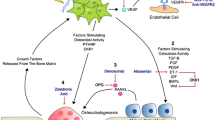
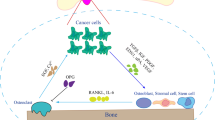
Abstract
Bone is the most common site of prostate cancer metastasis. Once prostate cancer cells metastasize to bone, the mortality rate of prostate cancer patients increases significantly. Furthermore, bone metastases produce multiple skeletal complications, including bone pain that impairs the patients’ quality of life. Effective therapies for bone metastatic disease are underdeveloped with most current therapies being primarily palliative with modest survival benefit. Although the exact mechanisms through which prostate cancer metastasizes to bone are unclear, growing evidence suggests that the bone marrow microenvironment, particularly its hematopoietic activity, is a significant mediator of prostate cancer bone tropism. Moreover, the bone microenvironment may regulate metastatic prostate cancer cells between dormant and proliferative states. In this review, we discuss (1) how prostate cancer cells interact with the bone microenvironment to establish bone metastases and (2) current and future potential treatments for prostate cancer patients with bone metastases.

Similar content being viewed by others
References
Jemal A, Siegel R, Ward E, Murray T, Xu J, Thun JM (2007) Cancer statistics. CA Cancer J Clin 57(1):43–66
Morash C, Tey R, Agbassi C, Klotz L, McGowan T, Srigley J, Evans A (2015) Active surveillance for the management of localized prostate cancer: guideline recommendations. Can Urol Assoc J 9(5–6):171–178. doi:10.5489/cuaj.2806
Bubendorf L, Schöpfer A, Wagner U, Sauter G, Moch H, Willi N, Gasser TC, Mihatsch MJ (2000) Metastatic patterns of prostate cancer: an autopsy study of 1,589 patients. Hum Pathol 31(5):578–583
Batson OV (1940) The function of the vertebral veins and their role in the spread of metastases. Ann Surg 112(1):138–149
Rahim F, Hajizamani S, Mortaz E, Ahmadzadeh A, Shahjahani M, Shahrabi S, Saki N (2014) Molecular regulation of bone marrow metastasis in prostate and breast cancer. Bone Marrow Res 2014:405920. doi:10.1155/2014/405920
Mazo IB, von Andrian UH (1999) Adhesion and homing of blood-borne cells in bone marrow microvessels. J Leukoc Biol 66(1):25–32
Miftakhova R, Hedblom A, Semenas J, Robinson B, Simoulis A, Malm J, Rizvanov A, Heery DM, Mongan NP, Maitland NJ, Allegrucci C, Persson JL (2016) Cyclin A1 and P450 aromatase promote metastatic homing and growth of stem-like prostate cancer cells in the bone marrow. Cancer Res 76(8):2453–2464. doi:10.1158/0008-5472.CAN-15-2340
Chu GC, Zhau HE, Wang R, Rogatko A, Feng X, Zayzafoon M, Liu Y, Farach-Carson MC, You S, Kim J, Freeman MR, Chung LW (2014) RANK- and c-Met-mediated signal network promotes prostate cancer metastatic colonization. Endocr Relat Cancer 21(2):311–326. doi:10.1530/ERC-13-0548
Zalucha JL, Jung Y, Joseph J, Wang J, Berry JE, Shiozawa Y, Taichman RS (2015) The role of osteoclasts in early dissemination of prostate cancer tumor cells. J Cancer Stem Cell Res 3:e1005
Shiozawa Y, Pedersen EA, Patel LR, Ziegler AM, Havens AM, Jung Y, Wang J, Zalucha S, Loberg RD, Pienta KJ, Taichman RS (2010) GAS6/AXL axis regulates prostate cancer invasion, proliferation, and survival in the bone marrow niche. Neoplasia 12(2):116–127
Chinni SR, Sivalogan S, Dong Z, Filho JC, Deng X, Bonfil RD, Cher ML (2006) CXCL12/CXCR4 signaling activates Akt-1 and MMP-9 expression in prostate cancer cells: the role of bone microenvironment-associated CXCL12. Prostate 66(1):32–48. doi:10.1002/pros.20318
Pound CR, Partin AW, Eisenberger MA, Chan DW, Pearson JD, Walsh PC (1999) Natural history of progression after PSA elevation following radical prostatectomy. JAMA 281(17):1591–1597
Tsuzuki S, Park SH, Eber MR, Peters CM, Shiozawa Y (2016) Skeletal complications in cancer patients with bone metastases. Int J Urol 23(10):825–832. doi:10.1111/iju.13170
