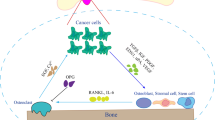
Abstract
The bone is a common site for metastasis in patients with advanced prostate carcinoma, and provides a ‘fertile’ milieu which stimulates tumour growth and associated bone disease. For years, the concept of treatment strategies has remained targeting the tumour itself; however, the occurrence of chemoresistance remains a challenge now more than ever. The attraction of targeting the bone microenvironment in order to disrupt tumour localisation and proliferation stems from the idea that stromal cells are superiorly stable at a genetic level, thus decreasing the risk of resistance manifestation. In this review, we will discuss recent findings with regards to the pathogenesis of prostate cancer-induced bone disease and recent therapeutic strategies in an aim to evaluate the ever increasing role of the microenvironment in disease progression.

Similar content being viewed by others
References
Papers of particular interest, published recently, have been highlighted as: • Of importance •• Of major importance
Coleman RE, 20 Pt 2. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–9.
Friedl P, Alexander S. Cancer invasion and the microenvironment: plasticity and reciprocity. Cell. 2011;147(5):992–1009.
Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350(16):1655–64.
Weilbaecher KN, Guise TA, McCauley LK. Cancer to bone: a fatal attraction. Nat Rev Cancer. 2011;11(6):411–25.
Hess KR et al. Metastatic patterns in adenocarcinoma. Cancer. 2006;106(7):1624–33.
Gundem G et al. The evolutionary history of lethal metastatic prostate cancer. Nature. 2015;520(7547):353–7. Uses whole genome sequencing to describe the pattern of metastatic spread in advanced prostate cancer.
Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2(8):584–93.
Jin JK, Dayyani F, Gallick GE. Steps in prostate cancer progression that lead to bone metastasis. Int J Cancer. 2011;128(11):2545–61.
Zheng X et al. Epithelial-to-mesenchymal transition is dispensable for metastasis but induces chemoresistance in pancreatic cancer. Nature. 2015;527(7579):525–30. Suggests that EMT is not critical for metastasis.
Fischer KR et al. Epithelial-to-mesenchymal transition is not required for lung metastasis but contributes to chemoresistance. Nature. 2015;527(7579):472–6. Suggests that EMT is not critical for metastasis.
Autio KA, Morris MJ. Targeting bone physiology for the treatment of metastatic prostate cancer. Clin Adv Hematol Oncol: H&O. 2013;11(3):134–43.
Buenrostro D, Park SI, Sterling JA. Dissecting the role of bone marrow stromal cells on bone metastases. Biomed Res Int. 2014;2014, 875305.
Taichman RS et al. The evolving biology and treatment of prostate cancer. J Clin Invest. 2007;117(9):2351–61.
Sun YX et al. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89(3):462–73.
Kaplan RN, Psaila B, Lyden D. Bone marrow cells in the ‘pre-metastatic niche’: within bone and beyond. Cancer Metastasis Rev. 2006;25(4):521–9.
Shiozawa Y et al. Human prostate cancer metastases target the hematopoietic stem cell niche to establish footholds in mouse bone marrow. J Clin Invest. 2011;121(4):1298–312.
Wang N et al. Prostate cancer cells preferentially home to osteoblast-rich areas in the early stages of bone metastasis: evidence from in vivo models. J Bone Miner Res. 2014;29(12):2688–96.
Wang N et al. Mitotic quiescence, but not unique “stemness,” marks the phenotype of bone metastasis-initiating cells in prostate cancer. FASEB J. 2015;29(8):3141–50.
Pedersen EA et al. The prostate cancer bone marrow niche: more than just ‘fertile soil’. Asian J Androl. 2012;14(3):423–7.
Shiozawa Y et al. Annexin II/annexin II receptor axis regulates adhesion, migration, homing, and growth of prostate cancer. J Cell Biochem. 2008;105(2):370–80.
Zheng Y et al. The role of the bone microenvironment in skeletal metastasis. J Bone Oncol. 2013;2(1):47–57.
Chantrain CF et al. Bone marrow microenvironment and tumor progression. Cancer Microenviron. 2008;1(1):23–35.
Hall CL et al. Prostate cancer cells promote osteoblastic bone metastases through Wnts. Cancer Res. 2005;65(17):7554–60.
Hall CL et al. Role of Wnts in prostate cancer bone metastases. J Cell Biochem. 2006;97(4):661–72.
Logothetis CJ, Lin SH. Osteoblasts in prostate cancer metastasis to bone. Nat Rev Cancer. 2005;5(1):21–8.
Shariat SF et al. Preoperative plasma levels of transforming growth factor beta(1) (TGF-beta(1)) strongly predict progression in patients undergoing radical prostatectomy. J Clin Oncol. 2001;19(11):2856–64.
Autzen P et al. Bone morphogenetic protein 6 in skeletal metastases from prostate cancer and other common human malignancies. Br J Cancer. 1998;78(9):1219–23.
Street J et al. Vascular endothelial growth factor stimulates bone repair by promoting angiogenesis and bone turnover. Proc Natl Acad Sci U S A. 2002;99(15):9656–61.
Sottnik JL, Keller ET. Understanding and targeting osteoclastic activity in prostate cancer bone metastases. Curr Mol Med. 2013;13(4):626–39.
Dougall WC et al. RANK is essential for osteoclast and lymph node development. Genes Dev. 1999;13(18):2412–24.
Keller ET, Brown J. Prostate cancer bone metastases promote both osteolytic and osteoblastic activity. J Cell Biochem. 2004;91(4):718–29.
