Abstract
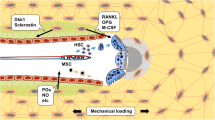
Mechanical loading is integral to the repair of bone damage. Osteocytes are mechanosensors in bone and participate in signaling through gap junction channels, which are primarily comprised of connexin 43 (Cx43). Nitric oxide (NO) and prostaglandin E2 (PGE2) have anabolic and catabolic effects on bone, and the secretion of these molecules occurs after mechanical stimulation. The effect of age on the repair of bone tissue after damage and on the ability of regenerated bone to transduce mechanical stimulation into a cellular response is unexplored. The goal of this study was to examine (1) osteocytes and their mineralized matrix within regenerated bone from aged and mature animals and (2) the ability of regenerated bone explants from aged and mature animals to transduce cyclic mechanical loading into a cellular response through NO and PGE2 secretion. Bilateral cortical defects were created in the diaphysis of aged (21-month-old) or mature (6-month-old) male rats, and new bone tissue was allowed to grow into a custom implant of controlled geometry. Mineralization and mineral-to-matrix ratio were significantly higher in regenerated bone from aged animals, while lacunar and osteocyte density and phosphorylated (pCx43) and total Cx43 protein were significantly lower, relative to mature animals. Regenerated bone from mature rats had increased pCx43 protein and PGE2 secretion with loading and greater NO secretion relative to aged animals. Reduced osteocyte density and Cx43 in regenerated bone in aged animals could limit the establishment of gap junctions as well as NO and PGE2 secretion after loading, thereby altering bone formation and resorption in vivo.




Similar content being viewed by others
References
Rubin CT, Lanyon LE (1984) Regulation of bone formation by applied dynamic loads. J Bone Joint Surg Am 66(3):397–402
Raggatt LJ, Partridge NC (2010) Cellular and molecular mechanisms of bone remodeling. J Biol Chem 285(33):25103–25108
Robling AG, Castillo AB, Turner CH (2006) Biomechanical and molecular regulation of bone remodeling. Annu Rev Biomed Eng 8:455–498
Szulc P, Garnero P, Munoz F, Marchand F, Delmas PD (2001) Cross-sectional evaluation of bone metabolism in men. J Bone Miner Res 16(9):1642–1650
Loiselle AE, Jiang JX, Donahue HJ (2013) Gap junction and hemichannel functions in osteocytes. Bone 54:205–212
Stains JP, Civitelli R (2005) Gap junctions in skeletal development and function. Biochim Biophys Acta 1719(1–2):69–81
Blackwell KA, Raisz LG, Pilbeam CC (2010) Prostaglandins in bone: bad cop, good cop? Trends Endocrinol Metab 21(5):294–301
Weinreb M, Rutledge SJ, Rodan GA (1997) Systemic administration of an anabolic dose of prostaglandin E2 induces early-response genes in rat bones. Bone 20(4):347–353
Gao Q, Xu M, Alander CB, Choudhary S, Pilbeam CC, Raisz LG (2009) Effects of prostaglandin E2 on bone in mice in vivo. Prostaglandins Other Lipid Mediat 89(1–2):20–25
Dekel S, Lenthall G, Francis MJ (1981) Release of prostaglandins from bone and muscle after tibial fracture: an experimental study in rabbits. J Bone Joint Surg Br 63-B(2):185–189
Naik AA, Xie C, Zuscik MJ, Kingsley P, Schwarz EM, Awad H et al (2009) Reduced COX-2 expression in aged mice is associated with impaired fracture healing. J Bone Miner Res 24(2):251–264
Chow JW, Fox SW, Lean JM, Chambers TJ (1998) Role of nitric oxide and prostaglandins in mechanically induced bone formation. J Bone Miner Res 13(6):1039–1044
