Abstract
Binaural beats represent the auditory experience of an oscillating sound that occurs when two sounds with neighboring frequencies are presented to one’s left and right ear separately. Binaural beats have been shown to impact information processing via their putative role in increasing neural synchronization. Recent studies of feature-repetition effects demonstrated interactions between perceptual features and action-related features: repeating only some, but not all features of a perception–action episode hinders performance. These partial-repetition (or binding) costs point to the existence of temporary episodic bindings (event files) that are automatically retrieved by repeating at least one of their features. Given that neural synchronization in the gamma band has been associated with visual feature bindings, we investigated whether the impact of binaural beats extends to the top-down control of feature bindings. Healthy adults listened to gamma-frequency (40 Hz) binaural beats or to a constant tone of 340 Hz (control condition) for ten minutes before and during a feature-repetition task. While the size of visuomotor binding costs (indicating the binding of visual and action features) was unaffected by the binaural beats, the size of visual feature binding costs (which refer to the binding between the two visual features) was considerably smaller during gamma-frequency binaural beats exposure than during the control condition. Our results suggest that binaural beats enhance selectivity in updating episodic memory traces and further strengthen the hypothesis that neural activity in the gamma band is critically associated with the control of feature binding.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Binaural beats represent the auditory experience of an oscillating sound that occurs when two sounds with neighboring frequencies are presented to one’s left and right ear separately. Binaural beats are perceived as periodic loudness fluctuations of a sound (Karino et al. 2006). The experience of such oscillations is described as hearing a sound with a frequency equal to the difference in frequencies between the original tones (Oster 1973). For instance, when the left ear is presented with a tone of 320 Hz, and the right ear with a tone of 360 Hz, the subject will perceive a tone that oscillates at a frequency of 40 Hz (i.e., 40 beats per second). In a seminal study, Karino et al. (2006) explored the cortical representation of binaural beat frequencies by applying modulation frequencies of 4.00–6.66 Hz while recording magnetic fields using magnetoencephalography. It was shown that the auditory steady-state responses (ASSR) to binaural beats emerged from the superior temporal, posterior parietal, and frontal cortices, in addition to the auditory cortex. However, beat-generated ASSR in the gamma-frequency seem to originate mainly in the primary auditory cortex (Pastor et al. 2002; Pantev et al. 1996). Even though direct causal links between neural activity and binaural beats are yet to be demonstrated, there is converging evidence that binaural beats are accompanied by, and systematically related to, neural synchronization. Indeed, it has been proposed that binaural beats represent a neural entrainment technique by means of which the brain “takes over” or synchronizes its activity based on external acoustic stimulation (Chaieb et al. 2015). The basic assumption is that listening to binaural beats in a specific frequency band will entrain the same frequency in the brain (Becher et al. 2015). The theoretical idea behind neural entrainment is that the rhythmic oscillatory activity within and between different brain regions can enhance cognitive functioning (see Chaieb et al. 2015 for a review on the effect of binaural beats on cognition and mood). Indeed, in recent years, it has been shown that binaural beats have an impact on the efficiency of allocating attention over time (Reedijk et al. 2015), attentional focusing (Colzato et al. 2015), dual-talk crosstalk effect (Hommel et al. 2016), and creativity (Reedijk et al. 2013). If binaural beats impact cognition via neural synchronization, it is most likely through the frequency of the beat. Whereas short-range communication within brain areas is often linked to neural synchronization in the gamma-frequency (i.e., centered on 40 Hz), long-range communication is related to neuronal phase locking in the slower frequency bands (von Stein and Sarnthein 2000; Schnitzler and Gross 2005). In line with this idea, the increase of gamma band power through neurofeedback improved the top-down control of feature bindings (Keizer et al. 2010a, b). Given this aforementioned link, in the current study, we were interested in searching for converging evidence of whether high-frequency binaural beats (gamma range) enhance the control and management of feature bindings.