Hardaway AL et al. Bone marrow fat: linking adipocyte-induced inflammation with skeletal metastases. Cancer Metastasis Rev. 2014;33(2–3):527–43.
Herroon MK et al. Bone marrow adipocytes promote tumor growth in bone via FABP4-dependent mechanisms. Oncotarget. 2013;4(11):2108–23.
Hardaway AL et al. Marrow adipocyte-derived CXCL1 and CXCL2 contribute to osteolysis in metastatic prostate cancer. Clin Exp Metastasis. 2015;32(4):353–68.
Luo J et al. Infiltrating bone marrow mesenchymal stem cells increase prostate cancer stem cell population and metastatic ability via secreting cytokines to suppress androgen receptor signaling. Oncogene. 2014;33(21):2768–78.
Jung Y et al. Recruitment of mesenchymal stem cells into prostate tumours promotes metastasis. Nat Commun. 2013;4:1795. Demonstrates a role for CXCR16 in recruiting MSCs into prostate tumours and promoting metastasis.
Olechnowicz SW, Edwards CM. Contributions of the host microenvironment to cancer-induced bone disease. Cancer Res. 2014;74(6):1625–31.
Li X et al. Loss of TGF-beta responsiveness in prostate stromal cells alters chemokine levels and facilitates the development of mixed osteoblastic/osteolytic bone lesions. Mol Cancer Res. 2012;10(4):494–503.
Ottewell PD et al. Castration-induced bone loss triggers growth of disseminated prostate cancer cells in bone. Endocr Relat Cancer. 2014;21(5):769–81.
Quail DF, Joyce JA. Microenvironmental regulation of tumor progression and metastasis. Nat Med. 2013;19(11):1423–37.
Sturge J, Caley MP, Waxman J. Bone metastasis in prostate cancer: emerging therapeutic strategies. Nat Rev Clin Oncol. 2011;8(6):357–68.
Saad F et al. A randomized, placebo-controlled trial of zoledronic acid in patients with hormone-refractory metastatic prostate carcinoma. J Natl Cancer Inst. 2002;94(19):1458–68.
Saad F et al. Long-term efficacy of zoledronic acid for the prevention of skeletal complications in patients with metastatic hormone-refractory prostate cancer. J Natl Cancer Inst. 2004;96(11):879–82.
Coleman RE, McCloskey EV. Bisphosphonates in oncology. Bone. 2011;49(1):71–6.
Ando K et al. RANKL/RANK/OPG: key therapeutic target in bone oncology. Curr Drug Discov Technol. 2008;5(3):263–8.
Smith MR et al. Denosumab and bone-metastasis-free survival in men with castration-resistant prostate cancer: results of a phase 3, randomised, placebo-controlled trial. Lancet. 2012;379(9810):39–46.
Lipton A et al. Superiority of denosumab to zoledronic acid for prevention of skeletal-related events: a combined analysis of 3 pivotal, randomised, phase 3 trials. Eur J Cancer. 2012;48(16):3082–92.
Roberts E, Cossigny DA, Quan GM. The role of vascular endothelial growth factor in metastatic prostate cancer to the skeleton. Prostate Cancer. 2013;2013:418340.
Di Lorenzo G et al. Combination of bevacizumab and docetaxel in docetaxel-pretreated hormone-refractory prostate cancer: a phase 2 study. Eur Urol. 2008;54(5):1089–94.
Kelly WK et al. Randomized, double-blind, placebo-controlled phase III trial comparing docetaxel and prednisone with or without bevacizumab in men with metastatic castration-resistant prostate cancer: CALGB 90401. J Clin Oncol. 2012;30(13):1534–40.
Huang D et al. Anti-angiogenesis or pro-angiogenesis for cancer treatment: focus on drug distribution. Int J Clin Exp Med. 2015;8(6):8369–76.
Wong PP et al. Dual-action combination therapy enhances angiogenesis while reducing tumor growth and spread. Cancer Cell. 2015;27(1):123–37.
Fang H, DeClerck YA. Targeting the tumor microenvironment: from understanding pathways to effective clinical trials. Cancer Res. 2013;73(16):4965–77.
Muralidharan A, Smith MT. Pathobiology and management of prostate cancer-induced bone pain: recent insights and future treatments. Inflammopharmacology. 2013;21(5):339–63.
Todenhofer T et al. Targeting bone metabolism in patients with advanced prostate cancer: current options and controversies. Int J Endocrinol. 2015;2015:838202.
Parker C et al. Alpha emitter radium-223 and survival in metastatic prostate cancer. N Engl J Med. 2013;369(3):213–23.
Shore ND. Radium-223 dichloride for metastatic castration-resistant prostate cancer: the urologist’s perspective. Urology. 2015;85(4):717–24.
Acknowledgments
This work was supported by the Prostate Cancer UK and Cancer Research UK (CR-UK) grant number C38302/A12278, through the Oxford Cancer Research Centre Development Fund and through the University of Oxford Medical Research Fund.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Christina J. Turner and Claire M. Edwards declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
This article does not contain any studies with human or animal subjects performed by any of the authors.
Additional information
This article is part of the Topical Collection on Osteoporosis and Cancer
Rights and permissions
About this article
Cite this article
Turner, C.J., Edwards, C.M. The Role of the Microenvironment in Prostate Cancer-Associated Bone Disease. Curr Osteoporos Rep 14, 170–177 (2016). https://doi.org/10.1007/s11914-016-0323-2
Published:
Issue Date:
DOI: https://doi.org/10.1007/s11914-016-0323-2