Abstract
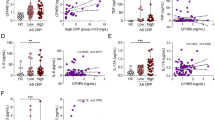
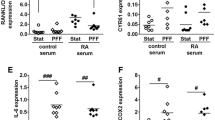
Bone remodeling can be disturbed in active rheumatoid arthritis (RA), possibly as a result of elevated levels of circulating inflammatory cytokines. Osteocyte-specific proteins and cytokines play a vital role in bone remodeling by orchestrating bone formation and/or bone resorption. Therefore, we aimed to investigate the effect of RA-serum or inflammatory cytokines on expression of human osteocyte-specific proteins and cytokines. Human trabecular bone chips were cultured with RA-serum or inflammatory cytokines for 7-days. Live-dead staining was performed to assess cell viability. Gene expression of osteocyte-specific proteins and cytokines was analyzed by qPCR. Immuno-staining was performed for osteocyte-specific markers. Approximately 60 % of the osteocytes on the bone chips were alive at day-7. Cells in or on the bone chips did express the gene for osteocyte markers SOST, FGF23, DMP1, and MEPE, and the cytokines IL-1β, IL-6, and TNFα at day 0 and 7. Active RA-serum treatment enhanced IL-1β, TNFα, SOST, and DKK1 gene expression. IL-1β treatment enhanced IL-1β, TNFα, IL-6, IL-8, FGF23, and SOST gene expression. TNFα treatment enhanced IL-1β, TNFα, IL-6, IL-8, and FGF23 gene expression. IL-8 treatment enhanced TNFα, IL-8, and FGF23 gene expression. A combination of IL-1β, IL-6, and TNFα treatment synergistically upregulated IL-1β, IL-6, and IL-8 gene expression, as well as enhanced TNFα, OPG, SOST, and FGF23, and inhibited DKK1 gene expression. In conclusion, gene expression of human osteocyte-specific proteins and cytokines was affected by RA-serum, and exogenous recombinant cytokines treatment suggesting that osteocytes could provide a new target to prevent systemic inflammation-induced bone loss in RA.






Similar content being viewed by others
References
Mielants H, Van den Bosch F (2009) Extra-articular manifestations. Clin Exp Rheumatol 27:S56–S61
Vis M, Güler-Yüksel M, Lems WF (2013) Can bone loss in rheumatoid arthritis be prevented? Osteoporos Int 10:2541–2553
Eggelmeijer F, Papapoulos SE, Westedt ML, Van Paassen HC, Dijkmans BA, Breedveld FC (1993) Bone metabolism in rheumatoid arthritis: relation to disease activity. Br J Rheumatol 32:387–391
Hardy R, Cooper MS (2009) Bone loss in inflammatory disorders. J Endocrinol 201:309–320
Luyten FP, Lories RJ, Verschueren P, de Vlam K, Westhovens R (2006) Contemporary concepts of inflammation, damage and repair in rheumatic diseases. Best Pract Res Clin Rheumatol 20:829–848
Alex P, Szodoray P, Knowlton N, Dozmorov IM, Turner M, Frank MB, Arthur RE, Willis L, Flinn D, Hynd RF, Carson C, Kumar A, El-Gabalawy HS, Centola M (2007) Multiplex serum cytokine monitoring as a prognostic tool in rheumatoid arthritis. Clin Exp Rheumatol 25:584–592
Kuan WP, Tam LS, Wong CK, Ko FW, Li T, Zhu T, Li EK (2010) CXCL 9 and CXCL 10 as sensitive markers of disease activity in patients with rheumatoid arthritis. J Rheumatol 37:257–264
Brennan FM, McInnes IB (2008) Evidence that cytokines play a role in rheumatoid arthritis. J Clin Invest 118:3537–3545
Kawashiri SY, Kawakami A, Iwamoto N, Fujikawa K, Aramaki T, Tamai M, Arima K, Kamachi M, Yamasaki S, Nakamura H, Tsurumoto T, Kono M, Shindo H, Ida H, Origuchi T, Eguchi K (2009) Proinflammatory cytokines synergistically enhance the production of chemokine ligand 20 (CCL20) from rheumatoid fibroblast-like synovial cells in vitro and serum CCL20 is reduced in vivo by biologic disease-modifying antirheumatic drugs. J Rheumatol 36:2397–2402
Chung SJ, Kwon YJ, Park MC, Park YB, Lee SK (2011) The correlation between increased serum concentrations of interleukin-6 family cytokines and disease activity in rheumatoid arthritis patients. Yonsei Med J 52:113–120
Schett G, Gravallese E (2012) Bone erosion in rheumatoid arthritis: mechanisms, diagnosis and treatment. Nat Rev Rheumatol 8:656–664
Bakker AD, Silva VC, Krishnan R, Bacabac RG, Blaauboer ME, Lin YC, Marcantonio RA, Cirelli JA, Klein-Nulend J (2009) Tumor necrosis factor α and interleukin-1β modulate calcium and nitric oxide signaling in mechanically stimulated osteocytes. Arthritis Rheum 60:3336–3345