Studies of feature-repetition effects commonly show interactions between perceptual features and action-related features: in contrast to complete repetitions and alternations, repeating only some but not all features of a perception–action episode (i.e., of a particular combination of stimulus and response features) hinders performance (Hommel 1998). Later studies have provided evidence that this effect is due to the fact that (a) the co-occurrence of stimulus and response features leads to the binding of the respective feature codes into the so-called event files (Hommel 2004), which are then (b) retrieved whenever at least one of the features is repeated (Beste et al. 2016; Colzato et al. 2005; Keizer et al. 2008; Frings et al. 2007; Kühn et al. 2011; Moeller and Frings 2014; Petruo et al. 2016). The binding part of this scenario seems to be rather immune to all sorts of attentional and instructional variation, while the retrieval part is systematically affected by the degree to which a particular stimulus dimension is attended (e.g., Hommel 2004, 2007). In particular, there is evidence that bindings including irrelevant features are less likely to be retrieved in individuals with high cognitive control abilities, such as individuals high in fluid intelligence (Colzato et al. 2006) and normally developing children as compared to children suffering from autistic spectrum disorder (Zmigrod et al. 2013). Of particular interest for the present investigation, two studies in which neurofeedback training was designed to increase local gamma band activity (Keizer et al. 2010a, b) found greater flexibility in handling (selectively retrieving) visual feature binding costs (which refer to the binding between the two visual features), but not visuomotor binding costs (indicating the binding of visual and action features).
If we assume that high-frequency binaural beats (gamma range) promote cognitive control (Hommel et al. 2016) and that neural synchronization in the gamma-frequency is associated with visual feature bindings (Keizer et al. 2010a, b), we would predict decreased visual feature but not visuomotor binding costs when listening to gamma-frequency beats as compared to a constant tone. If this were the case, we would expect an interaction between visual feature bindings and the kind of beats (gamma-frequency vs. control), with a greater flexibility in handling (selectively retrieving) visual feature binding costs with gamma-frequency beats than with a constant tone. Theoretically, such an interaction would suggest that binaural beats enhance selectivity in updating episodic memory traces. We tested this prediction by adopting a feature-repetition task (i.e., a task known to generate event file effects) and having participants perform it while listening to either high-frequency binaural beats (the gamma group) or to a continuous tone of 340 Hz (the control group).
Method
Participants
Forty Leiden University undergraduate students (30 females, 10 males, mean age = 22.10 years, SD = 2.82, range 18–28) without sensory problems participated in the experiment. Participants were recruited via an online recruiting system and were offered course credits for participating in the study. Once recruited, all participants were screened individually by the same lab assistant using the Mini International Neuropsychiatric Interview (MINI; Sheehan et al. 1998). The MINI is a short, structured interview that screens for several psychiatric disorders and drug use, often used in clinical and pharmacological research (Colzato et al. 2010, 2013a; Sheehan et al. 1998). Participants were randomly and equally distributed in two experimental groups. Twenty participants (4 males, mean age = 22.2 years, SD = 3.3) were exposed to gamma-frequency (40 Hz) binaural beats, and the other 20 (6 males, mean age = 22.0 years, SD = 2.4) were assigned to a control condition, in which they were exposed to a constant tone of 340 Hz.
All procedures performed were in accordance with the ethical standards of the institutional research committee (Leiden University, Institute for Psychological Research) and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. Informed consent was obtained from all individual participants included in the study.
Procedure
All participants took part in a single session and were tested individually. A double-blinded, sham/placebo-controlled, between-subject design was used to assess the effect of online gamma-frequency (40 Hz) binaural beats exposure on the top-down control of feature binding in healthy young volunteers. Upon arrival, after having read and signed the informed consent, participants familiarized with the event file task. Subsequently, they listened to gamma-frequency (40 Hz; 320 Hz left ear, 360 Hz right ear) binaural beats or to a constant tone of 340 Hz (control condition), for 10 min before (at rest) and during the event file task. Binaural beats were presented through in-ear headphones (Etymotic Research ER-4B microPro), which provide 35 dB noise attenuation. All tones were embedded in white noise, 20 Hz–10 kHz band filtered, to enhance clarity of the beats (Oster 1973; Reedijk et al. 2013). As beats are best perceived with a carrier frequency between 300 and 600 Hz (Licklider et al. 1950; Perrott and Nelson 1969), the binaural beats were centered around a 340 Hz carrier tone, which served as the constant tone in the control condition. After the event file task, the experimental session ended and participants were debriefed and dismissed.
