for particular vulnerability of the superolateral neck trabecular bone in the postmenopausal women with hip fractures. Bone 50:63–68
Maillard C, Berruyer M, Serre CM, Dechavanne M, Delmas PD (1992) Protein-S, a vitamin K-dependent protein, is a bone matrix component synthesized and secreted by osteoblasts. Endocrinology 130:1599–1604
Bellows CG, Reimers SM, Heersche JNM (1999) Expression of mRNAs for type-I collagen, bone sialoprotein, osteocalcin, and osteopontin at different stages of osteoblastic differentiation and their regulation by 1, 25 dihydroxyvitamin D3. Cell Tissue Res 297:249–259
Khosla S, Samakkarnthai P, Monroe DG, Farr JN (2021) Update on the pathogenesis and treatment of skeletal fragility in type 2 diabetes mellitus. Nat Rev Endocrinol 17:685–697
Farr JN, Drake MT, Amin S, Melton LJ 3rd, McCready LK, Khosla S (2014) In vivo assessment of bone quality in postmenopausal women with type 2 diabetes. J Bone Miner Res 29:787–795
Boivin G, Bala Y, Doublier A, Farlay D, Ste-Marie LG, Meunier PJ, Delmas PD (2008) The role of mineralization and organic matrix in the microhardness of bone tissue from controls and osteoporotic patients. Bone 43:532–538
Zani L, Erani P, Grassi L, Taddei F, Cristofolini L (2015) Strain distribution in the proximal human femur during in vitro simulated sideways fall. J Biomech 48:2130–2143
Cirovic A, Cirovic A, Djonic D, Zivkovic V, Nikolic S, Djuric M, Milovanovic P (2020) Three-dimensional microstructural basis for differential occurrence of subcapital versus basicervical hip fractures in men. Calcif Tissue Int 107:240–248
Jadzic J, Milovanovic P, Cvetkovic D, Ivovic M, Tomanovic N, Bracanovic M, Zivkovic V, Nikolic S, Djuric M, Djonic D (2021) Mechano-structural alteration in proximal femora of individuals with alcoholic liver disease: implications for increased bone fragility. Bone 150:116020
Yin B, Guo JL, Wang JZ, Li S, Liu YK, Zhang YZ (2019) Bone material properties of human phalanges using vickers indentation. Orthop Surg 11:487–492
Durlak JA (2009) How to select, calculate, and interpret effect sizes. J Pediatr Psychol 34:917–928
de Bakker PM, Manske SL, Ebacher V, Oxland TR, Cripton PA, Guy P (2009) During sideways falls proximal femur fractures initiate in the superolateral cortex: evidence from high-speed video of simulated fractures. J Biomech 42:1917–1925
Kral R, Osima M, Borgen TT, Vestgaard R, Richardsen E, Bjørnerem Å (2017) Increased cortical porosity and reduced cortical thickness of the proximal femur are associated with nonvertebral fracture independent of fracture risk assessment tool and garvan estimates in postmenopausal women. PLoS ONE 12:e0185363
McCalden RW, McGeough JA, Barker MB, Court-Brown CM (1993) Age-related changes in the tensile properties of cortical bone. The relative importance of changes in porosity, mineralization, and microstructure. J Bone Joint Surg Am 75:1193–1205
Endo D, Ogami-Takamura K, Imamura T, Saiki K, Murai K, Okamoto K, Tsurumoto T (2020) Reduced cortical bone thickness increases stress and strain in the female femoral diaphysis analyzed by a CT-based finite element method: implications for the anatomical background of fatigue fracture of the femur. Bone Rep 13:100733–100733
Sundh D, Mellström D, Nilsson M, Karlsson M, Ohlsson C, Lorentzon M (2015) Increased cortical porosity in older men with fracture. J Bone Miner Res 30:1692–1700
Sundh D, Nilsson AG, Nilsson M, Johansson L, Mellstrom D, Lorentzon M (2017) Increased cortical porosity in women with hip fracture. J Intern Med 281:496–506
Wölfel EM, Jähn-Rickert K, Schmidt FN, Wulff B, Mushumba H, Sroga GE, Püschel K, Milovanovic P, Amling M, Campbell GM, Vashishth D, Busse B (2020) Individuals with type 2 diabetes mellitus show dimorphic and heterogeneous patterns of loss in femoral bone quality. Bone 140:115556
Sassi F, Buondonno I, Luppi C, Spertino E, Stratta E, Di Stefano M, Ravazzoli M, Isaia G, Trento M, Passera P, Porta M, Isaia GC, D’Amelio P (2018) Type 2 diabetes affects bone cells precursors and bone turnover. BMC Endocr Disord 18:55
Ly FS, Proctor A, Hoffseth K, Yang HT, Hansma PK (2020) Significant correlation of bone material strength index as measured by the osteoprobe with vickers and rockwell hardness. Rev Sci Instrum 91:084102
Funding
This research was supported by the Science Fund of the Republic of Serbia (PROMIS, #6064549, DiaBoNet).
Author information
Authors and Affiliations
Contributions
Concept/Design of the study: PM, AC, MD, SN; Acquisition of data: AC, JJ, DD, VZ, SN; Data analysis: AC, JJ, PM; Data interpretation: AC, JJ, PM, DD, MD; Drafting of the manuscript: AC, PM. Critical revision of the manuscript: MD, JJ, DD, SN, VZ; Final approval of the article: all authors.
Corresponding author
Ethics declarations
Conflict of interest
Aleksandar Cirovic, Jelena Jadzic, Danica Djukic, Danijela Djonic, Vladimir Zivkovic, Slobodan Nikolic, Marija Djuric, Petar Milovanovic declare that they have no conflicts of interest.
Human and Animal Rights
The study complied with the relevant national and international standards, and all procedures were approved by the Ethics Committee of the Faculty of Medicine, University of Belgrade.
Informed Consent
N/A.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Cirovic, A., Jadzic, J., Djukic, D. et al. Increased Cortical Porosity, Reduced Cortical Thickness, and Reduced Trabecular and Cortical Microhardness of the Superolateral Femoral Neck Confer the Increased Hip Fracture Risk in Individuals with Type 2 Diabetes. Calcif Tissue Int 111, 457–465 (2022). https://doi.org/10.1007/s00223-022-01007-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-022-01007-6