Dimitroff CJ, Lechpammer M, Long-Woodward D, Kutok JL (2004) Rolling of human bone-metastatic prostate tumor cells on human bone marrow endothelium under shear flow is mediated by E-selectin. Cancer Res 64(15):5261–5269. doi:10.1158/0008-5472.CAN-04-0691
Dimitroff CJ, Descheny L, Trujillo N, Kim R, Nguyen V, Huang W, Pienta KJ, Kutok JL, Rubin MA (2005) Identification of leukocyte E-selectin ligands, P-selectin glycoprotein ligand-1 and E-selectin ligand-1, on human metastatic prostate tumor cells. Cancer Res 65(13):5750–5760. doi:10.1158/0008-5472.CAN-04-4653
Barthel SR, Wiese GK, Cho J, Opperman MJ, Hays DL, Siddiqui J, Pienta KJ, Furie B, Dimitroff CJ (2009) Alpha 1,3 fucosyltransferases are master regulators of prostate cancer cell trafficking. Proc Natl Acad Sci USA 106(46):19491–19496. doi:10.1073/pnas.0906074106
Chen C, Zhang Q, Liu S, Parajuli KR, Qu Y, Mei J, Chen Z, Zhang H, Khismatullin DB, You Z (2015) IL-17 and insulin/IGF1 enhance adhesion of prostate cancer cells to vascular endothelial cells through CD44-VCAM-1 interaction. Prostate 75(8):883–895. doi:10.1002/pros.22971
Cooper CR, Chay CH, Pienta KJ (2002) The role of alpha(v)beta(3) in prostate cancer progression. Neoplasia 4(3):191–194. doi:10.1038/sj/neo/7900224
Barthel SR, Hays DL, Yazawa EM, Opperman M, Walley KC, Nimrichter L, Burdick MM, Gillard BM, Moser MT, Pantel K, Foster BA, Pienta KJ, Dimitroff CJ (2013) Definition of molecular determinants of prostate cancer cell bone extravasation. Cancer Res 73(2):942–952. doi:10.1158/0008-5472.CAN-12-3264
Engl T, Relja B, Marian D, Blumenberg C, Muller I, Beecken WD, Jones J, Ringel EM, Bereiter-Hahn J, Jonas D, Blaheta RA (2006) CXCR4 chemokine receptor mediates prostate tumor cell adhesion through alpha5 and beta3 integrins. Neoplasia 8(4):290–301. doi:10.1593/neo.05694
Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL (2006) Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res 8(2):R20. doi:10.1186/bcr1398
Jung Y, Wang J, Schneider A, Sun YX, Koh-Paige AJ, Osman NI, McCauley LK, Taichman RS (2006) Regulation of SDF-1 (CXCL12) production by osteoblasts; a possible mechanism for stem cell homing. Bone 38(4):497–508. doi:10.1016/j.bone.2005.10.003
Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS (2007) Expression and activation of alpha v beta 3 integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate 67(1):61–73. doi:10.1002/pros.20500
Shiozawa Y, Taichman RS (2012) Getting blood from bone: an emerging understanding of the role that osteoblasts play in regulating hematopoietic stem cells within their niche. Exp Hematol 40(9):685–694. doi:10.1016/j.exphem.2012.05.004
Taichman RS, Cooper C, Keller ET, Pienta KJ, Taichman NS, McCauley LK (2002) Use of the stromal cell-derived factor-1/CXCR4 pathway in prostate cancer metastasis to bone. Cancer Res 62(6):1832–1837
Cojoc M, Peitzsch C, Trautmann F, Polishchuk L, Telegeev GD, Dubrovska A (2013) Emerging targets in cancer management: role of the CXCL12/CXCR4 axis. Onco Targets Ther 6:1347–1361. doi:10.2147/OTT.S36109
Wang J, Shiozawa Y, Wang J, Wang Y, Jung Y, Pienta KJ, Mehra R, Loberg R, Taichman RS (2008) The role of CXCR7/RDC1 as a chemokine receptor for CXCL12/SDF-1 in prostate cancer. J Biol Chem 283(7):4283–4294. doi:10.1074/jbc.M707465200
Conley-LaComb MK, Semaan L, Singareddy R, Li Y, Heath EI, Kim S, Cher ML, Chinni SR (2016) Pharmacological targeting of CXCL12/CXCR4 signaling in prostate cancer bone metastasis. Mol Cancer 15(1):68. doi:10.1186/s12943-016-0552-0
Karnoub AE, Dash AB, Vo AP, Sullivan A, Brooks MW, Bell GW, Richardson AL, Polyak K, Tubo R, Weinberg RA (2007) Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature 449(7162):557–563. doi:10.1038/nature06188