Event file task
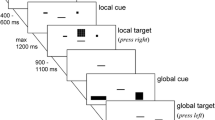
The task, which was originally developed by Hommel (1998), was adapted from Colzato et al. (2012, 2013b), see Fig. 1. During the task, participants were seated approximately 50 cm from a 17-inch monitor (96 dpi with a refresh rate of 120 Hz). The E-Prime 2.0 software system (Psychology Software Tools, Inc., Pittsburgh, PA) was used to generate the task and collect the responses.
Sequence of events in the event file task. A visual response cue signaled a left or right response (R1) that was to be delayed until presentation of the first stimulus S1 (S1 is used as a detection signal for R1). The second stimulus S2 appeared 1000 ms after S1. S2 signaled R2, a speeded left or right response according to the shape
The task measures binding-related effects by examining partial-repetition costs related to combinations of stimulus features (shape and color in our case) and combinations of stimulus features and the response. To manipulate the repetition versus alternation of stimulus features and responses, each trial involved a response to the presentation of a prime stimulus (S1 → R1) followed by a response to presentation of a probe stimulus (S2 → R2), see Fig. 1. Prime and probe stimuli consisted of yellow or green colored images of a banana or an apple. The probe trial required a manual binary left–right response (R2) to the shape of the second stimulus S2 (an apple or a banana). The prime trial required a manual response (R1) to the mere onset of the first stimulus (S1). The correct R1 was signaled in advance of S1 (through a left- or right-pointing arrowhead), so that S1 and R1 could be varied independently, which was necessary to create orthogonal repetitions and alternations of stimulus shape and response. An additional stimulus feature, namely color, was also varied by presenting the apple or banana in green or yellow (see Colzato et al. 2013b). So the following combinations were possible: green apple, green banana, yellow apple, and yellow banana.
Each trial began with the presentation of an arrowhead (stimulus duration = 1500 ms) that pointed to the left or to the right, and that signaled the response to be given upon the onset of the prime stimulus (S1), which appeared after a 1000 ms inter-stimulus period. The prime stimulus was presented for 1000 ms. Participants were instructed to press the left (“z”) key if the arrowhead preceding the prime stimulus pointed to the left, and the right (“m”) key if the arrowhead pointed to the right. After the response to the prime, the probe stimulus (S2) appeared (stimulus duration = 1500 ms). Participants were instructed to respond to the shape of the stimulus: the presentation of an “apple” required them to press the left (“z”) key, whereas the presentation of a “banana” required them to press the right (“m”) key. Participants were asked to respond as quickly and accurately as possible to both S1 and S2.
The task comprised a practice block of 10 trials, and an experimental block of 192 trials, presented in a random order. Experimental trials were equally distributed across eight conditions, resulting for the combinations of stimulus features (shape and color) and responses, which could all either repeat or alternate, thus creating a 2 × 2 × 2 factorial design.
Statistical analyses
First, an independent samples t test was performed to test age differences between the two groups. A Chi-square test was used to verify whether the two groups were comparable in terms of gender distribution.
The effect of binaural beats on the updating of stimulus–response episodes was assessed by submitting R2 correct reaction times (RTs) and percentage of errors (PEs) to separate 2 × 2 × 2 ANOVAs with Group (gamma vs. control) as a between-participant factor and the repetition vs. alternation of response (R1 → R2) and stimulus shape and color (S1 → S2) [hereafter referred to as Response, Shape, and Color, respectively] as within-participant factors. For the analysis of RTs, we excluded anticipatory responses, that is, RTs faster than 100 ms.