Pathak JL, Bravenboer N, Verschueren P, Lems WF, Luyten FP, Klein-Nulend J, Bakker AD (2014) Inflammatory factors in the circulation of patients with active rheumatoid arthritis stimulate osteoclastogenesis via endogenous cytokine production by osteoblasts. Osteoporos Int 25:2453–2463
Pathak JL, Bravenboer N, Luyten FP, Verschueren P, Lems WF, Klein-Nulend J, Bakker AD (2015) Mechanical loading reduces inflammation-induced human osteocyte-to-osteoclast communication. Calcif Tissue Int 97:169–178
Tu X, Delgado-Calle J, Condon KW, Maycas M, Zhang H, Carlesso N, Taketo MM, Burr DB, Plotkin LI, Bellido T (2015) Osteocytes mediate the anabolic actions of canonical Wnt/β-catenin signaling in bone. Proc Natl Acad Sci 112:E478–E486
Dallas SL, Prideaux M, Bonewald LF (2013) The osteocyte: an endocrine cell… and more. Endocr Rev 34:658–690
Feng JQ, Ward LM, Liu S, Lu Y, Xie Y, Yuan B, Yu X, Rauch F, Davis SI, Zhang S, Rios H, Drezner MK, Quarles LD, Bonewald LF, White KE (2006) Loss of DMP1 causes rickets and osteomalacia and identifies a role for osteocytes in mineral metabolism. Nat Genet 38:1310–1315
Poole KE, van Bezooijen RL, Loveridge N, Hamersma H, Papapoulos SE, Löwik CW, Reeve J (2005) Sclerostin is a delayed secreted product of osteocytes that inhibits bone formation. Faseb J 19:1842–1844
Ito N, Wijenayaka AR, Prideaux M, Kogawa M, Ormsby RT, Evdokiou A, Bonewald LF, Findlay DM, Atkins GJ (2015) Regulation of FGF23 expression in IDG-SW3 osteocytes and human bone by pro-inflammatory stimuli. Mol Cell Endocrinol 399:208–218
Kulkarni RN, Bakker AD, Everts V, Klein-Nulend J (2012) Mechanical loading prevents the stimulating effect of IL-1beta on osteocyte-modulated osteoclastogenesis. Biochem Biophys Res Commun 420:11–16
Brolese E, Buser D, Kuchler U, Schaller B, Gruber R (2014) Human bone chips release of sclerostin and FGF-23 into the culture medium: an in vitro pilot study. Clin Oral Implants Res 26:1211–1214
Zhao S, Zhang YK, Harris S, Ahuja SS, Bonewald LF (2002) MLO-Y4 osteocyte-like cells support osteoclast formation and activation. J Bone Miner Res 17:2068–2079
Nakashima T, Hayashi M, Fukunaga T, Kurata K, Oh-Hora M, Feng JQ, Bonewald LF, Kodama T, Wutz A, Wagner EF, Penninger JM, Takayanagi H (2011) Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat Med 17:1231–1234
Qiang YW, Barlogie B, Rudikoff S, Shaughnessy JD Jr (2008) Dkk1-induced inhibition of Wnt signaling in osteoblast differentiation is an underlying mechanism of bone loss in multiple myeloma. Bone 42:669–680
Quarles LD (2003) FGF23, PHEX, and MEPE regulation of phosphate homeostasis and skeletal mineralization. Am J Physiol Endocrinol Metab 285:E1–E9
Liu SG, Quarles LD (2007) How fibroblast growth factor 23 works. J Am Soc Nephrol 18:1637–1647
Juppner H (2011) Phosphate and FGF-23. Kidney Int Suppl 121:S24–S27
Bakker AD, Kulkarni RN, Klein-Nulend J, Lems WF (2014) IL-6 alters osteocyte signaling toward osteoblasts but not osteoclasts. J Dent Res 93:394–399
Pathak JL, Bakker AD, Verschueren P, Lems WF, Luyten FP, Klein-Nulend J, Bravenboer N (2015) CXCL8 and CCL20 enhance osteoclastogenesis via modulation of cytokine production by human primary osteoblasts. PLoS ONE 10(6):e0131041
Arnett FC, Edworthy SM, Bloch DA, McShane DJ, Fries JF, Cooper NS et al (1988) The American Rheumatism Association 1987 revised criteria for the classification of rheumatoid arthritis. Arthritis Rheum 31:315–324
Klein-Nulend J, Sterck JGH, Semeins CM, Lips P, Joldersma M, Baart JA, Burger EH (2002) Donor age and mechanosensitivity of human bone cells. Osteoporos Int 13:137–146
Theuns HM, Bekker H, Fokkenrood H, Offerman E (1993) Methyl-methacrylate embedding of undecalcified rat bone and simultaneous staining for alkaline and tartrate resistant acid phosphatase. Bone 14:545–550
Kennedy OD, Laudier DM, Majeska RJ, Sun HB, Schaffler MB (2014) Osteocyte apoptosis is required for production of osteoclastogenic signals following bone fatigue in vivo. Bone 64:132–137
Bianconi E, Piovesan A, Facchin F, Beraudi A, Casadei R, Frabetti F, Vitale L, Pelleri MC, Tassani S, Piva F, Perez-Amodio S, Strippoli P, Canaider S (2013) An estimation of the number of cells in the human body. Ann Hum Biol 40:463–471