Jung Y, Kim JK, Shiozawa Y, Wang J, Mishra A, Joseph J, Berry JE, McGee S, Lee E, Sun H, Wang J, Jin T, Zhang H, Dai J, Krebsbach PH, Keller ET, Pienta KJ, Taichman RS (2013) Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun 4:1795. doi:10.1038/ncomms2766
Singh R, Kapur N, Mir H, Singh N, Lillard JW Jr, Singh S (2016) CXCR6-CXCL16 axis promotes prostate cancer by mediating cytoskeleton rearrangement via Ezrin activation and alphavbeta3 integrin clustering. Oncotarget 7(6):7343–7353. doi:10.18632/oncotarget.6944
Lu Y, Wang J, Xu Y, Koch AE, Cai Z, Chen X, Galson DL, Taichman RS, Zhang J (2008) CXCL16 functions as a novel chemotactic factor for prostate cancer cells in vitro. Mol Cancer Res 6(4):546–554. doi:10.1158/1541-7786.MCR-07-0277
Ha HK, Lee W, Park HJ, Lee SD, Lee JZ, Chung MK (2011) Clinical significance of CXCL16/CXCR6 expression in patients with prostate cancer. Mol Med Rep 4(3):419–424. doi:10.3892/mmr.2011.446
Peinado H, Lavotshkin S, Lyden D (2011) The secreted factors responsible for pre-metastatic niche formation: old sayings and new thoughts. Semin Cancer Biol 21(2):139–146. doi:10.1016/j.semcancer.2011.01.002
Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D (2005) VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature 438(7069):820–827. doi:10.1038/nature04186
Costa-Silva B, Aiello NM, Ocean AJ, Singh S, Zhang H, Thakur BK, Becker A, Hoshino A, Mark MT, Molina H, Xiang J, Zhang T, Theilen TM, Garcia-Santos G, Williams C, Ararso Y, Huang Y, Rodrigues G, Shen TL, Labori KJ, Lothe IM, Kure EH, Hernandez J, Doussot A, Ebbesen SH, Grandgenett PM, Hollingsworth MA, Jain M, Mallya K, Batra SK, Jarnagin WR, Schwartz RE, Matei I, Peinado H, Stanger BZ, Bromberg J, Lyden D (2015) Pancreatic cancer exosomes initiate pre-metastatic niche formation in the liver. Nat Cell Biol 17(6):816–826. doi:10.1038/ncb3169
Valencia K, Luis-Ravelo D, Bovy N, Anton I, Martinez-Canarias S, Zandueta C, Ormazabal C, Struman I, Tabruyn S, Rebmann V, De Las Rivas J, Guruceaga E, Bandres E, Lecanda F (2014) miRNA cargo within exosome-like vesicle transfer influences metastatic bone colonization. Mol Oncol 8(3):689–703. doi:10.1016/j.molonc.2014.01.012
Di Vizio D, Morello M, Dudley AC, Schow PW, Adam RM, Morley S, Mulholland D, Rotinen M, Hager MH, Insabato L, Moses MA, Demichelis F, Lisanti MP, Wu H, Klagsbrun M, Bhowmick NA, Rubin MA, D’Souza-Schorey C, Freeman MR (2012) Large oncosomes in human prostate cancer tissues and in the circulation of mice with metastatic disease. Am J Pathol 181(5):1573–1584. doi:10.1016/j.ajpath.2012.07.030
Di Vizio D, Kim J, Hager MH, Morello M, Yang W, Lafargue CJ, True LD, Rubin MA, Adam RM, Beroukhim R, Demichelis F, Freeman MR (2009) Oncosome formation in prostate cancer: association with a region of frequent chromosomal deletion in metastatic disease. Cancer Res 69(13):5601–5609. doi:10.1158/0008-5472.CAN-08-3860
Shiozawa Y, Pedersen EA, Havens AM, Jung Y, Mishra A, Joseph J, Kim JK, Patel LR, Ying C, Ziegler AM, Pienta MJ, Song J, Wang J, Loberg RD, Krebsbach PH, Pienta KJ, Taichman RS (2011) Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Investig 121(4):1298–1312. doi:10.1172/JCI43414
Wang H, Yu C, Gao X, Welte T, Muscarella AM, Tian L, Zhao H, Zhao Z, Du S, Tao J, Lee B, Westbrook TF, Wong ST, Jin X, Rosen JM, Osborne CK, Zhang XH (2015) The osteogenic niche promotes early-stage bone colonization of disseminated breast cancer cells. Cancer Cell 27(2):193–210. doi:10.1016/j.ccell.2014.11.017
Shiozawa Y, Havens AM, Jung Y, Ziegler AM, Pedersen EA, Wang J, Wang J, Lu G, Roodman GD, Loberg RD, Pienta KJ, Taichman RS (2008) Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem 105(2):370–380. doi:10.1002/jcb.21835
Jung Y, Wang J, Song J, Shiozawa Y, Wang J, Havens A, Wang Z, Sun YX, Emerson SG, Krebsbach PH, Taichman RS (2007) Annexin II expressed by osteoblasts and endothelial cells regulates stem cell adhesion, homing, and engraftment following transplantation. Blood 110(1):82–90. doi:10.1182/blood-2006-05-021352