Bindings of stimulus features are indexed by a significant two-way interaction between Shape and Color, whereas stimulus–response bindings are reflected by significant two-way interactions between Shape and Response and between Color and Response (Hommel 1998). Partial-repetition costs were calculated as the difference between RTs for partial-repetitions (feature X repeated and feature Y alternated, or vice versa) and the RTs for complete repetitions and “complete” alternations. That is, if features X and Y repeated and alternated, their binding effect B XY would be calculated as B XY = [(RTX/alt, Y/rep + RTX/rep,Y/alt)/2) − ((RTX/rep,Y/rep + RTX/alt,Y/alt)/2]. A value close to zero means that the repetition effects of the two given features do not interact; a value greater than zero indicates a “binding-type” interaction.
A significance level of p < 0.05 was adopted for all statistical tests.
Results
Participants
No significant differences were found among groups with respect to age t(38) = 0.2, p = 0.83, or gender distribution, χ 2 (1, 40) = 0.53, p = 0.47.
Event file task
Table 1 provides an overview of the relevant ANOVA outcomes for RTs and PEs obtained for R2. The analysis of RTs did not reveal any significant main effects, all F s ≤ 3.6, all p s ≥ 0.07, all \(\eta_{\text{ps}}^{2}\) ≤ 0.09. Replicating earlier findings (Hommel 1998; Hommel and Colzato 2004; Colzato et al. 2012, 2013b), the analysis of RTs revealed a significant interaction between Response and Shape, F(1.38) = 65.48, p < 0.001, \(\eta_{\text{p}}^{2}\) = 0.63: repeating one but not the other feature slowed down responses (479 vs. 449 ms). The interactions between Response and Color and between Shape and Color were not significant, all F s ≤ 3.1, all p s ≥ 0.09, all \(\eta_{\text{ps}}^{2}\) ≤ 0.07—repeating one but not the other feature produced comparable responses (467 vs. 461 ms and 466 vs. 463 ms, respectively). Crucially, a significant three-way interaction involving Shape, Color, and Group was found, F(1,38) = 12.20, p = 0.001, \(\eta_{\text{p}}^{2}\) = 0.24: partial-repetition costs for color–shape binding occurred for the control group, but not for the gamma group, see Table 1. In contrast, partial-repetition costs for color–response and shape–response bindings were comparable across the two groups, as indicated by the absence of any significant three-way interaction involving Group with either Color and Response, or Shape and Response, all F s < 1, all p s ≥ 0.34, all \(\eta_{\text{ps}}^{2}\) ≤ 0.02, see Table 1. All the remaining interactions were not significant either, all F s ≤ 2.6, all p s ≥ 0.11, all \(\eta_{\text{ps}}^{2}\) ≤ 0.07.
The analysis of PEs revealed only two significant sources of variance. First, a significant main effect of Response was found, F(1,38) = 10.35, p = 0.003, \(\eta_{\text{p}}^{2}\) = 0.21: response repetition produced less errors than response alternation (3.3 vs. 5.1%). Second, a significant interaction between Shape and Response was found, F(1,38) = 63.16, p < 0.001, \(\eta_{\text{p}}^{2}\) = 0.62: repeating one but not the other feature elicited less accurate responses (1.6 vs. 6.8%). No other significant main effects or interactions were found, all F s ≤ 3.7, all p s ≥ 0.06, all \(\eta_{\text{ps}}^{2}\) ≤ 0.09.