Xiong J, Onal M, Jilka RL, Weinstein RS, Manolagas SC, O’Brien CA (2011) Matrix-embedded cells control osteoclast formation. Nat Med 17:1235–1241
Liu S, Zhou J, Tang W, Jiang X, Rowe DW, Quarles LD (2006) Pathogenic role of Fgf23 in Hyp mice. Am J Physiol Endocrinol Metab 291:E38–E49
Liu S, Guo R, Simpson LG, Xiao ZS, Burnham CE, Quarles LD (2003) Regulation of fibroblastic growth factor 23 expression but not degradation by PHEX. J Biol Chem 278:37419–37426
Thaler R, Sturmlechner I, Spitzer S, Riester SM, Rumpler M, Zwerina J, Klaushofer K, van Wijnen AJ, Varga F (2014) Acute-phase protein serum amyloid A3 is a novel paracrine coupling factor that controls bone homeostasis. FASEB J 9:1344–1359
Li X, Zhang Y, Kang H, Liu W, Liu P, Zhang J, Harris SE, Wu D (2005) Sclerostin binds to LRP5/6 and antagonizes canonical Wnt signaling. J Biol Chem 280:19883–19887
Bhat BM, Allen KM, Liu W, Graham J, Morales A, Anisowicz A, Lam HS, McCauley C, Coleburn V, Cain M, Fortier E, Bhat RA, Bex FJ, Yaworsky PJ (2007) Structure-based mutation analysis shows the importance of LRP5 beta-propeller 1 in modulating Dkk1-mediated inhibition of Wnt signaling. Gene 391:103–112
Wang Y, Li YP, Paulson C, Shao JZ, Zhang X, Wu M, Chen W (2014) Wnt and the Wnt signaling pathway in bone development and disease. Front Biosci (Landmark Ed) 19:379–407
Heiland GR, Zwerina K, Baum W, Kireva T, Distler JH, Grisanti M, Asuncion F, Li X, Ominsky M, Richards W, Schett G, Zwerina J (2010) Neutralisation of Dkk-1 protects from systemic bone loss during inflammation and reduces sclerostin expression. Ann Rheum Dis 69:2152–2159
Dalal M, Sun K, Cappola AR, Ferrucci L, Crasto C, Fried LP, Semba RD (2011) Relationship of serum fibroblast growth factor 23 with cardiovascular disease in older community-dwelling women. Eur J Endocrinol 165:797–803
Skeoch S, Bruce IN (2015) Atherosclerosis in rheumatoid arthritis: is it all about inflammation? Nat Rev Rheumatol 11:390–400
Dessein PH, Hsu HC, Tsang L, Millen AM, Woodiwiss AJ, Norton GR, Solomon A, Gonzalez-Gay MA (2015) Kidney function, endothelial activation and atherosclerosis in black and white Africans with rheumatoid arthritis. PLoS ONE 10(3):e0121693
Evenepoel P, D’Haese P, Brandenburg V (2015) Sclerostin and DKK1: new players in renal bone and vascular disease. Kidney Int 88:235–240
El-Gabalawy HS, Robinson DB, Smolik I, Hart D, Elias B, Wong K, Peschken CA, Hitchon CA, Li X, Bernstein CN, Newkirk MM, Fritzler MJ (2012) Familial clustering of the serum cytokine profile in the relatives of rheumatoid arthritis patients. Arthritis Rheum 64:1720–1729
Mosedale DE, Grainger DJ (1999) An antibody present in normal human serum inhibits the binding of cytokines to their receptors in an in vitro system. Biochem J 343:125–133
Cappellano G, Orilieri E, Woldetsadik AD, Boggio E, Soluri MF, Comi C, Sblattero D, Chiocchetti A, Dianzani U (2012) Anti-cytokine autoantibodies in autoimmune diseases. Am J Clin Exp Immunol 1:136–146
Acknowledgments
We thank Mr. Huib W. van Essen and Mrs Jolanda M.A. Hogervorst for technical support. This research was funded by the European Commission through MOVE-AGE, an Erasmus Mundus Joint Doctorate programme (2011-0015).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of Interest
Janak L. Pathak, Astrid D. Bakker, Frank P. Luyten, Patrick Verschueren, Willem F. Lems, Jenneke Klein-Nulend, and Nathalie Bravenboer declare that they have no conflict of interest.
Human and Animal Rights and Informed Consent
The protocol was approved by the Ethical Review Board of the VU University Medical Center, Amsterdam, The Netherlands, and all subjects gave informed consent.
Additional information
J. Klein-Nulend and N. Bravenboer have equally contributed to this study and also shared last authorship.
Rights and permissions
About this article
Cite this article
Pathak, J.L., Bakker, A.D., Luyten, F.P. et al. Systemic Inflammation Affects Human Osteocyte-Specific Protein and Cytokine Expression. Calcif Tissue Int 98, 596–608 (2016). https://doi.org/10.1007/s00223-016-0116-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-016-0116-8