Jung Y, Shiozawa Y, Wang J, Patel LR, Havens AM, Song J, Krebsbach PH, Roodman GD, Taichman RS (2011) Annexin-2 is a regulator of stromal cell-derived factor-1/CXCL12 function in the hematopoietic stem cell endosteal niche. Exp Hematol 39(2):151–166e151. doi:10.1016/j.exphem.2010.11.007
Jung Y, Wang J, Lee E, McGee S, Berry JE, Yumoto K, Dai J, Keller ET, Shiozawa Y, Taichman RS (2015) Annexin 2-CXCL12 interactions regulate metastatic cell targeting and growth in the bone marrow. Mol Cancer Res 13(1):197–207. doi:10.1158/1541-7786.MCR-14-0118
Broxmeyer HE, Orschell CM, Clapp DW, Hangoc G, Cooper S, Plett PA, Liles WC, Li X, Graham-Evans B, Campbell TB, Calandra G, Bridger G, Dale DC, Srour EF (2005) Rapid mobilization of murine and human hematopoietic stem and progenitor cells with AMD3100, a CXCR4 antagonist. J Exp Med 201(8):1307–1318. doi:10.1084/jem.20041385
Taichman RS, Emerson SG (1998) The role of osteoblasts in the hematopoietic microenvironment. Stem Cells 16(1):7–15. doi:10.1002/stem.160007
Kerr BA, Miocinovic R, Smith AK, West XZ, Watts KE, Alzayed AW, Klink JC, Mir MC, Sturey T, Hansel DE, Heston WD, Stephenson AJ, Klein EA, Byzova TV (2015) CD117(+) cells in the circulation are predictive of advanced prostate cancer. Oncotarget 6(3):1889–1897. doi:10.18632/oncotarget.2796
Wiesner C, Nabha SM, Dos Santos EB, Yamamoto H, Meng H, Melchior SW, Bittinger F, Thuroff JW, Vessella RL, Cher ML, Bonfil RD (2008) C-kit and its ligand stem cell factor: potential contribution to prostate cancer bone metastasis. Neoplasia 10(9):996–1003
Smith DC, Smith MR, Sweeney C, Elfiky AA, Logothetis C, Corn PG, Vogelzang NJ, Small EJ, Harzstark AL, Gordon MS, Vaishampayan UN, Haas NB, Spira AI, Lara PN Jr, Lin CC, Srinivas S, Sella A, Schoffski P, Scheffold C, Weitzman AL, Hussain M (2013) Cabozantinib in patients with advanced prostate cancer: results of a phase II randomized discontinuation trial. J Clin Oncol 31(4):412–419. doi:10.1200/JCO.2012.45.0494
Shiozawa Y, Pienta KJ, Taichman RS (2011) Hematopoietic stem cell niche is a potential therapeutic target for bone metastatic tumors. Clin Cancer Res 17(17):5553–5558. doi:10.1158/1078-0432.CCR-10-2505
Psaila B, Lyden D (2009) The metastatic niche: adapting the foreign soil. Nat Rev Cancer 9(4):285–293. doi:10.1038/nrc2621
Ghajar CM (2015) Metastasis prevention by targeting the dormant niche. Nat Rev Cancer 15(4):238–247. doi:10.1038/nrc3910
Lam HM, Vessella RL, Morrissey C (2014) The role of the microenvironment-dormant prostate disseminated tumor cells in the bone marrow. Drug Discov Today Technol 11:41–47. doi:10.1016/j.ddtec.2014.02.002
Kobayashi A, Okuda H, Xing F, Pandey PR, Watabe M, Hirota S, Pai SK, Liu W, Fukuda K, Chambers C, Wilber A, Watabe K (2011) Bone morphogenetic protein 7 in dormancy and metastasis of prostate cancer stem-like cells in bone. J Exp Med 208(13):2641–2655. doi:10.1084/jem.20110840
Aguirre-Ghiso JA, Estrada Y, Liu D, Ossowski L (2003) ERK(MAPK) activity as a determinant of tumor growth and dormancy; regulation by p38(SAPK). Cancer Res 63(7):1684–1695
Aguirre-Ghiso JA, Liu D, Mignatti A, Kovalski K, Ossowski L (2001) Urokinase receptor and fibronectin regulate the ERK(MAPK) to p38(MAPK) activity ratios that determine carcinoma cell proliferation or dormancy in vivo. Mol Biol Cell 12(4):863–879
Sharma S, Xing F, Liu Y, Wu K, Said N, Pochampally R, Shiozawa Y, Lin HK, Balaji KC, Watabe K (2016) Secreted protein acidic and rich in cysteine (SPARC) mediates metastatic dormancy of prostate cancer in bone. J Biol Chem 291(37):19351–19363. doi:10.1074/jbc.M116.737379