Discussion
The aim of this study was to investigate whether high-frequency binaural beats (gamma range) would show a specific effect in the top-down control of feature bindings, that is, of bindings between codes that represent the features of experienced objects and stimulus–response episodes (Hommel 1998, 2004). As expected, the size of visuomotor binding costs (indicating the binding of visual and action features) was unaffected by the binaural beats, while the size of visual feature binding costs (which refer to the binding between the two visual features) was considerably smaller in the gamma-frequency binaural beats group than in the control group. Our findings suggest that binaural beats enhance selectivity in updating episodic memory traces. Our results fit with previous neurofeedback training studies in which increased local gamma band activity led to greater flexibility in handling (selectively retrieving) only of visual feature binding costs, but not of visuomotor binding costs (Keizer et al. 2010a, b). Even though direct causal links between gamma activity and feature integration are yet to be confirmed, there is converging evidence that processes involved in the creation and maintenance of visual feature bindings are systematically associated with neural activity in the gamma band. In particular, gamma band power has been linked to visual awareness (Engel and Singer 2001; Wyar and Tallon-Baudry 2008) and visual working memory (Tallon-Baudry et al. 1998). Further, neural synchronization in gamma band and visual feature integration seem to be linked to the same neurotransmitter system. Gamma synchronization in the primary visual cortex of the cat is promoted by muscarinic–cholinergic agonists and impaired by muscarinic–cholinergic antagonists (Rodriguez-Bermudez et al. 2004). This is in line with the findings in healthy young humans showing that caffeine—a muscarinic–cholinergic agonist—enhances the updating of visual feature bindings (Colzato et al. 2005), while alcohol—a muscarinic–cholinergic antagonist—hampers such selective retrieval (Colzato et al. 2004). Future studies should investigate whether the concomitant administration of muscarinic–cholinergic agonists and high-frequency binaural beats (gamma range) might have an additive effect on enhancing the updating of visual feature bindings compared to the separate administration of the single factors. The fact that the effect of gamma-frequency beats was limited to visual feature bindings is consistent with previous research, demonstrating that while visual feature integration is associated with gamma band activity, visuomotor integration relies on beta band activity (Roelfsema et al. 1997). It would be interesting in future studies to investigate whether beta-frequency beats might impact visuomotor bindings but not visual feature bindings.
The finding of greater flexibility in handling visual feature binding costs when listening to gamma-frequency beats may be of particular interest for some clinical conditions and intoxication state. Previous studies have found impairment in the updating of feature bindings in children with Autism Spectrum Disorder (ASD) (Zmigrod et al. 2013), patients suffering from Gilles de la Tourette syndrome (Beste et al. 2016), after acute alcohol consumption (Colzato et al. 2004), and in elderly as compared to young adults (Hommel et al. 2011). That is, binaural beats, by enhancing selectivity in updating episodic memory traces, may slow down and (partially) compensate for the cognitive negative consequences associated with ASD, Gilles de la Tourette syndrome, alcohol consumption, and aging.
Our study used a between-subject design to avoid possible practice effects on task performance. However, a between-subject design can be sensitive to differences between the individuals in the two groups. Hence, follow-up investigations should point out whether our findings can be replicated in a within-subject comparison (where the same participants will be exposed to both the control and binaural beats conditions) and extended using different versions of the feature-repetition task across different modalities.
Our findings bring converging evidence on the idea that binaural beats act as a neural entrainment technique that works by moderating brain oscillations that specific cognitive processes require or profit from (Chaieb et al. 2015), and oscillations in the gamma-frequency band might be particularly relevant for this purpose (Schwarz and Taylor 2005; Pastor et al. 2002). Accordingly, future studies should make use of electro- or magnetoencephalographic methods (e.g., Picton et al. 1987; Galambos et al. 1981, Becher et al. 2015), which would permit directly assessing the relationship between binaural beats, the auditory entrainment of brain oscillations, and cognitive processes.