van’t Hof RJ, Ralston SH (2001) Nitric oxide and bone. Immunology 103(3):255–261
Damoulis PD, Hauschka PV (1997) Nitric oxide acts in conjunction with proinflammatory cytokines to promote cell death in osteoblasts. J Bone Miner Res 12(3):412–422
Zhu W, Diwan AD, Lin JH, Murrell GA (2001) Nitric oxide synthase isoforms during fracture healing. J Bone Miner Res 16(3):535–540
Diwan AD, Wang MX, Jang D, Zhu W, Murrell GA (2000) Nitric oxide modulates fracture healing. J Bone Miner Res 15(2):342–351
Baldik Y, Diwan AD, Appleyard RC, Fang ZM, Wang Y, Murrell GA (2005) Deletion of iNOS gene impairs mouse fracture healing. Bone 37(1):32–36
Cau SB, Carneiro FS, Tostes RC (2012) Differential modulation of nitric oxide synthases in aging: therapeutic opportunities. Front Physiol 3:218
Waldorff EI, Christenson KB, Cooney LA, Goldstein SA (2010) Microdamage repair and remodeling requires mechanical loading. J Bone Miner Res 25(4):734–745
Turner CH, Takano Y, Owan I (1995) Aging changes mechanical loading thresholds for bone formation in rats. J Bone Miner Res 10(10):1544–1549
Donahue SW, Jacobs CR, Donahue HJ (2001) Flow-induced calcium oscillations in rat osteoblasts are age, loading frequency, and shear stress dependent. Am J Physiol Cell Physiol 281(5):C1635–C1641
Rubin CT, Bain SD, McLeod KJ (1992) Suppression of the osteogenic response in the aging skeleton. Calcif Tissue Int 50(4):306–313
Silbermann M, Bar-Shira-Maymon B, Coleman R, Reznick A, Weisman Y, Steinhagen-Thiessen E et al (1990) Long-term physical exercise retards trabecular bone loss in lumbar vertebrae of aging female mice. Calcif Tissue Int 46(2):80–93
Buhl KM, Jacobs CR, Turner RT, Evans GL, Farrell PA, Donahue HJ (2001) Aged bone displays an increased responsiveness to low-intensity resistance exercise. J Appl Physiol 90(4):1359–1364
Srinivasan S, Agans SC, King KA, Moy NY, Poliachik SL, Gross TS (2003) Enabling bone formation in the aged skeleton via rest-inserted mechanical loading. Bone 33(6):946–955
Pruitt LA, Taaffe DR, Marcus R (1995) Effects of a 1 year high-intensity versus low-intensity resistance training program on bone mineral density in older women. J Bone Miner Res 10(11):1788–1795
Allison SJ, Folland JP, Rennie WJ, Summers GD, Brooke-Wavell K (2013) High impact exercise increased femoral neck bone mineral density in older men: a randomised unilateral intervention. Bone 53:321–328
Hoffler CE, Hankenson KD, Miller JD, Bilkhu SK, Goldstein SA (2006) Novel explant model to study mechanotransduction and cell–cell communication. J Orthop Res 24(8):1687–1698
Meganck JA, Kozloff KM, Thornton MM, Broski SM, Goldstein SA (2009) Beam hardening artifacts in micro-computed tomography scanning can be reduced by X-ray beam filtration and the resulting images can be used to accurately measure BMD. Bone 45(6):1104–1116
Volkman SK, Galecki AT, Burke DT, Paczas MR, Moalli MR, Miller RA et al (2003) Quantitative trait loci for femoral size and shape in a genetically heterogeneous mouse population. J Bone Miner Res 18(8):1497–1505
Draper ER, Morris MD, Camacho NP, Matousek P, Towrie M, Parker AW et al (2005) Novel assessment of bone using time-resolved transcutaneous Raman spectroscopy. J Bone Miner Res 20(11):1968–1972
Mazzali M, Kipari T, Ophascharoensuk V, Wesson JA, Johnson R, Hughes J (2002) Osteopontin—a molecule for all seasons. QJM 95(1):3–13
Cheng MZ, Zaman G, Rawlinson SC, Pitsillides AA, Suswillo RF, Lanyon LE (1997) Enhancement by sex hormones of the osteoregulatory effects of mechanical loading and prostaglandins in explants of rat ulnae. J Bone Miner Res 12(9):1424–1430