Jung Y, Shiozawa Y, Wang J, McGregor N, Dai J, Park SI, Berry JE, Havens AM, Joseph J, Kim JK, Patel L, Carmeliet P, Daignault S, Keller ET, McCauley LK, Pienta KJ, Taichman RS (2012) Prevalence of prostate cancer metastases after intravenous inoculation provides clues into the molecular basis of dormancy in the bone marrow microenvironment. Neoplasia 14(5):429–439
Taichman RS, Patel LR, Bedenis R, Wang J, Weidner S, Schumann T, Yumoto K, Berry JE, Shiozawa Y, Pienta KJ (2013) GAS6 receptor status is associated with dormancy and bone metastatic tumor formation. PLoS ONE 8(4):e61873. doi:10.1371/journal.pone.0061873
Cackowski FC, Eber MR, Rhee J, Decker AM, Yumoto K, Berry JE, Lee E, Shiozawa Y, Jung Y, Aguirre-Ghiso JA, Taichman RS (2016) Mer tyrosine kinase regulates disseminated prostate cancer cellular dormancy. J Cell Biochem. doi:10.1002/jcb.25768
Cook LM, Shay G, Araujo A, Aruajo A, Lynch CC (2014) Integrating new discoveries into the “vicious cycle” paradigm of prostate to bone metastases. Cancer Metastasis Rev 33(2–3):511–525. doi:10.1007/s10555-014-9494-4
Body JJ, Casimiro S, Costa L (2015) Targeting bone metastases in prostate cancer: improving clinical outcome. Nat Rev Urol 12(6):340–356. doi:10.1038/nrurol.2015.90
Sottnik JL, Keller ET (2013) Understanding and targeting osteoclastic activity in prostate cancer bone metastases. Curr Mol Med 13(4):626–639
Lynch CC, Hikosaka A, Acuff HB, Martin MD, Kawai N, Singh RK, Vargo-Gogola TC, Begtrup JL, Peterson TE, Fingleton B, Shirai T, Matrisian LM, Futakuchi M (2005) MMP-7 promotes prostate cancer-induced osteolysis via the solubilization of RANKL. Cancer Cell 7(5):485–496. doi:10.1016/j.ccr.2005.04.013
Hall CL, Daignault SD, Shah RB, Pienta KJ, Keller ET (2008) Dickkopf-1 expression increases early in prostate cancer development and decreases during progression from primary tumor to metastasis. Prostate 68(13):1396–1404. doi:10.1002/pros.20805
Thudi NK, Martin CK, Murahari S, Shu ST, Lanigan LG, Werbeck JL, Keller ET, McCauley LK, Pinzone JJ, Rosol TJ (2011) Dickkopf-1 (DKK-1) stimulated prostate cancer growth and metastasis and inhibited bone formation in osteoblastic bone metastases. Prostate 71(6):615–625. doi:10.1002/pros.21277
Dai J, Hall CL, Escara-Wilke J, Mizokami A, Keller JM, Keller ET (2008) Prostate cancer induces bone metastasis through Wnt-induced bone morphogenetic protein-dependent and independent mechanisms. Cancer Res 68(14):5785–5794. doi:10.1158/0008-5472.CAN-07-6541
Armstrong AP, Miller RE, Jones JC, Zhang J, Keller ET, Dougall WC (2008) RANKL acts directly on RANK-expressing prostate tumor cells and mediates migration and expression of tumor metastasis genes. Prostate 68(1):92–104. doi:10.1002/pros.20678
Zhang J, Dai J, Qi Y, Lin DL, Smith P, Strayhorn C, Mizokami A, Fu Z, Westman J, Keller ET (2001) Osteoprotegerin inhibits prostate cancer-induced osteoclastogenesis and prevents prostate tumor growth in the bone. J Clin Investig 107(10):1235–1244. doi:10.1172/JCI11685
Zheng Y, Basel D, Chow SO, Fong-Yee C, Kim S, Buttgereit F, Dunstan CR, Zhou H, Seibel MJ (2014) Targeting IL-6 and RANKL signaling inhibits prostate cancer growth in bone. Clin Exp Metastasis 31(8):921–933. doi:10.1007/s10585-014-9680-3
Mach DB, Rogers SD, Sabino MC, Luger NM, Schwei MJ, Pomonis JD, Keyser CP, Clohisy DR, Adams DJ, O’Leary P, Mantyh PW (2002) Origins of skeletal pain: sensory and sympathetic innervation of the mouse femur. Neuroscience 113(1):155–166
Patel MS, Elefteriou F (2007) The new field of neuroskeletal biology. Calcif Tissue Int 80(5):337–347. doi:10.1007/s00223-007-9015-3
Elefteriou F, Campbell P, Ma Y (2014) Control of bone remodeling by the peripheral sympathetic nervous system. Calcif Tissue Int 94(1):140–151. doi:10.1007/s00223-013-9752-4
Elefteriou F (2005) Neuronal signaling and the regulation of bone remodeling. Cell Mol Life Sci 62(19–20):2339–2349. doi:10.1007/s00018-005-5175-3