Change history
19 January 2021
A Correction to this paper has been published: https://doi.org/10.1007/s00221-020-06018-z
References
Becher AK, Höhne M, Axmacher N, Chaieb L, Elger CE, Fell J (2015) Intracranial electroencephalography power and phase synchronization changes during monaural and binaural beat stimulation. Eur J Neurosci 41(2):254–263
Beste C, Tübing J, Seeliger H, Bäumer T, Brandt V, Stock AK, Münchau A (2016) Altered perceptual binding in Gilles de la Tourette syndrome. Cortex 83:160–166
Chaieb L, Wilpert EC, Reber TP, Fell J (2015) Auditory beat stimulation and its effects on cognition and mood states. Front Psychiat 6:70
Colzato LS, Erasmus V, Hommel B (2004) Moderate alcohol consumption in humans impairs feature binding in visual perception but not across perception and action. Neurosci Lett 360:103–105
Colzato LS, Fagioli S, Erasmus V, Hommel B (2005) Caffeine, but not nicotine enhances visual feature binding. Eur J Neurosci 21:591–595
Colzato LS, van Wouwe NC, Lavender TJ, Hommel B (2006) Intelligence and cognitive flexibility: fluid intelligence correlates with feature “unbinding” across perception and action. Psychon B Rev 13:1043–1048
Colzato LS, Pratt J, Hommel B (2010) Dopaminergic control of attentional flexibility: inhibition of Return is associated with the dopamine transporter gene (DAT1). Front Hum Neurosci 14:53. doi:10.3389/fnhum.2010.00053
Colzato LS, van Wouwe NC, Hommel B, Zmigrod S, Ridderinkhof KR, Wylie SA (2012) Dopaminergic modulation of the updating of stimulus–response episodes in Parkinson’s disease. Behav Brain Res 228(1):82–86
Colzato LS, van den Wildenberg WP, Hommel B (2013a) The genetic impact (C957T-DRD2) on inhibitory control is magnified by aging. Neuropsychologia 51(7):1377–1381
Colzato LS, Zmigrod S, Hommel B (2013b) Dopamine, norepinephrine, and the management of sensorimotor bindings: individual differences in updating of stimulus–response episodes are predicted by DAT1, but not DBH5′-ins/del. Exp Brain Res 228(2):213–220
Colzato LS, Barone H, Sellaro R, Hommel B (2015) More attentional focusing through binaural beats: evidence from the global-local task. Psychol Res. doi:10.1007/s00426-015-0727-0
Engel AK, Singer W (2001) Temporal binding and the neural correlates of sensory awareness. Trends Cogn Sci 5:16–25
Frings C, Rothermund K, Wentura D (2007) Distractor repetitions retrieve previous responses to targets. Q J Exp Psychol 60(10):1367–1377
Galambos R, Makeig S, Talmachoff PJ (1981) A 40-Hz auditory potential recorded from the human scalp. Proc Natl Acad Sci USA 78(4):2643–2647
Hommel B (1998) Event files: evidence for automatic integration of stimulus response episodes. Vis Cogn 5:183–216
Hommel B (2004) Event files: feature binding in and across perception and action. Trends Cogn Sci 8:494–500
Hommel B (2007) Feature integration across perception and action: event files affect response choice. Psychol Res 71:42–63
Hommel B, Kray J, Lindenberger U (2011) Feature integration across the lifespan: stickier stimulus-response bindings in children and older adults. Front Psychol 2:268
Hommel B, Sellaro R, Fischer R, Borg S, Colzato LS (2016) High-frequency binaural beats increase cognitive flexibility: evidence from dual-task crosstalk. Front Psychol 7:1287
Karino S, Yumoto M, Itoh K, Uno A, Yamakawa K, Sekimoto S, Kaga K (2006) Neuromagnetic responses to binaural beat in human cerebral cortex. J Neurophysiol 96:1927–1938
Keizer AW, Colzato LS, Hommel B (2008) Integrating faces, houses, motion, and action: spontaneous binding across ventral and dorsal processing streams. Acta Psychol 127(1):177–185
Keizer AW, Verment R, Hommel B (2010a) Enhancing cognitive control through neurofeedback: a role of gamma-band activity in managing episodic retrieval. Neuroimage 49:3404–3413
Keizer AW, Verschoor M, Verment R, Hommel B (2010b) The effect of gamma enhancing neurofeedback on measures of feature-binding flexibility and intelligence. Int J Psychophysiol 75:25–32
Kühn S, Keizer AW, Colzato LS, Rombouts SA, Hommel B (2011) The neural underpinnings of event-file management: evidence for stimulus-induced activation of and competition among stimulus–response bindings. J Cogn Neurosci 23(4):896–904