Waldorff EI, Goldstein SA, McCreadie BR (2007) Age-dependent microdamage removal following mechanically induced microdamage in trabecular bone in vivo. Bone 40(2):425–432
Bonar LC, Roufosse AH, Sabine WK, Grynpas MD, Glimcher MJ (1983) X-ray diffraction studies of the crystallinity of bone mineral in newly synthesized and density fractionated bone. Calcif Tissue Int 35(2):202–209
Currey JD (1984) Effects of differences in mineralization on the mechanical properties of bone. Philos Trans R Soc Lond B Biol Sci 304(1121):509–518
Donnelly E, Chen DX, Boskey AL, Baker SP, van der Meulen MC (2010) Contribution of mineral to bone structural behavior and tissue mechanical properties. Calcif Tissue Int 87(5):450–460
Burr DB (2002) The contribution of the organic matrix to bone’s material properties. Bone 31(1):8–11
Raghavan M, Sahar ND, Kohn DH, Morris MD (2012) Age-specific profiles of tissue-level composition and mechanical properties in murine cortical bone. Bone 50(4):942–953
Yerramshetty JS, Akkus O (2008) The associations between mineral crystallinity and the mechanical properties of human cortical bone. Bone 42(3):476–482
Mehta M, Strube P, Peters A, Perka C, Hutmacher D, Fratzl P et al (2010) Influences of age and mechanical stability on volume, microstructure, and mineralization of the fracture callus during bone healing: is osteoclast activity the key to age-related impaired healing? Bone 47(2):219–228
Joiner DM, Tayim RJ, Kadado A, Goldstein SA (2012) Bone marrow stromal cells from aged male rats have delayed mineralization and reduced response to mechanical stimulation through nitric oxide and ERK1/2 signaling during osteogenic differentiation. Biogerontology 13(5):467–478
Justesen J, Stenderup K, Eriksen EF, Kassem M (2002) Maintenance of osteoblastic and adipocytic differentiation potential with age and osteoporosis in human marrow stromal cell cultures. Calcif Tissue Int 1:36–44
Busse B, Djonic D, Milovanovic P, Hahn M, Puschel K, Ritchie RO et al (2010) Decrease in the osteocyte lacunar density accompanied by hypermineralized lacunar occlusion reveals failure and delay of remodeling in aged human bone. Aging Cell 9(6):1065–1075
Vashishth D, Verborgt O, Divine G, Schaffler MB, Fyhrie DP (2000) Decline in osteocyte lacunar density in human cortical bone is associated with accumulation of microcracks with age. Bone 26(4):375–380
Xia X, Batra N, Shi Q, Bonewald LF, Sprague E, Jiang JX (2010) Prostaglandin promotion of osteocyte gap junction function through transcriptional regulation of connexin 43 by glycogen synthase kinase 3/beta-catenin signaling. Mol Cell Biol 30(1):206–219
Acknowledgements
The authors thank XiXi Wang, Sharon Reske, Kathy Sweet, and Bonnie Nolan for animal care; Dennis Kayner and Charles Roehm for their assistance with the three-point bending system; and David Nadziejka for technical editing of the manuscript. An NSF Graduate Student Research Fellowship (D.M.J.) and NIH grant R01 AR51504 (S.A.G.) supported this work.
Author information
Authors and Affiliations
Corresponding author
Additional information
The authors have stated that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Joiner, D.M., Tayim, R.J., McElderry, JD. et al. Aged Male Rats Regenerate Cortical Bone with Reduced Osteocyte Density and Reduced Secretion of Nitric Oxide After Mechanical Stimulation. Calcif Tissue Int 94, 484–494 (2014). https://doi.org/10.1007/s00223-013-9832-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00223-013-9832-5