Katayama Y, Battista M, Kao WM, Hidalgo A, Peired AJ, Thomas SA, Frenette PS (2006) Signals from the sympathetic nervous system regulate hematopoietic stem cell egress from bone marrow. Cell 124(2):407–421. doi:10.1016/j.cell.2005.10.041
Chow A, Lucas D, Hidalgo A, Mendez-Ferrer S, Hashimoto D, Scheiermann C, Battista M, Leboeuf M, Prophete C, van Rooijen N, Tanaka M, Merad M, Frenette PS (2011) Bone marrow CD169 + macrophages promote the retention of hematopoietic stem and progenitor cells in the mesenchymal stem cell niche. J Exp Med 208(2):261–271. doi:10.1084/jem.20101688
Mendez-Ferrer S, Michurina TV, Ferraro F, Mazloom AR, Macarthur BD, Lira SA, Scadden DT, Ma’ayan A, Enikolopov GN, Frenette PS (2010) Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature 466(7308):829–834. doi:10.1038/nature09262
Elefteriou F (2016) Role of sympathetic nerves in the establishment of metastatic breast cancer cells in bone. J Bone Oncol 5(3):132–134. doi:10.1016/j.jbo.2016.03.003
Ayala GE, Dai H, Powell M, Li R, Ding Y, Wheeler TM, Shine D, Kadmon D, Thompson T, Miles BJ, Ittmann MM, Rowley D (2008) Cancer-related axonogenesis and neurogenesis in prostate cancer. Clin Cancer Res 14(23):7593–7603. doi:10.1158/1078-0432.CCR-08-1164
Ayala GE, Wheeler TM, Shine HD, Schmelz M, Frolov A, Chakraborty S, Rowley D (2001) In vitro dorsal root ganglia and human prostate cell line interaction: redefining perineural invasion in prostate cancer. Prostate 49(3):213–223
Ciftci S, Yilmaz H, Ciftci E, Simsek E, Ustuner M, Yavuz U, Muezzinoglu B, Dillioglugil O (2015) Perineural invasion in prostate biopsy specimens is associated with increased bone metastasis in prostate cancer. Prostate 75(15):1783–1789. doi:10.1002/pros.23067
Magnon C, Hall SJ, Lin J, Xue X, Gerber L, Freedland SJ, Frenette PS (2013) Autonomic nerve development contributes to prostate cancer progression. Science 341(6142):1236361. doi:10.1126/science.1236361
Palm D, Lang K, Niggemann B, Drell TL 4th, Masur K, Zaenker KS, Entschladen F (2006) The norepinephrine-driven metastasis development of PC-3 human prostate cancer cells in BALB/c nude mice is inhibited by beta-blockers. Int J Cancer 118(11):2744–2749. doi:10.1002/ijc.21723
Hassan S, Karpova Y, Baiz D, Yancey D, Pullikuth A, Flores A, Register T, Cline JM, D’Agostino R Jr, Danial N, Datta SR, Kulik G (2013) Behavioral stress accelerates prostate cancer development in mice. J Clin Investig 123(2):874–886. doi:10.1172/JCI63324
Grytli HH, Fagerland MW, Fosså SD, Taskén KA (2014) Association between use of β-blockers and prostate cancer-specific survival: a cohort study of 3561 prostate cancer patients with high-risk or metastatic disease. Eur Urol 65(3):635–641. doi:10.1016/j.eururo.2013.01.007
Jimenez-Andrade JM, Bloom AP, Stake JI, Mantyh WG, Taylor RN, Freeman KT, Ghilardi JR, Kuskowski MA, Mantyh PW (2010) Pathological sprouting of adult nociceptors in chronic prostate cancer-induced bone pain. J Neurosci 30(44):14649–14656. doi:10.1523/JNEUROSCI.3300-10.2010
Benyamin R, Trescot AM, Datta S, Buenaventura R, Adlaka R, Sehgal N, Glaser SE, Vallejo R (2008) Opioid complications and side effects. Pain Phys 11(2 Suppl):S105–S120
Zylla D, Gourley BL, Vang D, Jackson S, Boatman S, Lindgren B, Kuskowski MA, Le C, Gupta K, Gupta P (2013) Opioid requirement, opioid receptor expression, and clinical outcomes in patients with advanced prostate cancer. Cancer 119(23):4103–4110. doi:10.1002/cncr.28345
Lutz S, Berk L, Chang E, Chow E, Hahn C, Hoskin P, Howell D, Konski A, Kachnic L, Lo S, Sahgal A, Silverman L, von Gunten C, Mendel E, Vassil A, Bruner DW, Hartsell W, American Society for Radiation O (2011) Palliative radiotherapy for bone metastases: an ASTRO evidence-based guideline. Int J Radiat Oncol Biol Phys 79(4):965–976. doi:10.1016/j.ijrobp.2010.11.026