Licklider JCR, Webster JC, Hedlun JM (1950) On the frequency limits of binaural beats. J Acoust Soc Am 22(4):468–473
Moeller B, Frings C (2014) Long-term response-stimulus associations can influence distractor-response bindings. Adv Cogn Psychol 10(2):68–80
Oster G (1973) Auditory beats in the brain. Sci Am 229:94–102
Pantev C, Roberts LE, Elbert T, Roß B, Wienbruch C (1996) Tonotopic organization of the sources of human auditory steady-state responses. Hear Res 101(1):62–74
Pastor MA, Artieda J, Arbizu J, Marti-Climent JM, Peñuelas I, Masdeu JC (2002) Activation of human cerebral and cerebellar cortex by auditory stimulation at 40 Hz. J Neurosci 22(23):10501–10506
Perrott DR, Nelson MA (1969) Limits for the detection of binaural beats. J Acoust Soc Am 46(6B):1477–1481
Petruo VA, Stock AK, Münchau A, Beste C (2016) A systems neurophysiology approach to voluntary event coding. Neuroimage 135:324–332
Picton TW, Skinner CR, Champagne SC, Kellett AJ, Maiste AC (1987) Potentials evoked by the sinusoidal modulation of the amplitude or frequency of a tone. J Acoust Soc Am 82(1):165–178
Reedijk SA, Bolders A, Hommel B (2013) The impact of binaural beats on creativity. Front Hum Neurosci 7:786
Reedijk SA, Bolders A, Colzato LS, Hommel B (2015) Eliminating the attentional blink through binaural beats: a case for tailored cognitive enhancement. Front Psychiatry 6:82
Rodriguez-Bermudez R, Kallenbach U, Singer W, Munk MH (2004) Short- and long-term effects of cholinergic modulation on gamma oscillations and response synchronization in the visual cortex. J Neurosci 24:10369–10378
Roelfsema PR, Engel AK, König P, Singer W (1997) Visuomotor integration is associated with zero time-lag synchronization among cortical areas. Nature 385(6112):157–161
Schnitzler A, Gross J (2005) Normal and pathological oscillatory communication in the brain. Nat Rev Neurosci 6:285–296
Schwarz DW, Taylor P (2005) Human auditory steady state responses to binaural and monaural beats. Clin Neurophysiol 116(3):658–668
Sheehan DV, Lecrubier Y, Sheehan KH et al (1998) The Mini-International Neuropsychiatric Interview (MINI): the development and validation of a structured diagnostic psychiatric interview for DSM-IV and ICD-10. J Clin Psychiatry 59:22–23
Tallon-Baudry C, Bertrand O, Peronnet F, Pernier J (1998) Induced γ-band activity during the delay of a visual short-term memory task in humans. J Neurosci 18:4244–4254
von Stein A, Sarnthein J (2000) Different frequencies for different scales of cortical integration: from local gamma to long range alpha/theta synchronization. Int J Psychophysiol 38:301–313
Wyar V, Tallon-Baudry C (2008) Neural dissociation between visual awareness and spatial attention. J Neurosci 28:2667–2679
Zmigrod S, de Sonneville LMJ, Colzato LS, Swaab H, Hommel B (2013) Cognitive control of feature bindings: evidence from children with autistic spectrum disorder. Psychol Res 77:147–154
Acknowledgements
This work was supported by a research grant from the Netherlands Organization for Scientific Research (NWO; www.nwo.nl) awarded to L.S.C. (Vidi grant: #452-12-001). The NWO had no further role in study design; in the collection, analysis, and interpretation of data; in the writing of the report; and in the decision to submit the paper for publication. We thank Andres von Schnehen for his enthusiasm and invaluable assistance in the data collection.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Additional information
This article has been retracted. Please see the retraction notice for more detail:https://doi.org/10.1007/s00221-020-06018-z
Rights and permissions
Open Access This article is distributed under the terms of the Creative Commons Attribution 4.0 International License (http://creativecommons.org/licenses/by/4.0/), which permits unrestricted use, distribution, and reproduction in any medium, provided you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons license, and indicate if changes were made.
About this article
Cite this article
Colzato, L.S., Steenbergen, L. & Sellaro, R. RETRACTED ARTICLE: The effect of gamma-enhancing binaural beats on the control of feature bindings. Exp Brain Res 235, 2125–2131 (2017). https://doi.org/10.1007/s00221-017-4957-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00221-017-4957-9