Chow E, van der Linden YM, Roos D, Hartsell WF, Hoskin P, Wu JS, Brundage MD, Nabid A, Tissing-Tan CJ, Oei B, Babington S, Demas WF, Wilson CF, Meyer RM, Chen BE, Wong RK (2014) Single versus multiple fractions of repeat radiation for painful bone metastases: a randomised, controlled, non-inferiority trial. Lancet Oncol 15(2):164–171. doi:10.1016/S1470-2045(13)70556-4
Hartsell WF, Scott CB, Bruner DW, Scarantino CW, Ivker RA, Roach M 3rd, Suh JH, Demas WF, Movsas B, Petersen IA, Konski AA, Cleeland CS, Janjan NA, DeSilvio M (2005) Randomized trial of short- versus long-course radiotherapy for palliation of painful bone metastases. J Natl Cancer Inst 97(11):798–804. doi:10.1093/jnci/dji139
El-Amm J, Aragon-Ching JB (2016) Targeting bone metastases in metastatic castration-resistant prostate cancer. Clin Med Insights Oncol 10(Suppl 1):11–19. doi:10.4137/CMO.S30751
Harrison MR, Wong TZ, Armstrong AJ, George DJ (2013) Radium-223 chloride: a potential new treatment for castration-resistant prostate cancer patients with metastatic bone disease. Cancer Manag Res 5:1–14. doi:10.2147/CMAR.S25537
Bruland OS, Nilsson S, Fisher DR, Larsen RH (2006) High-linear energy transfer irradiation targeted to skeletal metastases by the alpha-emitter 223Ra: adjuvant or alternative to conventional modalities? Clin Cancer Res 12(20 Pt 2):6250s–6257s. doi:10.1158/1078-0432.CCR-06-0841
Nilsson S, Larsen RH, Fossa SD, Balteskard L, Borch KW, Westlin JE, Salberg G, Bruland OS (2005) First clinical experience with alpha-emitting radium-223 in the treatment of skeletal metastases. Clin Cancer Res 11(12):4451–4459. doi:10.1158/1078-0432.CCR-04-2244
Parker C, Nilsson S, Heinrich D, Helle SI, O’Sullivan JM, Fossa SD, Chodacki A, Wiechno P, Logue J, Seke M, Widmark A, Johannessen DC, Hoskin P, Bottomley D, James ND, Solberg A, Syndikus I, Kliment J, Wedel S, Boehmer S, Dall’Oglio M, Franzen L, Coleman R, Vogelzang NJ, O’Bryan-Tear CG, Staudacher K, Garcia-Vargas J, Shan M, Bruland OS, Sartor O, Investigators A (2013) Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med 369(3):213–223. doi:10.1056/NEJMoa1213755
Saad F, Carles J, Gillessen S, Heidenreich A, Heinrich D, Gratt J, Levy J, Miller K, Nilsson S, Petrenciuc O, Tucci M, Wirth M, Federhofer J, O’Sullivan JM, Radium-223 International Early Access Program I (2016) Radium-223 and concomitant therapies in patients with metastatic castration-resistant prostate cancer: an international, early access, open-label, single-arm phase 3b trial. Lancet Oncol 17(9):1306–1316. doi:10.1016/S1470-2045(16)30173-5
Vuong W, Sartor O, Pal SK (2014) Radium-223 in metastatic castration resistant prostate cancer. Asian J Androl 16(3):348–353. doi:10.4103/1008-682X.127812
Drake MT, Clarke BL, Khosla S (2008) Bisphosphonates: mechanism of action and role in clinical practice. Mayo Clin Proc 83(9):1032–1045. doi:10.4065/83.9.1032
Coxon FP, Helfrich MH, Van’t Hof R, Sebti S, Ralston SH, Hamilton A, Rogers MJ (2000) Protein geranylgeranylation is required for osteoclast formation, function, and survival: inhibition by bisphosphonates and GGTI-298. J Bone Miner Res 15(8):1467–1476. doi:10.1359/jbmr.2000.15.8.1467
Benford HL, McGowan NW, Helfrich MH, Nuttall ME, Rogers MJ (2001) Visualization of bisphosphonate-induced caspase-3 activity in apoptotic osteoclasts in vitro. Bone 28(5):465–473
Saad F, Gleason DM, Murray R, Tchekmedyian S, Venner P, Lacombe L, Chin JL, Vinholes JJ, Goas JA, Chen B, Zoledronic Acid Prostate Cancer Study G (2002) A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst 94(19):1458–1468
Paller CJ, Carducci MA, Philips GK (2012) Management of bone metastases in refractory prostate cancer–role of denosumab. Clin Interv Aging 7:363–372. doi:10.2147/CIA.S27930
Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C (2011) Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet 377(9768):813–822. doi:10.1016/S0140-6736(10)62344-6
Smith MR, Saad F, Coleman R, Shore N, Fizazi K, Tombal B, Miller K, Sieber P, Karsh L, Damiao R, Tammela TL, Egerdie B, Van Poppel H, Chin J, Morote J, Gomez-Veiga F, Borkowski T, Ye Z, Kupic A, Dansey R, Goessl C (2012) Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet 379(9810):39–46. doi:10.1016/S0140-6736(11)61226-9
Thellenberg-Karlsson C, Nyman C, Nilsson S, Blom R, Marquez M, Castellanos E, Holmberg AR (2016) Bone-targeted novel cytotoxic polybisphosphonate conjugate in castration-resistant prostate cancer: a multicenter phase 1 study. Anticancer Res 36(12):6499–6504. doi:10.21873/anticanres.11249
Alaiya A, Fox J, Bobis S, Matic G, Shinwari Z, Barhoush E, Marquez M, Nilsson S, Holmberg AR (2014) Proteomic analysis of soft tissue tumor implants treated with a novel polybisphosphonate. Cancer Genom Proteom 11(1):39–49
Kantoff PW, Higano CS, Shore ND, Berger ER, Small EJ, Penson DF, Redfern CH, Ferrari AC, Dreicer R, Sims RB, Xu Y, Frohlich MW, Schellhammer PF, Investigators IS (2010) Sipuleucel-T immunotherapy for castration-resistant prostate cancer. N Engl J Med 363(5):411–422. doi:10.1056/NEJMoa1001294
Nilsson S (2016) Radionuclide therapies in prostate cancer: integrating radium-223 in the treatment of patients with metastatic castration-resistant prostate cancer. Curr Oncol Rep 18(2):14. doi:10.1007/s11912-015-0495-4
Morris MJ, Higano CS, Scher HI, Sweeney C, Antonarakis ES, Shevrin DH, Ryan CJ, Loriot Y, Fizazi K, Pandit-Taskar N, Garcia-Vargas JE, Lyseng K, Bloma M, Carrasquillo JA (2015) Effects of radium-223 dichloride (Ra-223) with docetaxel (D) vs D on prostate-specific antigen (PSA) and bone alkaline phosphatase (bALP) in patients (pts) with castration-resistant prostate cancer (CRPC) and bone metastases (mets): a phase 1/2a clinical trial. J Clin Oncol 33(7 Suppl):202. doi:10.1200/jco.2015.33.7_suppl.202
O’Sullivan J GS, Heidenreich A, Heinrich D, Gratt J, Lévy J et al (2015) Effects of concomitant use of abiraterone and/or enzalutamide with radium-223 on safety and overall survival in metastatic castration-resistant prostate cancer (mCRPC) patients treated in an international early access program (EAP). In: European society for medical oncology, Vienna, Austria, 2015. Abstract 2561
Acknowledgements
This work is directly supported by National Cancer Institute Grants CA163124 (Y. Shiozawa) and P01 CA093900 (E. Keller), Department of Defense (W81XWH-14-1-0403 and W81XWH-17-1-0541, Y. Shiozawa), the Wake Forest School of Medicine Internal Pilot Funding (Y. Shiozawa), and the Wake Forest Baptist Comprehensive Cancer Center Internal Pilot Funding (Y. Shiozawa). Y Shiozawa is supported as the Translational Research Academy which is supported by the National Center for Advancing Translational Sciences (NCATS), National Institutes of Health, through Grant Award Number UL1TR001420. This work is also supported by the National Cancer Institute’s Cancer Center Support Grant award number P30CA012197 issued to the Wake Forest Baptist Comprehensive Cancer Center. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Cancer Institute.
Author information
Authors and Affiliations
Corresponding authors
Ethics declarations
Conflict of interest
Sun H. Park, Evan T. Keller, and Yusuke Shiozawa declare that they have no conflicts of interest.
Additional information
Evan T. Keller and Yusuke Shiozawa: Co-senior authors.
Rights and permissions
About this article
Cite this article
Park, S.H., Keller, E.T. & Shiozawa, Y. Bone Marrow Microenvironment as a Regulator and Therapeutic Target for Prostate Cancer Bone Metastasis. Calcif Tissue Int 102, 152–162 (2018). https://doi.org/10.1007/s00223-017-0350-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-017-0350-8